Published online Nov 28, 2014. doi: 10.3748/wjg.v20.i44.16665
Revised: April 10, 2014
Accepted: June 21, 2014
Published online: November 28, 2014
AIM: To investigate the effects of 17β-estradiol via estrogen receptors (ER) or direct administration of ER agonists on human colorectal cancer.
METHODS: LoVo cells were established from the Bioresource Collection and Research Center and cultured in phenol red-free DMEM (Sigma, United States). To investigate the effects of E2 and/or ER selective agonists on cellular proliferation, LoVo colorectal cells were treated with E2 or ER-selective agonists for 24 h and 48 h and subjected to the MTT (Sigma) assay to find the concentration. And investigate the effects of E2 and/or ER selective agonists on cell used western immunoblotting to find out the diversification of signaling pathways. In order to observe motility and migration the wound healing assay and a transwell chamber (Neuro Probe) plate were tased. For a quantitative measure, we counted the number of migrating cells to the wound area post-wounding for 24 h. We further examined the cellular migration-regulating factors urokinase-type plasminogen activator (u-PA), tissue-type plasminogen activator (t-PA) and matrix metalloproteinase (MMP)-9 in human LoVo cells so gelatin zymography that we used and gelatinolytic activity was visualized by Coomassie blue staining. And these results are presented as means ± SE, and statistical comparisons were made using Student’s t-test.
RESULTS: The structure was first compared with E2 and ER agonists. We then treated the LoVo cells with E2 and ER agonists (10-8 mol/L) for 24 h and 48 h and subsequently measured the cell viability using MTT assay. Our results showed that treatment with 17β-estradiol and/or ER agonists in human LoVo colorectal cancer cells activated p53 and then up-regulated p21 and p27 protein levels, subsequently inhibiting the downstream target gene, cyclin D1, which regulates cell proliferation. Taken together, our findings demonstrate the anti-tumorigenesis effects of 17β-estradiol and/or ER agonists and suggest that these compounds may prove to be a potential alternative therapy in the treatment of human colorectal cancer. These results demonstrate that 17β-estradiol and/or ER agonists downregulate migration-related proteins through the p53 signaling pathway in human LoVo colorectal cancer cells. These findings suggest that p53 plays a critical role in the 17β-estradiol and/or ER agonist-mediated protective activity against colorectal cancer progression. In addition, 17β-estradiol and/or ER agonists dramatically inhibited cell migration and reduced the expression of u-PA, t-PA and MMP-9 as well as MMP-2/9 activity in LoVo cells, which regulate cell metastasis. Moreover, we observed that pretreatment with a p53 inhibitor significantly blocked the anti-migration effects of E2 and/or ER agonists on LoVo cells. That E2 and/or ER agonists may impair LoVo cell migration by modulating migration-related factors via the p53 tumor suppressor gene.
CONCLUSION: Direct ER treatment may prove to be an attractive alternative therapy in the treatment of human colorectal tumors in the future.
Core tip: The present study is to investigate the effects of 17β-estradiol via estrogen receptors or directly administration of ERs agonist on the development of human colorectal cancer, and to elucidate whether the effect was regulated by tumor suppressor gene p53. Here, our results showed that 17β-estradiol and/or ERs agonist treatment in human LoVo colorectal cancer cells could active p53, then up-regulated p21 and p27 protein levels, subsequently inhibited downstream target gene, cyclin D1, which regulated the cell proliferation.
- Citation: Hsu HH, Kuo WW, Ju DT, Yeh YL, Tu CC, Tsai YL, Shen CY, Chang SH, Chung LC, Huang CY. Estradiol agonists inhibit human LoVo colorectal-cancer cell proliferation and migration through p53. World J Gastroenterol 2014; 20(44): 16665-16673
- URL: https://www.wjgnet.com/1007-9327/full/v20/i44/16665.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i44.16665
Epidemiological studies suggest that cancers of the lung/bronchus, prostate and colon/rectum in men and cancers of the lung/bronchus, breast and colon/rectum in women continue to be the most common cancers in the United States. Colorectal cancer is the third most common cause of cancer death[1]. Colorectal cancers include nonhereditary and hereditary types. Hereditary colon cancers include familial adenomatous polyposis and hereditary non-polyposis colon cancer (HNPCC). HNPCC is the most common form of colorectal cancer, accounting for 5%-10% of total hereditarycolorectal cancers, and it occurs as early as age 25 with an average age of 45 years at diagnosis[2].
The role of the female sex hormone, 17β-estradiol, in tumorigenesis has been studied for many years. It has been proposed that the lower incidence of colorectal cancer (CRC) in women might be due to the influence of female sex steroid hormones[3]. Many studies have confirmed that hormone replacement therapy (HRT) in postmenopausal women reduces the incidence of colorectal cancer[4], whereas only one study has reported an adverse effect of HRT[5].
One might ask what is the mechanism behind this protective effect of female sex hormones against cancer cell proliferation and carcinogenesis? The biological activity of 17β-estradiol is mediated mainly by its binding to two specific receptors: estrogen receptor alpha (ERα), the prevalent form in the breast, cardiovascular system and liver,and estrogen receptor beta (ERβ), the prevalent form in the gastrointestinal tract[6]. Both ERα and ERβ exist in colorectal cancer cells[7].
Various proteases are expressed in cancer progression and metastasis[8]. The systems primarily responsible for extracellular matrix (ECM) degradation in vivo are matrix metalloproteinase (MMP) and plasminogen activator (PA) systems[9]. MMPs are a family of functionally related zinc-containing enzymes that include interstitial collagenases, gelatinases,metalloelastase and membrane-type MMPs[10,11]. The gelatinases MMP-2 and MMP-9 have been implicated in colorectal cancer progression and metastasis in animal models and patients[12]. In the proteolytic plasminogen system, the up-regulation of urokinase-type plasminogen activators (u-PAs) and tissue-type plasminogen activators (t-PAs) has been shown to activate MMPs and is involved in colon cancer progression[13,14]. In addition, a mutation in the adenomatous polyposis coli (APC) tumor suppressor gene occurs in most colorectal tumors, resulting in the accumulation of β-catenin due to reduced ubiquitin-mediated proteolysis, which may play a causal role in promoting carcinogenesis[15,16]. The current results indicate that the accumulation of nuclear β-catenin can be used as a prognostic marker in patients with stage IIA colon cancer[17].
The p53 tumor suppressor gene mediates many cellular processes, including cell cycle regulation, DNA repair, differentiation and apoptosis, in response to various extracellular and intracellular signals[18,19]. In contrast, it is well known that p53 mutations contribute to the malignant progression of colorectal cancer and resistance to anticancer therapy[20-22]. Interestingly, the precise anti-metastasis mechanismsunderlying the protective effects of 17β-estradiol/ERs on colorectal cancer via the p53 tumor suppressor protein remain unclear. This study examines the effects of 17β-estradiol and/or ER agonists on the regulation of cell proliferation and migration in human LoVo colorectal cancer cells. The roles of p53 and the precise molecular mechanisms behind this protective property are identified.
The human colon cancer cell lineLoVo was obtained from the Bioresource Collection and Research Center (BCRC). LoVo cells were established from a metastatic nodule resected from a 56-year-old Caucasian malecolon adenocarcinoma patient.
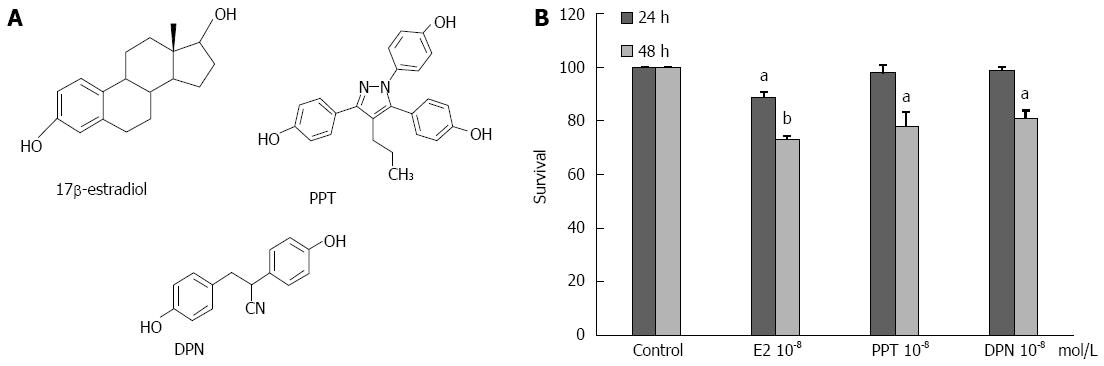
The following reagents were used for experiment: 17β-estradiol (E2) (Sigma, Louis), an ERα-selective agonist [propylpyrazole-triol (PPT)], an ERβ-selective agonist [diarylpropionitrile (DPN)] (Figure 1A), an ERα-selective antagonist [methyl-piperidinopyrazoledihydrochloride (MPP)], an ERβ-selective antagonist 4-[2-Phenyl-5,7-bis (trifluoromethyl) pyrazolo [1,5-a] pyrimidin-3-yl] phenol (PHTPP), the ER antagonist ICI 182780 (ICI) (all from TOCRIS), and the p53 inhibitor Pifithrin-a, p-Nitro, Cyclic (Merck).
LoVo colon cancer cells were cultured inphenol red-free DMEM (Sigma, United States) supplemented with 1.5 g/L sodium bicarbonate, 3.5 g/L glucose, 1% penicillin-streptomycin and 10% cosmic calf serum (Hyclone, United States) in a humidified atmosphere at 37 °C with 5% CO2. The medium was changed to phenol red-free DMEM with 0% serum 4 h before the experiment was started.
To investigate the effects of E2 and/or ER selective agonists on cellular proliferation, LoVo colorectal cells were treated with E2 or ER-selective agonists for 24h and 48h and subjected to the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) (Sigma) assay. The blue formazan crystal absorbance was measured at 570 nm using an enzyme-linked immunosorbent assay plate reader.
Total proteins were extracted using lysis buffer [50 mmol/L Tris-base, pH = 7.5, 0.5 mol/L NaCl, 1.0 mmol/L EDTA, pH = 7.5, 10% glycerol, 1 mmol/L β-Mercaptoethanol and a proteinase inhibitor cocktail (Roche Molecular Biochemicals)]. The cell lysate proteins were analyzed using SDS-PAGE. The following primary antibodies were used for incubations: MMP-9 (Chemicon), Cyclin D1 sc-246, β-Catenin sc-7963, GSK-3β sc-9166, p53 sc-1311, u-PA sc-14019, t-PA sc-5239, α-Tubulin sc-5286,and β-actin sc-47778 (all from Santa Cruz Biotechnology). Following primary antibody incubations, membranes were incubated with horseradish peroxidase-linked secondary antibodies (anti-rabbit, anti-mouse, or anti-goat IgG) (all from Santa Cruz Biotechnology).
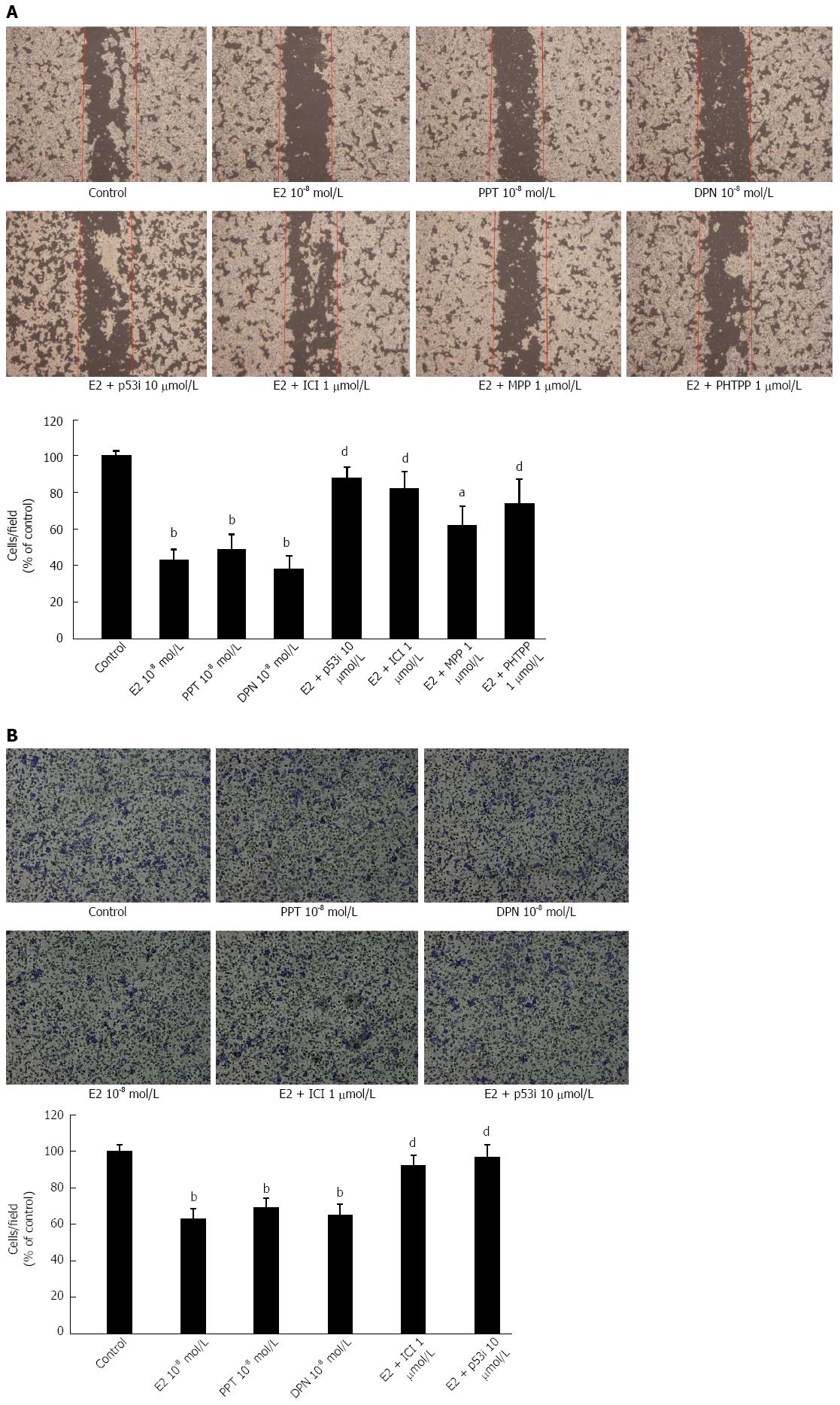
LoVo cells were seeded into six-well plates at 1 × 105 cells/well in culture medium. Confluent monolayers were scratched with a sterile micro-pipette tip and then washed with PBS to remove floating cells in serum-free medium. Then, the cells were serum starved for 4 h. Wound healing was performed by treatment with E2, ER-selective agonists, a p53 inhibitor (10 μmol/L), ER-selective antagonists or ICI182780 (1 μmol/L), respectively. For a quantitative measure, we counted the number of migrating cells to the wound area post-wounding for 24 h.
The cell migration assay was carried out using a modified Boyden chamber consisting of a trans well chamber (Neuro Probe) plate with 8-μm pore size polycarbonate membrane filters[23]. Serum-deprived LoVo cells were added to the upper part of the Boyden chamber, and the bottom chamber was filled with DMEM containing 10% serum. After incubation for 48 h, the cells were allowed to migrate to the underside of the membrane. The cells onthe membrane filter were then fixed with methanol and stained with 0.05% Giemsa (Sigma). The number of migrated cells was quantified by cell counting in at least three random fields (magnification, × 200) per filter.
LoVo cells cultured in DMEM were treated with E2 (10-8 mol/L) for 24h and subsequently collected in conditional medium. Samples were electrophoresed without reduction (no DTT) on 8% SDS polyacrylamide gels copolymerized with 0.1% gelatin. When the tracking dye at the front reached the bottom of the gel, the gel was removed and shaken gently for 30 min in 2.5% Triton X-100 to remove SDS. The gels were then transferred to a bath (without Triton X-100) and washed for 30 min to remove Triton X-100. The gels were then incubated overnight (37 °C) in reaction buffer containing 40 mmol/L Tris-HCl (pH = 8.0), 0.01% NaCl and 10 mol/L CaCl2. Finally, gelatinolytic activity was visualized by Coomassie blue staining.
Each experiment was repeated at least twice with identical results. The resultsare presented as means ± SEs, and statistical comparisons were made using Student’s t-test. Significance was presented as a P < 0.05 or P < 0.01.
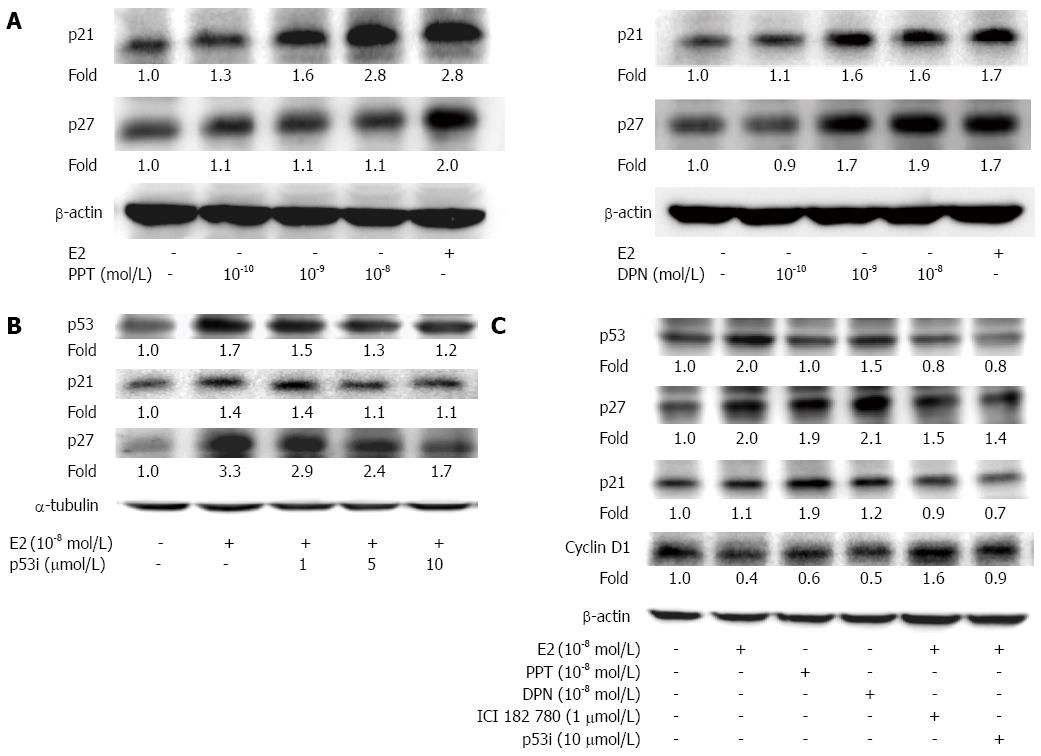
To determine the effects of E2 and ER-selective agonists on the proliferation of human LoVo colorectal cancer cells, the structure was first compared with E2 and ER agonists.We then treated the LoVo cells with E2 and ER agonists (10-8 mol/L) for 24 h and 48 h and subsequently measured the cell viability using MTT assay. The results showed a significant reduction in LoVo colorectal cancer cell viability, with a reduction of approximately 28.0% following E2 treatment for 48 h, 21.0% following PPT treatment for 48 h and 15.8% following DPN treatment for 48 h (Figure 1B). We further examined the level of p53 signaling and downstream proteins through Western blotting. After LoVo cells were treated with E2 (10-8 mol/L) or various concentrations (10-10 mol/L, 10-9 mol/L and 10-8 mol/L) of PPT or DPN for 24 h, we observed a significant dose-dependent reduction in the expression of p21 and p27 (Figure 2A). In LoVo cells, administration of a p53 inhibitor (1 μmol/L, 5 μmol/L, 10 μmol/L) significantly inhibited the E2-induced activation of p53, p27 and p21 in a dose-dependent manner (Figure 2B). The serum-starved human LoVo colorectal cancer cells were pretreated with ICI or p53 inhibitor (1 μmol/L), which significantly inhibited the E2-induced increases in p53, p27 and p21 protein levels and blocked the E2-dependent reduction in the protein levels of cell cycle-regulating proteins such as cyclin D1. At the same time, we observed that treatment with the ER agonists PPT or DPN alone also had the same effect as E2 treatment on human LoVo cells (Figure 2C). These results suggest that the administration of 17β-estradiol or ER agonist scan inhibit cell proliferation in human LoVo cells by modulating p53 signaling and its downstream cell cycle target gene, cyclin D1.
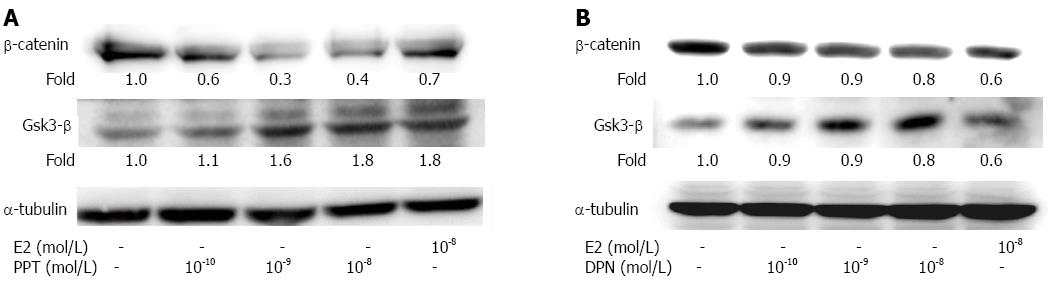
After LoVo cells were treated with various concentrations (10-10 mol/L, 10-9 mol/L and 10-8 mol/L) of PPT or DPN for 24 h, the level of the Wnt-β-catenin complex protein was investigated. The GSK-3β protein content was dramatically increased, and the β-catenin level was decreased in a dose-dependent manner (Figure 3). These results might indicate that E2 or ER agonist treatment can inhibit colorectal cancer cells growth via Wnt/wingless signaling suppression.
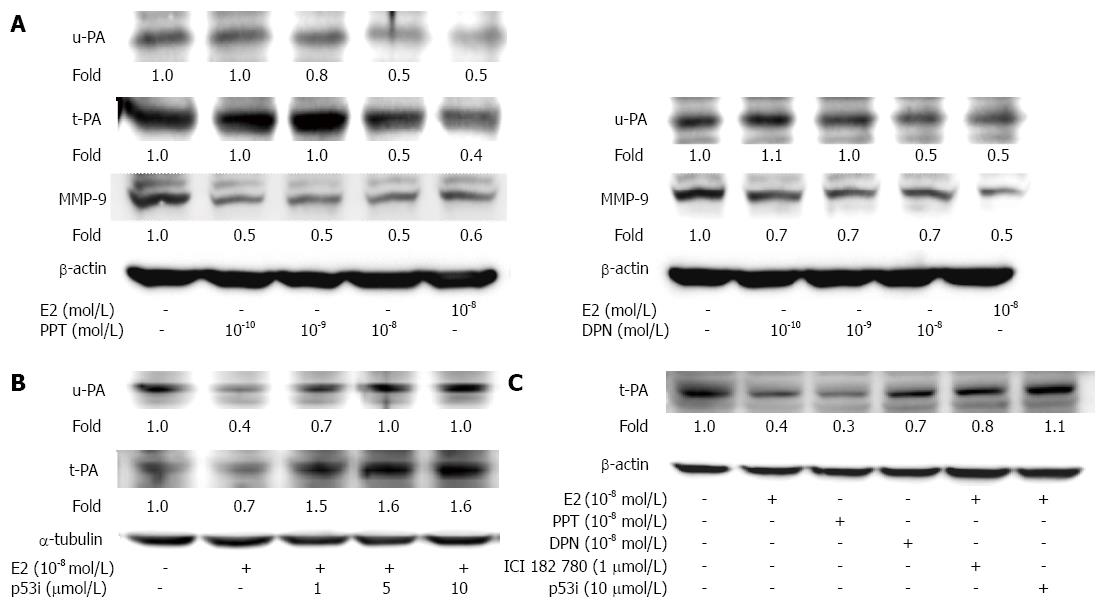
It is known that proteolytic plasminogen system activation by u-PA and t-PA is involved in the up-regulation of downstream MMPs in cancer cells[24]. We therefore further examined the cellular migration-regulating factors u-PA, t-PA and MMP-9 in human LoVo cells. In our studies, we observed that a significant reduction in u-PA, t-PA, MMP-2 and MMP-9 protein expression was induced by E2 (10-8 mol/L) treatment within 24 h. Subsequently, the quantitative results showed that PPT (10-10 mol/L, 10-9 mol/L and 10-8 mol/L) treatment alone also significantly reduced the u-PA, t-PA and MMP-9 levels. DPN (10-10 mol/L, 10-9 mol/L and 10-8 mol/L) treatment alone reduced the u-PA and MMP-9 levels in a dose-dependent manner (Figure 4A). Administration of a p53 inhibitor (1 μmol/L, 5 μmol/L, 10 μmol/L) to LoVo cells significantly blocked the E2-induced inhibition of u-PA and t-PA in a dose-dependent manner (Figure 4B).The serum-starved human LoVo colorectal cancer cells that were pretreated with ICI or p53 inhibitor (1 μmol/L) significantly blocked the E2-dependent reduction in t-PA protein levels (Figure 4C). Therefore, these results suggest that 17β-estradiol or ER agonistadministration can inhibit cellular migration-regulating factors, including u-PA, t-PA and MMP-9, in a p53-dependent manner.
It is known that MMPs function as proteases in extracellular matrix protein degradation[25]. Thus, MMP activities play important roles in cell cycle regulation. We therefore examined whether E2 can suppress MMP-2/9 expression and activity in LoVo cells. In Figure 5, gel images from gelatin zymography show that MMP-2 (72 kDa) and MMP-9 (92 kDa) activities were significantly suppressed in LoVo cells treated with E2.
We observed that the migration ability of LoVo cells could be inhibited after E2 (10-8 mol/L) treatment. We further determined that E2 and/or ER agonists played a role in LoVo cell migration ability. We then cultured LoVo cells with E2 (10-8 mol/L) in the presence or absence of ICI 182780, a p53 inhibitor, ERα antagonists (MPP) or ERβ antagonists (PHTPP).Weal so treated LoVo cells with PPT or DPN (10-8 mol/L), which are ER agonists, alone for 48 h. We observed the migration ability in LoVo cells using scratch motility and migration assays. In the scratch motility (Figure 6A) and migration assays (Figure 6B), we observed that ICI 182780 and the p53 inhibitor dramatically blocked the inhibitory effects of E2 on cellar migration in LoVo cells. In addition, treatment with either of the ER agonists, i.e., PPT or DPN, alone also significantly inhibited LoVo colorectal cancer cell migration by approximately 24% and 39%, respectively. Both ERα and ERβ are involved in down regulating cellular mobility in human LoVo colorectal cancer cells. These findings showed that p53 mediates the E2-ER-modulated migration ability of human LoVo cells and that ER agonist treatment alone has the same ability to inhibit LoVo cell migration.
Previous studies have shown that E2 binding to ERs can regulate tissue and cellular responses via multiple signaling pathways[26]. The ERs act at estrogen-responsive target genes to either trans-activate or trans-repress gene expression[27]. In our previous studies, we found that over-expressed ERα induces apoptosis and inhibits the proliferation of human LoVo colorectal cancer cells[28].The apoptotic effects of over-expressed ERβ acted in a ligand-dependent manner[29]. These published results provide evidence that E2 and/or the over-expression of ERs plays a critical role in inducing the apoptosis of human LoVo colorectal cancer cells. Here, we were interested in further determining the anti-motility effects of the ERα- and ERβ-selective agonists PPT and DPN on LoVo colorectal cancer cells.
The major findings of this study can be summarized as follows: (1) treatment with E2 and/or ER agonists (10-10 mol/L, 10-9 mol/L and 10-8 mol/L) significantly inhibits human LoVo colon cancer cell proliferation by increasing p53, p21 and p27 protein levels and decreasing the expression of the downstream target gene cyclin D1. These results suggest that E2 and/or ER agonists (10-8 mol/L) greatly suppress cell proliferation by activating the p53 signaling pathway and modulating cell cycle-regulating factors in human LoVo cells; (2) the motility of LoVo colorectal cancer cells is significantly suppressed by E2 and/or ER agonist treatment. We simultaneously observed that the decrease in the migratory ability of LoVo cells due to treatment with E2 (10-8 mol/L) and/or ER agonists (10-10 mol/L, 10-9 mol/L and 10-8 mol/L) was accompanied by the down regulation of migration-related proteins, including u-PA, t-PA and MMP-9, as well as the suppression of MMP-2/9 activities (Figure 5); and (3) the inhibitory effects of u-PA, t-PA and MMP-9 in human LoVo cells that had been treated with E2 and/or ER agonists were completely inhibited by a p53 inhibitor. These results demonstrate that E2 and/or ER agonists down regulate migration-related proteins through the p53 signaling pathway in human LoVo colorectal cancer cells. These findings suggest that p53 plays a critical role in the E2- and/or ER agonist-mediated protective activity against colorectal cancer progression.
Changes in the expression of cell cycle regulators, such as cyclin D1, are a critical step in tumor development and progression, which are the most critical events in colorectal cancer[30]. The cell cycle regulator Cyclin D1 is a key intracellular regulator that is involved in cell cycle progression through the G1 phase and is over-expressed in colorectal carcinoma, leading to a worse prognosis[31]. In our study, we observed that cell proliferation and the expression of cyclin D1 in LoVo cells was significantly inhibited by E2 and/or ER agonist treatment. These findings suggest that E2 and/or ER agonists may protect against colorectal cancer proliferation by modulating cell cycle regulators.
Many studies have reported that increased MMP levels contribute to ECM remodeling and tumor cell motility, thus leading to the progression of malignant tumors[8,12]. Activation of the plasminogen activator system, which includes u-PA and t-PA, is reported to be involved in MMP activation and colorectal cancer development. Expression of u-PA and t-PA is considered a marker of malignant colon cancer[13,14,32,33]. Here, we found that the administration of E2 and/or ER agonists dramatically inhibited cell migration and reduced the expression of u-PA, t-PA and MMP-9 as well as MMP-2/9 activity in LoVo cells. Moreover, we observed that pretreatment with a p53 inhibitor significantly blocked the anti-migration effects of E2 and/or ER agonists on LoVo cells. These findings suggest that E2 and/or ER agonists may impair LoVo cell migration by modulating migration-related factors via the p53 tumor suppressor gene.
Taken together, our results suggest that the tumor suppression protein p53 may mediate downstream signaling when E2 binds to ERs and, further, that it modulates E2-mediated anti-tumorigenic properties by inhibiting the expression of u-PA, t-PA and MMP-9. In addition, ER agonists directly activate ERα or ERβ Anti-migration effects were observed after E2 treatment of human LoVo colorectal cancer cells. These results show that direct ER treatment may prove to be an attractive alternative therapy in the treatment of human colorectal tumors in the future.
Epidemiological studies suggest that cancers of the lung/bronchus, prostate and colon/rectum in men and cancers of the lung/bronchus, breast and colon/rectum in women continue to be the most common cancers in the United States. Colorectal cancer is the third most common cause of cancer death.
This study examines the effects of 17β-estradiol and/or ER agonists on the regulation of cell proliferation and migration in human LoVo colorectal cancer cells. The roles of p53 and the precise molecular mechanisms behind this protective property are identified.
ER agonists directly activate ERα or ERβ Anti-migration effects were observed after E2 treatment of human LoVo colorectal cancer cells. These results show that direct ER treatment may prove to be an attractive alternative therapy in the treatment of human colorectal tumors in the future.
The authors mainly focus on to explore whether estrogen or estradiol agonists inhibit human LoVo colorectal-cancer cell proliferation and migration through p53. Their findings showed that treatment with17β-estradiol and/or ER agonists in human LoVo colorectal cancer cells activated p53 and then up-regulated p21 and p27 protein levels, subsequently inhibiting the downstream target gene, cyclin D1, which regulates cell proliferation.
P- Reviewer: Handa O, Wang YD S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7953] [Cited by in F6Publishing: 8022] [Article Influence: 534.8] [Reference Citation Analysis (2)] |
| 2. | Razmus I, Jackson J, Wilson D. Hereditary non-polyposis colon cancer: change the name to protect the innocent. Medsurg Nurs. 2008;17:400-44, 410. [PubMed] [Cited in This Article: ] |
| 3. | Wingo PA, Ries LA, Rosenberg HM, Miller DS, Edwards BK. Cancer incidence and mortality, 1973-1995: a report card for the U.S. Cancer. 1998;82:1197-1207. [PubMed] [Cited in This Article: ] |
| 4. | Kampman E, Potter JD, Slattery ML, Caan BJ, Edwards S. Hormone replacement therapy, reproductive history, and colon cancer: a multicenter, case-control study in the United States. Cancer Causes Control. 1997;8:146-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 5. | Crandall CJ. Estrogen replacement therapy and colon cancer: a clinical review. J Womens Health Gend Based Med. 1999;8:1155-1166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Gustafsson JA. Estrogen receptor beta--a new dimension in estrogen mechanism of action. J Endocrinol. 1999;163:379-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 304] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 7. | Campbell-Thompson M, Lynch IJ, Bhardwaj B. Expression of estrogen receptor (ER) subtypes and ERbeta isoforms in colon cancer. Cancer Res. 2001;61:632-640. [PubMed] [Cited in This Article: ] |
| 8. | Liotta LA, Stetler-Stevenson WG. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res. 1991;51:5054s-5059s. [PubMed] [Cited in This Article: ] |
| 9. | Yoon WH, Jung YJ, Kim TD, Li G, Park BJ, Kim JY, Lee YC, Kim JM, Park JI, Park HD. Gabexate mesilate inhibits colon cancer growth, invasion, and metastasis by reducing matrix metalloproteinases and angiogenesis. Clin Cancer Res. 2004;10:4517-4526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387-2392. [PubMed] [Cited in This Article: ] |
| 11. | Ellerbroek SM, Stack MS. Membrane associated matrix metalloproteinases in metastasis. Bioessays. 1999;21:940-949. [PubMed] [Cited in This Article: ] |
| 12. | Mook OR, Frederiks WM, Van Noorden CJ. The role of gelatinases in colorectal cancer progression and metastasis. Biochim Biophys Acta. 2004;1705:69-89. [PubMed] [Cited in This Article: ] |
| 13. | Berger DH. Plasmin/plasminogen system in colorectal cancer. World J Surg. 2002;26:767-771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Seetoo DQ, Crowe PJ, Russell PJ, Yang JL. Quantitative expression of protein markers of plasminogen activation system in prognosis of colorectal cancer. J Surg Oncol. 2003;82:184-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA. 1995;92:3046-3050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 759] [Cited by in F6Publishing: 819] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 16. | Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286-3305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1965] [Cited by in F6Publishing: 1942] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 17. | Horst D, Reu S, Kriegl L, Engel J, Kirchner T, Jung A. The intratumoral distribution of nuclear beta-catenin is a prognostic marker in colon cancer. Cancer. 2009;115:2063-2070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Hofseth LJ, Hussain SP, Harris CC. p53: 25 years after its discovery. Trends Pharmacol Sci. 2004;25:177-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 306] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 19. | Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2:594-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2420] [Cited by in F6Publishing: 2380] [Article Influence: 108.2] [Reference Citation Analysis (0)] |
| 20. | Morselli E, Tasdemir E, Maiuri MC, Galluzzi L, Kepp O, Criollo A, Vicencio JM, Soussi T, Kroemer G. Mutant p53 protein localized in the cytoplasm inhibits autophagy. Cell Cycle. 2008;7:3056-3061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 206] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 21. | Toscano F, Fajoui ZE, Gay F, Lalaoui N, Parmentier B, Chayvialle JA, Scoazec JY, Micheau O, Abello J, Saurin JC. P53-mediated upregulation of DcR1 impairs oxaliplatin/TRAIL-induced synergistic anti-tumour potential in colon cancer cells. Oncogene. 2008;27:4161-4171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Bossi G, Lapi E, Strano S, Rinaldo C, Blandino G, Sacchi A. Mutant p53 gain of function: reduction of tumor malignancy of human cancer cell lines through abrogation of mutant p53 expression. Oncogene. 2006;25:304-309. [PubMed] [Cited in This Article: ] |
| 23. | Hsieh YH, Wu TT, Huang CY, Hsieh YS, Hwang JM, Liu JY. p38 mitogen-activated protein kinase pathway is involved in protein kinase Calpha-regulated invasion in human hepatocellular carcinoma cells. Cancer Res. 2007;67:4320-4327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Danø K, Andreasen PA, Grøndahl-Hansen J, Kristensen P, Nielsen LS, Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1740] [Cited by in F6Publishing: 1816] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 25. | Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562-573. [PubMed] [Cited in This Article: ] |
| 26. | Marino M, Galluzzo P, Ascenzi P. Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics. 2006;7:497-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 424] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 27. | Valentine JE, Kalkhoven E, White R, Hoare S, Parker MG. Mutations in the estrogen receptor ligand binding domain discriminate between hormone-dependent transactivation and transrepression. J Biol Chem. 2000;275:25322-25329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Hsu HH, Cheng SF, Chen LM, Liu JY, Chu CH, Weng YJ, Li ZY, Lin CS, Lee SD, Kuo WW. Over-expressed estrogen receptor-alpha up-regulates hTNF-alpha gene expression and down-regulates beta-catenin signaling activity to induce the apoptosis and inhibit proliferation of LoVo colon cancer cells. Mol Cell Biochem. 2006;289:101-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Hsu HH, Cheng SF, Wu CC, Chu CH, Weng YJ, Lin CS, Lee SD, Wu HC, Huang CY, Kuo WW. Apoptotic effects of over-expressed estrogen receptor-beta on LoVo colon cancer cell is mediated by p53 signalings in a ligand-dependent manner. Chin J Physiol. 2006;49:110-116. [PubMed] [Cited in This Article: ] |
| 30. | Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821-1828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1744] [Cited by in F6Publishing: 1714] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 31. | Maeda K, Chung YS, Kang SM, Ogawa M, Onoda N, Nakata B, Nishiguchi Y, Ikehara T, Okuno M, Sowa M. Overexpression of cyclin D1 and p53 associated with disease recurrence in colorectal adenocarcinoma. Int J Cancer. 1997;74:310-315. [PubMed] [Cited in This Article: ] |
| 32. | Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004;23:101-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 370] [Cited by in F6Publishing: 377] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 33. | Murakami A. Modulation of protein quality control systems by food phytochemicals. J Clin Biochem Nutr. 2013;52:215-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |