Published online Mar 14, 2017. doi: 10.3748/wjg.v23.i10.1780
Peer-review started: September 19, 2016
First decision: October 20, 2016
Revised: November 6, 2016
Accepted: December 2, 2016
Article in press: December 2, 2016
Published online: March 14, 2017
To compare the expression levels of interleukin (IL)-6 in colorectal cancer (CRC) tissues and adjacent non-cancerous tissues, and analyse the correlation of IL-6 expression with the clinicopathological parameters of CRC.
Fifty CRC tissue specimens and 50 matched adjacent mucosa specimens were collected. The expression of IL-6 in these clinical samples was examined by immunohistochemical staining. The correlation between IL-6 expression and clinicopathological parameters was assessed by statistical analysis.
IL-6 expression was significantly elevated in CRC tissues compared with noncancerous tissues (P < 0.001). IL-6 expression was positively correlated with tumour TNM stage (P < 0.001), but a negative correlation was detected between IL-6 expression and tumor histological differentiation in CRC (P < 0.05). Furthermore, IL-6 expression was associated with invasion depth and lymph node metastasis in CRC.
IL-6 might be a useful marker for predicting a poor prognosis in patients with CRC and might be used as a potential therapeutic target in CRC.
Core tip: Colorectal cancer (CRC) is the fourth leading cause of cancer-related death worldwide. Previous studies have demonstrated that interleukin (IL)-6 is a critical tumor promoter during early CRC tumorigenesis. However, there have been few studies regarding the expression of IL-6 and its prognostic role in CRC. Therefore, we explored the correlation between the expression of IL-6 and the clinicopathological features in CRC. To the best of our knowledge, this is the first analysis of the expression of IL-6 in resected CRC samples using immunohistochemistry combined with biostatistics. The results showed that the expression of IL-6 was correlated with tumour TNM stage and histological differentiation. Furthermore, IL-6 in tumour cells showed stronger immunoreactivity as tumour cells invaded deeply. These data indicated that IL-6 might be used as a potential therapeutic target in patients with CRC.
- Citation: Zeng J, Tang ZH, Liu S, Guo SS. Clinicopathological significance of overexpression of interleukin-6 in colorectal cancer. World J Gastroenterol 2017; 23(10): 1780-1786
- URL: https://www.wjgnet.com/1007-9327/full/v23/i10/1780.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i10.1780
Colorectal cancer (CRC) is one of the most common malignancies and the fourth leading cause of cancer-related death worldwide[1-3]. Over one million new cases of CRC are diagnosed each year, and its incidence is second only to lung cancer[4,5]. CRC has been observed to be quite prevalent in Western industrialized countries in the past. In recent decades, a growing number of developing countries have exhibited an acute increase in the incidence of CRC. With the improvement in living conditions, the incidence rate of CRC in China has leapt, and CRC has become the fifth most common cancer. Despite radiotherapeutic and chemotherapeutic regimens and significantly improved surgical outcomes, approximately half of CRC patients will suffer from CRC again within five years of treatment and inevitably surrender to the disease[6]. The prognosis evaluation and treatment of CRC currently depend mostly on the pathologic stage of disease when diagnosed and primary surgical therapy. Unfortunately, no specific biomarker that allows for the accurate prediction of outcomes for individual patients currently exists.
Many previous studies have shown that neoplasms arise at sites of chronic inflammation[7]. The upregulation of inflammatory cytokines secreted by inflammatory cells, other mesenchymal cells and tumour cells could facilitate tumour initiation and enhance tumour cell proliferation and invasion[8-10]. Among the numerous inflammatory cytokines, interleukin (IL)-6 has continually attracted extensive attention.
IL-6 is a pleiotropic cytokine that is involved in tumour growth, invasion, and metastasis in human malignancies[11,12]. There is abundant mechanistic evidence suggesting a significant role of IL-6 in the tumour initiation and progression of a variety of cancers. For instance, Nguyen et al[13] demonstrated that IL-6 is a pivotal modulator in the initiation of prostate tumourigenesis, tumour growth, metastasis, and resistance to chemotherapy. Zhang et al[14] reported that IL-6 is necessary for pancreatic intraepithelial neoplasia (PanIN) maintenance and progression. Taniguchi et al[15] summarized that serum IL-6 levels correlate with poor prognosis, tumour burden, survival and advanced stages of disease in cancers of the lung, esophagus, mammary gland, ovary, and kidney, among others. In addition, several recent studies have suggested a potential role for IL-6 in colon cancer initiation and progression. It has been shown that serum levels of IL-6 are elevated in CRC patients[16]. Furthermore, IL-6 has been shown to promote the growth of colorectal cancer epithelial cells in vitro. However, there is relatively little understanding of the correlation between IL-6 expression and clinicopathological features in CRC.
The present study was designed to examine the difference in IL-6 expression between CRC tissues and matched adjacent normal mucosa tissues, and the association between IL-6 expression and clinicopathological features in CRC.
Fifty primary CRC tissues and 50 matched adjacent normal mucosa tissues were collected from the patients who underwent surgical resection at the Sichuan Provincial People’s Hospital (Chengdu, China). The original tumours were staged on the basis of the tumour-node-metastasis (TNM) classification system of the International Union Against Cancer[17]. Tumour differentiation was scored according to Edmondson Steiner scoring by senior pathologists. Detailed clinicopathological information was excerpted from the clinical data and a summary of the specific CRC demographics is displayed in Table 1. Informed prior to analysis, all patients consented to the tissue procurement, and the study was approved by the Institutional Ethics Committee of Sichuan Provincial People’s Hospital.
| Characteristic | Value |
| Total number of patients | 50 |
| Age (yr) | |
| Median | 61 |
| Range | 25-88 |
| Sex, n | |
| Male | 28 |
| Female | 22 |
| TNM stage | |
| I/II | 29 |
| III/IV | 21 |
| Pathologic grade | |
| Well | 14 |
| Moderate | 27 |
| Poor | 9 |
Human cancer tissue sections were subjected to immunohistochemistry analysis using a Dako Envision System (Dako Cytomation GmbH, Hamburg, Germany Denmark) according to the manufacturer’s instructions. In brief, tumour blocks were formalin-fixed, paraffin-embedded, and cut into 4-μm-thick sections. The sections were deparaffinized in xylene and rehydrated through diminishing concentrations of ethanol (100%, 95%, 85%, and 75%). This was followed by subsequent incubation in 3% H2O2 for 10 min in the dark at room temperature to eliminate endogenous peroxidase activity. Antigen-retrieval was performed by heating the sections for 5 min in citrate buffer (pH 6.0) using the autoclave sterilizer method. The sections were then allowed to cool at room temperature for 60 min, and rinsed twice for 5 min with fresh PBS. Thereafter, the slides were preincubated with healthy bovine or goat serum albumin diluted in PBS (pH 7.4) for 15 min at 37 °C, and then incubated overnight at 4 °C with primary antibody specific for IL-6 (mouse anti-IL-6, dilution 1:100, Proteintech). After three rinses in fresh PBS, the slides were incubated for 40 min at 37 °C with horseradish peroxidase-coupled secondary antibody. Following three additional washes, all specimens were stained with 3,3’-diaminobenzidine (DAB) substrate chromogen system (Dako Cytomation GmbH). Finally, the sections were rinsed in distilled water, and counterstained with Mayer’s haematoxylin according to the manufacturer’s instructions. Non-immune rabbit IgG at the same dilution as the primary antibody was used as a negative control.
Cells with observable brown particles in the cytoplasm were taken as positive. All sections were assessed by two professional pathologists who were blinded to patient outcomes and all clinicopathologic data. The immunohistochemical staining was evaluated according to the intensity (weak = 1, intense = 2) of IL-6 immunostaining and the density (0% = 0, 1%-50% = 1, 51%-75% = 2, > 76% = 3) of positive carcinoma cells[5]. The eventual score of each specimen was calculated by multiplying intensity and density, and the tumours were finally determined as negative expression: score = 0; low expression: score ≤ 3; or high expression: score > 3. If the two assessments did not agree for a sample, the sample was re-evaluated and classified based on the evaluations given most frequently by the experts.
The data were analysed with the SPSS 16.0 for Windows (SPSS Inc). Pearson χ2 test and Fisher’s exact test were used to compare qualitative variables, and quantitative variables were analysed by the t-test. The correlation between clinicopathological factors and IL-6 expression was evaluated by the Spearman test for non-parametric variables. P-values less than 0.05 were considered statistically significant.
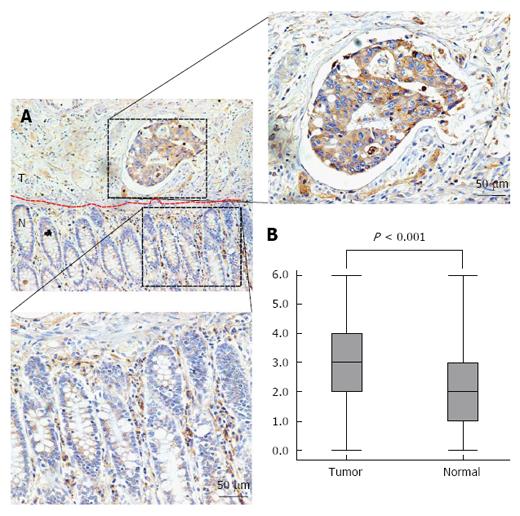
To explore the underlying clinical role of IL-6 in CRC, the expression of IL-6 was examined by immunohistochemical staining in 50 CRC tissue samples and 50 matched adjacent normal mucosa tissue samples. Strong IL-6 staining was mostly located in the cytoplasm of CRC cells (Figure 1A), while partial IL-6 staining was observed in the normal mucosa. Strong IL-6 expression was observed in 8% (4/50) of the normal colorectal mucosa samples and in 46% (23/50) of primary CRC samples. IL-6 expression was significantly increased in the CRC tissues compared with the normal mucosa tissues (P < 0.001; Figure 1B).
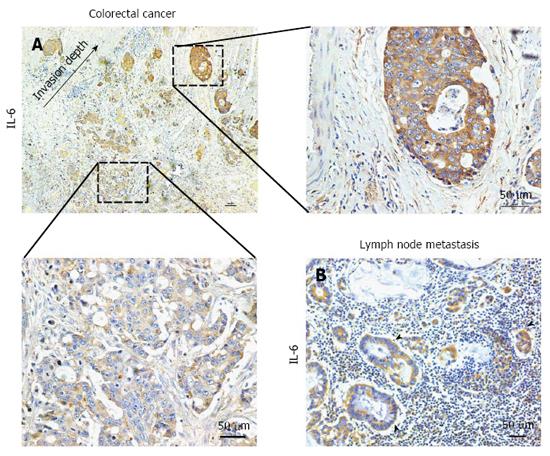
When analysing the levels of IL-6 expression in colorectal tumour cells by immunohistochemistry, we observed that IL-6 in tumour cells showed stronger immunoreactivity as tumour cells invaded more deeply (Figure 2A). This means that the tumour regions that were closer to the invasion front showed higher IL-6 expression levels. In addition, the majority of tumor cells in lymph node metastases were also IL-6-immunopositive (Figure 2B).
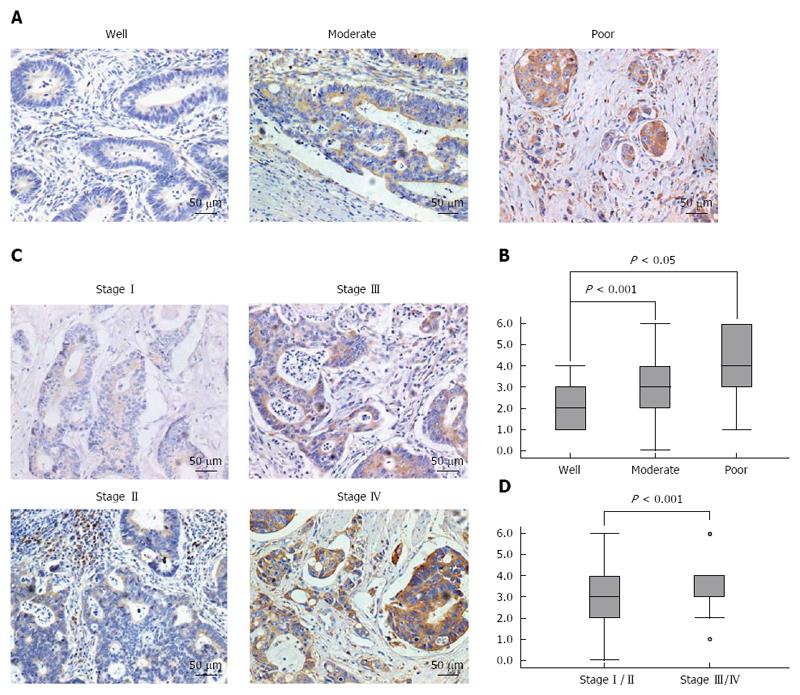
We next analysed the association between the levels of IL-6 expression and clinicopathologic parameters in CRC, including TNM stage (stages I, II, III, and IV) and histological differentiation (well, moderately, and poorly differentiated). The results showed that the levels of IL-6 expression were inversely associated with histological differentiation (P < 0.05, Figure 3A and B), but positively correlated with TNM stage (P < 0.001, Figure 3C and D). Of the 50 IL-6-positive CRC cases, 14 were well-differentiated, 27 moderately differentiated and 9 poorly differentiated. In addition, 29 cases were classified as stage I-II, while 21 cases were classified as stage III-IV. Taken together, these analyses indicated that the upregulation of IL-6 in CRC cells correlates with tumour progression.
Chronic inflammation is thought to be the leading cause of many human cancers including CRC[13]. In patients with inflammatory bowel disease, the risk of developing CRC is much higher than in the general population[18]. However, even in sporadic CRC with no preceding chronic inflammation, inflammatory cells infiltrate the tumour region and secrete inflammatory cytokines, which contribute to cancer development. Such “tumour-elicited inflammation” further emphasizes the importance of chronic inflammation in cancer progression. IL-6 is an NF-κB-regulated inflammatory cytokine that enforces proliferation and anti-apoptotic effects in tumour cells[2,13]. It has been reported that IL-6 expression in serum samples from patients was associated with an increased risk of colorectal adenoma[19,20]. Until now, to the best of our knowledge, there have been no relevant studies analysing the levels of IL-6 expression in resected CRC samples by immunohistochemistry combined with biostatistics. In this study, we found that IL-6 expression was elevated in CRC compared with normal mucosa, which is consistent with previous studies[21,22]. In addition, we found that the levels of IL-6 expression were inversely associated with histological differentiation, but positively associated with TNM stage. These results imply that IL-6 may be involved in CRC progression. Interestingly, the tumour regions that were closer to the invasion front showed higher IL-6 expression levels. Furthermore, the majority of cancer cells in lymph node metastases were also IL-6-immunopositive. These results suggested that IL-6 might be associated with CRC invasion and metastasis.
The mechanisms underlying IL-6-mediated CRC initiation and development have been elucidated comprehensively. IL-6 is a critical tumour promoter during early CRC tumourigenesis[20]. In mice with colitis-associated cancer, anti-IL-6 receptor antibody treatment reduced the incidence of colitis-associated cancer (CAC) by decreasing the expression of key genes in aerobic glycolysis[23]. In an experimental CAC mouse model, researchers found that the expression levels of IL-6 protein were gradually increased after the induction of dysplastic lesions over time. These data suggested that IL-6 might be a therapeutic target in CAC[24]. Activation of the IL-6/Stat3 pathway via IL-6 trans-signaling plays an important role not only in CRC initiation but also in CRC development[25,26]. According to the growing evidence supporting a critical role for IL-6 signaling in the development of both sporadic and inflammation-associated CRC, therapeutics targeting this pathway could be promising options for CRC patients.
The authors thank Professors Ke Xie and Dan-Dan Dong and Technician Fang-Hua Li at the Sichuan Provincial People’s Hospital (Chengdu, China) for providing CRC tissue samples.
Colorectal cancer (CRC) is the fourth leading cause of cancer-related death worldwide. Previous studies have demonstrated that IL-6 is a critical tumour promoter during early CRC tumourigenesis. However, there have been few studies regarding the expression of IL-6 and its prognostic role in CRC. The correlation between the expression levels of IL-6 and the clinicopathological features of CRC specimens has not yet been investigated.
Previous studies have demonstrated that IL-6 is a critical tumour promoter during early CRC tumourigenesis and that the IL-6/STAT3 signaling pathway plays an important role in the progression of CRC.
To the best of our knowledge, this is the first analysis of the expression of IL-6 in resected CRC samples and its prognostic role using immunohistochemistry combined with biostatistics.
The results showed that the expression levels of IL-6 were correlated with TNM stage and histological differentiation. Furthermore, IL-6 in tumour cells showed stronger immunoreactivity as tumour cells invaded more deeply. These data indicate that IL-6 might be used as a potential target for postoperative adjuvant therapy in patients with CRC.
Invasion depth, also called the depth of tumour invasion, is the most significant histological predictor of lymph node metastasis in colorectal cancer. Increasing evidence indicates that colorectal cancer with submucosal deep invasion correlates with a poor prognosis.
This is an interesting paper. The method they used to quantify IL-6 in CRC specimens is only a qualitative method.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lakatos PL, Sterpetti AV S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Khan S, Cameron S, Blaschke M, Moriconi F, Naz N, Amanzada A, Ramadori G, Malik IA. Differential gene expression of chemokines in KRAS and BRAF mutated colorectal cell lines: role of cytokines. World J Gastroenterol. 2014;20:2979-2994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1534] [Cited by in F6Publishing: 1664] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 3. | Lu CC, Kuo HC, Wang FS, Jou MH, Lee KC, Chuang JH. Upregulation of TLRs and IL-6 as a marker in human colorectal cancer. Int J Mol Sci. 2014;16:159-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Jiang Q, Li Q, Chen H, Shen A, Cai Q, Lin J, Peng J. Scutellaria barbata D. Don inhibits growth and induces apoptosis by suppressing IL-6-inducible STAT3 pathway activation in human colorectal cancer cells. Exp Ther Med. 2015;10:1602-1608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Zeng J, Yang X, Cheng L, Liu R, Lei Y, Dong D, Li F, Lau QC, Deng L, Nice EC. Chemokine CXCL14 is associated with prognosis in patients with colorectal carcinoma after curative resection. J Transl Med. 2013;11:6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Abajo A, Bitarte N, Zarate R, Boni V, Lopez I, Gonzalez-Huarriz M, Rodriguez J, Bandres E, Garcia-Foncillas J. Identification of colorectal cancer metastasis markers by an angiogenesis-related cytokine-antibody array. World J Gastroenterol. 2012;18:637-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 29] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Kraus S, Arber N. Inflammation and colorectal cancer. Curr Opin Pharmacol. 2009;9:405-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 8. | Knüpfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review). Breast Cancer Res Treat. 2007;102:129-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 288] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 9. | Hyun YS, Lee AR, Eun CS, Youn JH, Kim HY. This close link between IL-6 and cancer suggests that the IL-6 might be induce tumorigenesis. 2012;1-25. [Cited in This Article: ] |
| 10. | Wang K, Kim Min K, Di Caro G, Wong J, Shalapour S, Wan J, Zhang W, Zhong Z, Sanchez-Lopez E, Wu L-W. Interleukin-17 Receptor A Signaling in Transformed Enterocytes Promotes Early Colorectal Tumorigenesis. Immunity. 2014;41:1052-1063. [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 230] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 11. | Nie W, Xue L, Sun G, Ning Y, Zhao X. Interleukin-6 -634C/G polymorphism is associated with lung cancer risk: a meta-analysis. Tumour Biol. 2014;35:4581-4587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Esfandi F, Mohammadzadeh Ghobadloo S, Basati G. Interleukin-6 level in patients with colorectal cancer. Cancer Lett. 2006;244:76-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Nguyen DP, Li J, Tewari AK. Inflammation and prostate cancer: the role of interleukin 6 (IL-6). BJU Int. 2014;113:986-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 254] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 14. | Zhang Y, Yan W, Collins MA, Bednar F, Rakshit S, Zetter BR, Stanger BZ, Chung I, Rhim AD, di Magliano MP. Interleukin-6 is required for pancreatic cancer progression by promoting MAPK signaling activation and oxidative stress resistance. Cancer Res. 2013;73:6359-6374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 15. | Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26:54-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 390] [Cited by in F6Publishing: 475] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 16. | Galizia G, Orditura M, Romano C, Lieto E, Castellano P, Pelosio L, Imperatore V, Catalano G, Pignatelli C, De Vita F. Prognostic significance of circulating IL-10 and IL-6 serum levels in colon cancer patients undergoing surgery. Clin Immunol. 2002;102:169-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 170] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | Wittekind C, Compton CC, Greene FL, Sobin LH. TNM residual tumor classification revisited. Cancer. 2002;94:2511-2516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 331] [Cited by in F6Publishing: 326] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 18. | Langholz E, Munkholm P, Davidsen M, Binder V. Colorectal cancer risk and mortality in patients with ulcerative colitis. Gastroenterology. 1992;103:1444-1451. [PubMed] [Cited in This Article: ] |
| 19. | Kim S, Keku TO, Martin C, Galanko J, Woosley JT, Schroeder JC, Satia JA, Halabi S, Sandler RS. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer Res. 2008;68:323-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 20. | Sasaki Y, Takeda H, Sato T, Orii T, Nishise S, Nagino K, Iwano D, Yaoita T, Yoshizawa K, Saito H. Serum Interleukin-6, insulin, and HOMA-IR in male individuals with colorectal adenoma. Clin Cancer Res. 2012;18:392-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Kakourou A, Koutsioumpa C, Lopez DS, Hoffman-Bolton J, Bradwin G, Rifai N, Helzlsouer KJ, Platz EA, Tsilidis KK. Interleukin-6 and risk of colorectal cancer: results from the CLUE II cohort and a meta-analysis of prospective studies. Cancer Causes Control. 2015;26:1449-1460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Nagasaki T, Hara M, Nakanishi H, Takahashi H, Sato M, Takeyama H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br J Cancer. 2014;110:469-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 285] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 23. | Han J, Meng Q, Xi Q, Zhang Y, Zhuang Q, Han Y, Jiang Y, Ding Q, Wu G. Interleukin-6 stimulates aerobic glycolysis by regulating PFKFB3 at early stage of colorectal cancer. Int J Oncol. 2016;48:215-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Yang H, Qi H, Ren J, Cui J, Li Z, Waldum HL, Cui G. Involvement of NF-κB/IL-6 Pathway in the Processing of Colorectal Carcinogenesis in Colitis Mice. Int J Inflam. 2014;2014:130981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Matsumoto S, Hara T, Mitsuyama K, Yamamoto M, Tsuruta O, Sata M, Scheller J, Rose-John S, Kado S, Takada T. Essential roles of IL-6 trans-signaling in colonic epithelial cells, induced by the IL-6/soluble-IL-6 receptor derived from lamina propria macrophages, on the development of colitis-associated premalignant cancer in a murine model. J Immunol. 2010;184:1543-1551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 191] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 26. | Waldner MJ, Foersch S, Neurath MF. Interleukin-6--a key regulator of colorectal cancer development. Int J Biol Sci. 2012;8:1248-1253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 271] [Article Influence: 22.6] [Reference Citation Analysis (0)] |