- 1Rheumatology Research Group, Institute of Inflammation and Ageing, University of Birmingham, Birmingham, UK
- 2Department of Biochemistry, Center for Immunity and Infection, University of Lausanne, Lausanne, Switzerland
Tertiary lymphoid structures (TLS) are organized aggregates of lymphocytes, myeloid, and stromal cells that provide ectopic hubs for acquired immune responses. TLS share phenotypical and functional features with secondary lymphoid organs (SLO); however, they require persistent inflammatory signals to arise and are often observed at target sites of autoimmune disease, chronic infection, cancer, and organ transplantation. Over the past 10 years, important progress has been made in our understanding of the role of stromal fibroblasts in SLO development, organization, and function. A complex and stereotyped series of events regulate fibroblast differentiation from embryonic life in SLOs to lymphoid organ architecture observed in adults. In contrast, TLS-associated fibroblasts differentiate from postnatal, locally activated mesenchyme, predominantly in settings of inflammation and persistent antigen presentation. Therefore, there are critical differences in the cellular and molecular requirements that regulate SLO versus TLS development that ultimately impact on stromal and hematopoietic cell function. These differences may contribute to the pathogenic nature of TLS in the context of chronic inflammation and malignant transformation and offer a window of opportunity for therapeutic interventions in TLS associated pathologies.
Introduction
Organs are defined as collection of cells, extracellular structures, and fluids, joined into an operational unit to serve a common function. The anatomy of an organ is designed by its structural elements, or resident stromal cells, which provide shape and compartmentalization to the tissues. Secondary lymphoid organs (SLOs), which include spleen, lymph nodes (LN), and Peyer’s patches (PP), largely conform to this. SLOs hold a network of fibroblasts, vessels, and nerves that support a large mobile population of leukocyte and which support immune surveillance and response to noxious agents (1, 2). This elegant organization of SLOs develops in a highly conserved and regulated process that largely occurs, both in mice and humans, in embryonic and early postnatal life (3, 4). SLOs evolved simultaneously with the development of an adaptive immune system in vertebrates, with hundreds of LN being distributed at strategic sites, thereby providing a platform for immune cell clustering at well-defined areas. This enables rapid and more efficient adaptive immune responses that outpace pathogen replication, spread, and pathology (5).
Well-developed tertiary or ectopic lymphoid structures (TLS or ELS) resemble SLOs anatomically, as complex aggregates of leukocytes and specialized stromal cells. However, TLS are not capsulated and lack an independent vascular network. TLS form within non-lymphoid tissue in response to specific pathogenic events (6–8) and are commonly found, in adult life, at sites of chronic inflammation and cancer (9–12). It is likely that the capacity to form TLS preceded the development of SLO during evolution, as a tool to accumulate innate immune cells at sites of inflammation in non-vertebrates and lower vertebrates, such as birds, amphibians, and reptiles (13, 14).
Mucosa-associated lymphoid tissues (MALT), such as cryptopatches (CP), fat-associated lymphoid clusters (FALC), and induced nasopharynx-associated lymphoid tissue (iNALT), may be placed, both anatomically and developmentally, between SLOs and TLSs. These are pre-programed in time and space, developing either pre- or postnatally at predetermined sites but are able to expand and accommodate specialized immune responses if required. In CPs, their development into IgA plasma cell-rich isolated lymphoid follicles (ILF) is a classic example of this phenomenon (5). Interestingly, ILF are reversible structures, as indicated by their disaggregation upon antibiotic treatment (15, 16). Similarly, TLS are considered reversible once the antigen source and inflammatory signals are cleared, as will be discussed later.
TLS anatomy is plastic and highly variable, as is its cellular composition. While a certain degree of T/B cell segregation, vascular specialization, and lymphoid tissue chemokine expression is often observed, the level of organization and formation of germinal centers (GC) is dependent on the context, stage, and site of the immune response. Unraveling the mechanisms responsible for the differential maturation of the stromal and leukocyte compartments in TLS and the functional differences between SLO and TLS has provided key information on the biology, clinical relevance, and role of those structures as potential therapeutic targets. In this review, we discuss in detail the contribution of stromal cells, most notably fibroblasts, to both SLO and TLS development and function, and the potential to target therapeutically this specific cell type.
In a “resting state,” all organs of the body contain fibroblasts that provide structure and mechanical strength to the tissue. Fibroblast phenotype and function greatly differ between various anatomical sites, as shown by the extensive transcriptional differences detected in fibroblasts isolated from different compartments in diverse locations of the body (17). This specialization is further enhanced by the specific ability of fibroblasts to respond to a series of cytokines and inflammatory stimuli, which increase their proliferative capacity and induce functional adaptations to the environment (18–22). This review will focus on a subset of fibroblasts defined as “lymphoid tissue fibroblasts,” which inhabit SLOs and TLS, are characterized by extensive plasticity and specialized functions, and have recently emerged as important regulators of adaptive immunity (8, 23–25).
Lymphoid Stromal Cell Differentiation in SLOs and TLS
In order to understand the TLS development, it is useful to review the development of LN and PP, which is initiated in the sterile environment of the embryo, at approximately E11 and E15, respectively. The locations at which LN develop are predetermined, at least in part, by endothelial expression of the lymphotoxin β receptor (LTβR) (26). Activation of the LTβR signaling pathway enables the clustering of CD45+CD4+CD3− hematopoietic lymphoid tissue inducer (LTi), also known as type 3 innate lymphoid cells (ILC3) expressing LTα1β2, TRANCE, and RORγt (3, 4).
The origin and identity of the signals that induce specification of the mesenchymal progenitor cells prior to LTi arrival remain largely unknown. It is clear that a close anatomical and functional connection between immune cells, mesenchyme, and vascular structures is critical for the establishment of the anlage. At around E13.5, it is possible to identify the nascent LN anlage as small clusters of endothelial cells expressing both podoplanin (gp38) and ICAM-1. The anlage are surrounded by a layer of mesenchymal cells expressing PDGFRα, fibronectin, and ER-TR7 and is separated from the outer layers of fibroblastic cells by a thin Perlecan+ basement membrane (27, 28). The early differentiation of the mesenchyme and the initial upregulation of ICAM-1 and VCAM-1, CXCL13 and IL-7, occurs at this stage in the absence of LTα1β2 or LTi cells and is thought to be regulated by retinoic acid signals released by neurons and/or ILC3 (29, 30). Nonetheless, LTα1β2 is required for the further upregulation of adhesion molecules and of lymphoid chemokines. LTα1β2 binds specifically to LTβR and activates the alternative pathway of the NF-κB cascade, while TNFα and LTα3 have TNFR1 and TNFR2 as main receptors and activate the canonical NF-κB pathway (1, 3). The combined activation of TNFR1- and LTβR-signaling pathways not only leads to the activation of both the classical and alternative NF-κB pathways, which act in synergy but also shows complex cross-regulation. In addition, LTβR can be also activated by LIGHT (31). LTβ-receptor regulates in fibroblasts and endothelial cells the expression of various chemokines (CCL19/21, CXCL13) and survival factors (BAFF), while TNF receptor is required for the production of adhesion molecules, such as VCAM-1, ICAM-1, and MAdCAM (32–35). The activation of the alternative pathway, demonstrated by the expression of NIK and the transcription factor RelB, is a specific requirement for the activated mesenchyme to mature in a lymphoid tissue organizer cell (LTo) (1–3). Mature LTo specification facilitates, in turn, further attraction and retention of more LTi cells through the binding of CXCR5, CCR7, α4β1, α4β7, and LTα1β2 with their respective ligands (2, 3). These later steps coincide with the ingrowth of the mesenchymal layer into the endothelial bud (27, 28, 36, 37). The physical interaction between hematopoietic LTi and stromal LTo cells establishes a positive feedback loop that reinforces the formation of the cluster and leads to the stabilization of the anlagen with vascular differentiation, development of high endothelial venules (HEVs), and eventually attraction and compartmentalization of mature lymphoid and myeloid cells (2–4).
Utilizing a CCL19-Cre dependent LTβR ablation (Ccl19-Cre × Ltbrfl/fl mice), Ludewig and colleagues have recently shown that CCL19+ myofibroblastic stromal cell precursor cells can develop the basic LN infrastructure even in absence of LTβR triggering (38). Nonetheless, fibroblastic LTo cells require LTβR signaling to reach full maturation and immunological competence that includes strong expression of ICAM-1, VCAM-1, CCL19, CCL21, IL-7, and RANKL (28, 38, 39). Of note, LTo responsible for the aggregation of different lymphoid tissues are not uniform. This is suggested by the observation that embryonic LTo cells in PP, mesenteric, and peripheral LN display transcriptional differences as well as differential cellular and molecular requirements (40, 41).
Interestingly, LN development is associated with but not fully dependent on a functional lymphatic vasculature network. As a consequence, embryos lacking the major transcriptional regulator for lymphatic cell development, Prox1, either due to full or conditional Prox1 deletion, fail to form mature LN. Both mutants develop hypoplastic LN anlagen containing small LTi clusters in areas of activated mesenchyme (42). Similarly, Clec-2 knockout mice, which exhibit a defect in lymphatic endothelial cell proliferation late in embryogenesis, form hypoplastic LNs with a mixture of blood and lymphatic flow and reduced LTi and LTo numbers (43).
Evolutionarily more ancient than LNs is the spleen that, together with gut-associated lymphoid tissue (GALT), represents the oldest SLO. The spleen is present in bony fish, amphibians, and reptiles, although in a less complex organization than that observed in mammals (14, 44). The development of the splenic white pulp cords that starts at birth in mice (45–48) and after 15 weeks of gestation in humans (49) does not require LTi cells or LTα1β2 (14, 44, 50, 51). However, as observed in the LN, stromal cell maturation, chemokine expression, and lymphocyte compartmentalization still require LTα1β2 and TNFα (1, 3, 52–56). Those ligands are likely to be provided by B cells and, as a consequence, B cell-deficient mice display smaller spleens, with poorly developed T zones (47). In conclusion, spleen and LN development depend on different types of inducer cells but show a similar hematopoietic–mesenchymal cell interaction, which eventually leads to a similar pathway of fibroblast maturation and lymphoid tissue compartmentalization.
Lymph nodes and PP anlagen formation in the embryo resemble a “sterile inflammation” (5, 13) aimed at forming organs before and independently from the encounter of danger signals. Thereby, these organs collate in a single, highly organized space antigen-presenting cells, naïve lymphocytes, and stromal cells that enable the rapid generation of adaptive immune responses against pathogens.
Tertiary lymphoid structures formation in the adult shares many similarities with SLO development; however, the order of events and molecular mechanisms responsible for TLS development are significantly different from those regulating LN development and partially different from those of the spleen. First, TLS form in the presence of lymphocytes that are absent during embryonic SLO formation. Second, TLS do not develop as separate encapsulated organs but arise as part of highly inflamed tissues, in response to a requirement for lymphocytes to cluster, survive, and generate local, efficient antigen-driven responses. Activation of the resident vascular structures including the upregulation of homing molecules to enable lymphocyte recruitment is therefore a prerequisite of TLS assembly (7, 8). However, while influenced by increased recruitment and defective lymphatic drainage of leukocytes, TLS formation is not simply determined by retention of activated cells in the tissue (57).
Modification of tissue-resident stromal cells into functional lymphoid tissue-like fibroblasts represents another hallmark feature of TLS, specifically, the ectopic and largely segregated expression of chemokines, such as CXCL13, CCL21, CCL19, and CXCL12, and of lymphocyte survival factors, such as IL-7, BAFF, and APRIL (7, 8). Once fully matured, TLS can display a specialized network of follicular dendritic cells (FDCs) capable of driving a functional GC response, and HEVs, differentiated from perivenular capillaries (57). Local upregulation of PNAd, MAdCAM, ICAM-1, and CCL21, first on flat, and later on high endothelial cells, enables the recirculation through the inflamed tissue of naïve T and B lymphocytes, previously excluded by the absence of cognate ligands for CCR7 and L-selectin (8, 57–59).
As opposed to SLO that arise on the backbone of a poorly differentiated mesenchyme, TLS form within organs whose mesenchyme is postnatal and specialized to support local anatomical and functional requirements (8). Accordingly, TLS assembly relies on a larger variety of mesenchymal organizer cells, hematopoietic inducer cells, and cytokines (6–8, 60). TLS preferentially arise in close proximity to vascular or epithelial ductal structures adjacent to pericytes, smooth muscle cells, or myofibroblast-like cells that share many functional and phenotypic features of LTo cells. For example, leukocyte aggregates in the synovium of patients with rheumatoid arthritis (RA) form in close proximity to networks of αSMA+ fibroblastic cells (61), and LTβR+ aortic smooth muscle cells have been observed in vascular TLS that form in murine models of atherosclerosis (62). Focal lymphocytic aggregations that define Sjogren’s syndrome (SS) assume a classical periductal structure in close contact to a layer of gp38+ myofibroblastic or pericytic cells that define the ductal basal membrane (63). Similarly, fibroblastic reticular stromal cells that share LTo and lymphoid tissue features have been identified in several murine and human TLS, including SS, primary biliary cirrhosis, insulitis, RA, and lungs infected by Pseudomonas aeruginosa (Table 1). More recently, a marked increase in the frequency of PDGFRα+PDGFRβ+Cadherin-11+ICAM-1+ fibroblasts has also been identified within cerebral lesions of mice that develop experimental autoimmune encephalomyelitis (EAE) (64). Recently, endothelial cells and perivascular fibroblasts of the brain were shown to nucleate local CD8+T cell responses to a neurotropic virus by expressing CCR7 ligands (65). As TLS-associated LTo-like cells are considered resident and long-lived, in comparison to the circulating and short-lived hematopoietic cells, they may hold the key to the reversibility of TLS assembly, providing an interesting therapeutic target for this process.
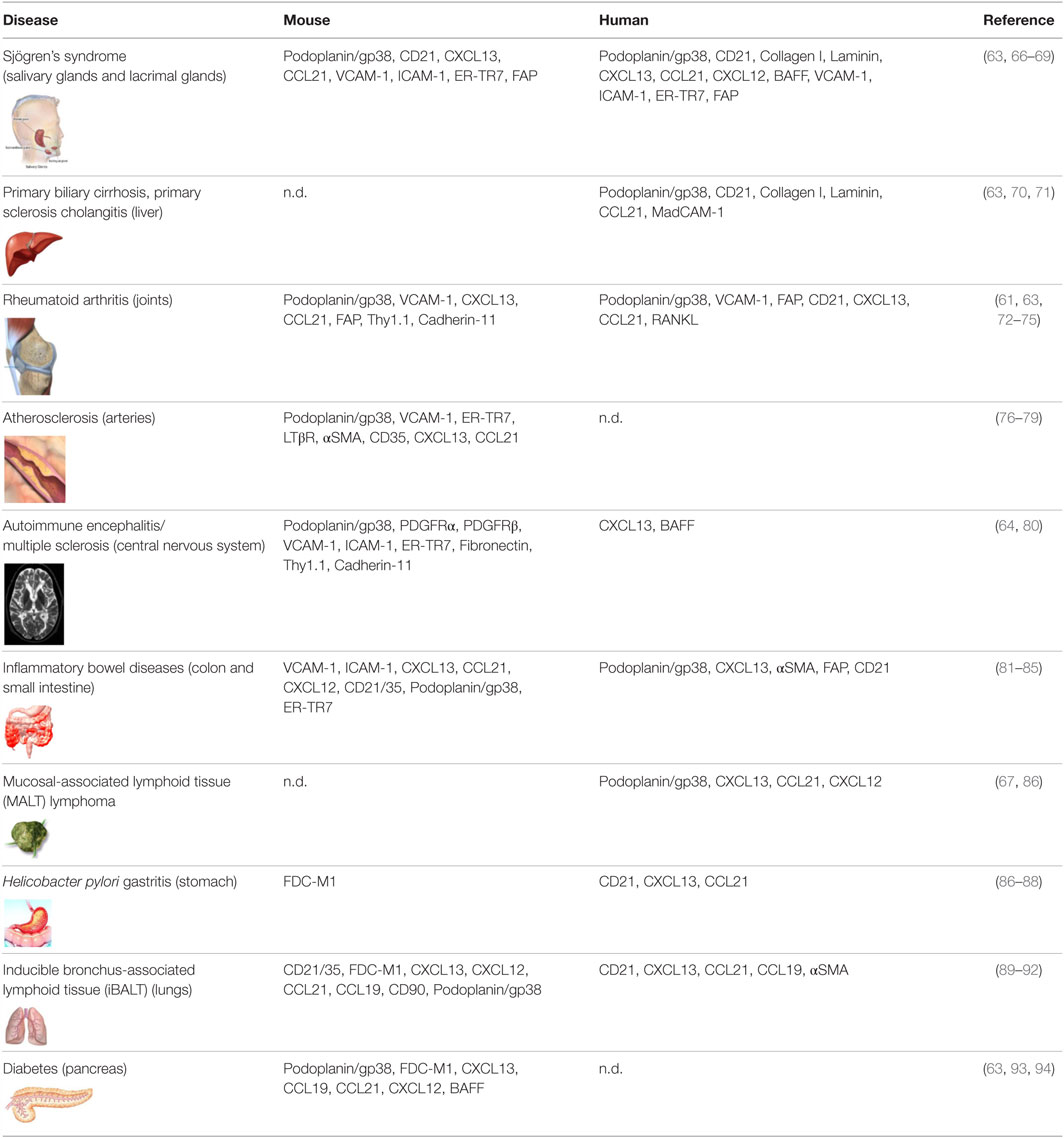
Table 1. Markers associated with fibroblasts found in tertiary lymphoid structures observed in disease settings in mice and human.
Priming of Fibroblastic Stromal Cells at Sites of Inflammation
The cellular and molecular requirement for mesenchymal cell priming, leading to cell differentiation and specialization during TLS establishment, is debated. Transgenic expression of lymphoid tissue chemokines such as CCL21 and CXCL13 overcomes the requirement for LTi cells, but not of LTα1β2 in non–spontaneous models of TLS that form in the eye, thyroid, and pancreas (95–98). The absence of LTi cells, in a CXCL13 transgenic model, leads to the development of smaller and less organized infiltrates suggesting a specific role for those cells in developing larger and more complex infiltrates (63). However, because the aggregates in these models develop perinatally and in the absence of inflammation, this model cannot be considered a classical model of TLS, and conclusions on its elements should be carefully drawn.
The hierarchy of requirement of TNF family members in physiological lymphoneogenesis is clear, with LTα−/− mice lacking both LTα3 and LTα1β2 expression showing the most severe phenotype, characterized by lack of all LNs and PPs, and a disorganized splenic white pulps (99, 100). In contrast, LTβ−/− mice, which specifically lack LTα1β2 function, retain MLNs and cervical LNs, and their splenic defects are less pronounced than those of LTα−/− mice (101, 102). A similar phenotype is observed in pregnant mice treated with LTβR–Ig fusion protein, whose progeny lack most PLNs and PP but retain MLNs (103–105). Ruddle and her group have clearly shown that ectopic expression of either TNFα, LTα, or LTα1β2 regulates the assembly of organized TLS, with the formation of MAdCAM+ (in LTα transgenic) and PNAd+ HEV (in LTα1β2 transgenic mice) and a complex network of lymphoid tissue chemokine expression (58, 106). In general, the combined expression of both LTα and β goes along with the formation of better organized lymphoid structures (58).
In spontaneous models of TLS formation, LTα1β2 is not absolutely required to prime the stromal cell compartment. Accordingly, the upregulation of adhesion molecules and the transient expression of lymphoid chemokines can occur in the absence of LTα1β2, or LTi and lymphocytes. Peduto and colleagues first demonstrated that a population of αSMA+ podoplanin+ fibroblasts, which express lymphoid chemokines and survival factors, classically associated with lymphoid stroma in SLOs, can differentiate in non-lymphoid tissue during inflammation and cancer. This phenomenon occurs prior to lymphocyte infiltration in the tissue and is conserved in RORγ-deficient mice (27). Other leukocytes, which are more abundant in the earliest phases of inflammation, such as myeloid cells or granulocytes, might therefore assume a “initiator role” in the formation of TLS by releasing proinflammatory cytokines capable of inducing activation of resident fibroblasts (27, 69, 107–109).
Since, by definition, TLS arise at sites of inflammation, it is virtually impossible to exclude the contribution of one or more of the TNF family members to the early phases of TLS formation. Engagement of TNFR on inflammatory or lymphoid tissue fibroblasts is known to upregulate chemokines, cytokines (including BAFF and IL-6), and adhesion molecules that largely define a primed stroma (110, 111). Moreover, TNF is known to upregulate the receptors for some of the inflammatory cytokines proposed to be involved in TLS establishment (112, 113). One may therefore put forward the hypothesis that the engagement of TNF is key prior to, or synergistically, with the expression of other proinflammatory cytokines to drive the initial priming of resident fibroblasts into functional LTo cells.
Other members of the TNF family have been implicated in lymphoneogenesis. Transgenic expression of LIGHT has been shown to induce TLS formation in models of melanoma and fibrosarcoma (114, 115) and to exacerbate disease in NOD mice (93). Overexpression of RANKL can also support the establishment of lymphoid tissue characterized by stromal cell production of lymphoid chemokines and lymphocyte recruitment (116–118). However, the mechanism by which RANKL regulates TLS assembly is not clear.
Besides TNF and LT family members, the types of cytokines involved in the first phase of stromal cell priming in TLS vary according to the tissues and types of responses (Figure 1). IL-6 has been associated with the perivascular accumulation of B cells and mature plasma cells (119). In a model of subcutaneous tumor apoptosis, TGF-β has been demonstrated to induce CXCL13 expression (120), while IL-5 expression has been associated with iBALT development and lung disease (121). IL-4 and IL-13 are known to stimulate, to different extents, the upregulation of adhesion molecules and transient chemokine expression on fibroblasts (122). Finally, IL-4, IL-7, and to a lesser extent IL-15, are known to stimulate expression of LTα1β2 on naïve T lymphocytes that might lead to TLS formation [reviewed in Ref. (95, 123)].
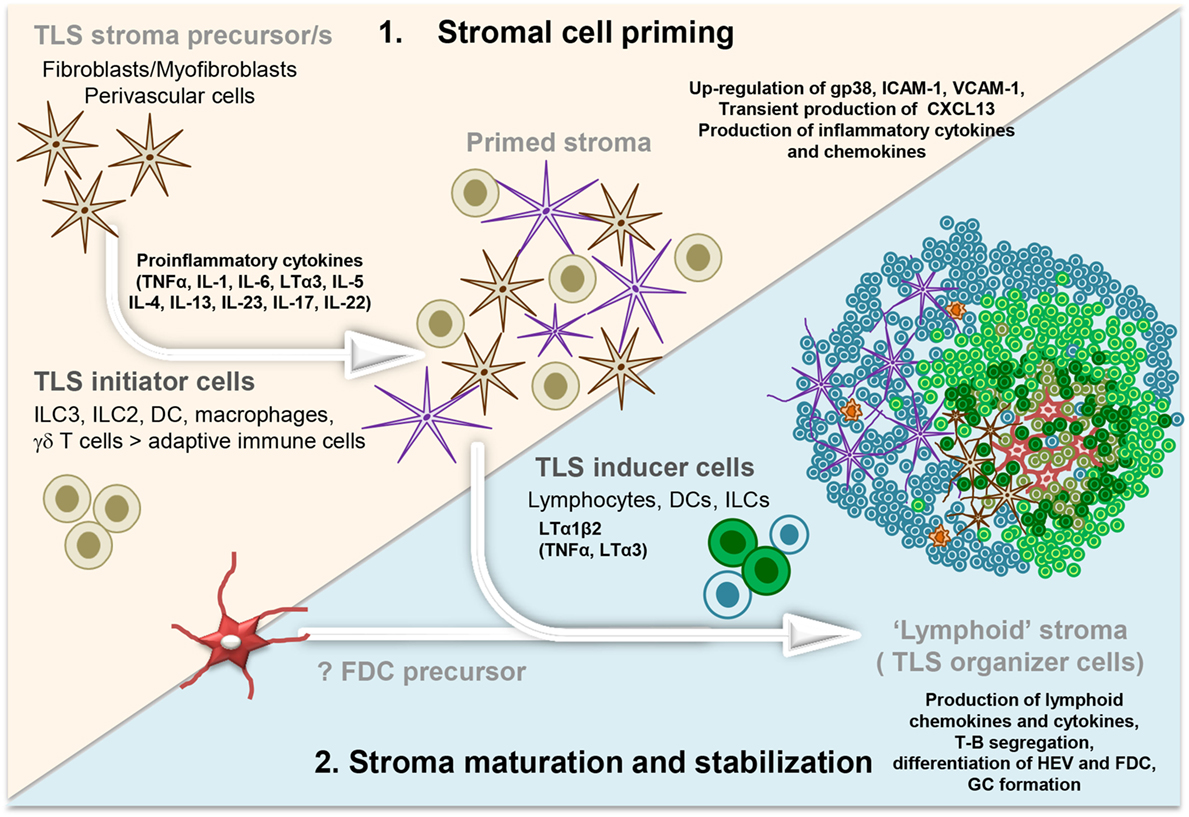
Figure 1. Development and maturation of lymphoid tissue-like stromal fibroblasts in tertiary lymphoid structure (TLS). Multistep model illustrating the priming followed by the stabilization and maturation of the fibroblasts allowing a lymphoid tissue-like organization and function of the lymphocyte infiltrate. Acute inflammation within a tissue results in the localized production of several different proinflammatory cytokines by infiltrating leukocyte populations (initiators) and tissue-resident cells. These signals elicit “priming” of local stromal fibroblasts, which may include the upregulation of gp38, adhesion molecules, and inflammatory cytokines. Prolonged inflammation can lead to local production of LTα1β2, along with TNFα and LTα by hematopoietic inducer cells. This triggers changes in the stromal fibroblast phenotype and function, including the production of chemokines typically expressed in lymphoid organs. FDC differentiation from local fibroblasts might only occur at this stage, when a critical mass of LTα3/LTα1β2/TNFα signals are provided by the co-localizing B cells, possibly inducing a positive feedback loop. Organization of the resident stroma and hematopoietic cells in a T cell (blue cells) and B cell (green cells) rich zone enables priming and activation of T and B cells toward locally displayed antigens. The formation of GC supports affinity maturation and expansion of B cells clones and plasma cells. Not Illustrated: the presence and differentiation of the vascular network [reviewed in Ref. (57)].
A separate case needs to be made for cytokines belonging to the IL-23 family. IL-17 production by γδ T cells has been demonstrated to provide the trigger for priming of lung fibroblasts in iBALT (92). During EAE, the development of a PDGFRα+PDGFRβ+ podoplanin+CD31− stromal cell network has been shown to be dependent on both IL-17 and IL-22 (64). Within murine salivary glands IL-22, but not IL-17, was deemed a key requirement for stromal cell activation in TLS that form (69). IL-23 expression has been associated with TLS formation in a model of arthritis (124), and the transfer of Th17 cells is sufficient to induce TLS during central nervous system tissue inflammation (109). Interestingly, the production of these cytokines and their relative source appears to be linked to the presence of the transcription factor RORγ that is also required for development and function of ILC3, γδ T cells, and Th17 cells (125–130). Several factors, like IL-23, may act upstream of RORγ, with IL-17 being downstream (131), thus increasing the level of complexity of this system. In some settings, RORγ is deemed dispensable, such as in virus-induced salivary gland infection, where TLS formation and autoantibody generation strongly depend on IL-22 but not IL-17 or RORγ (69). In a model of airway damage and inflammation, it has been shown that IL-17A might regulate both the expression and the proinflammatory properties of IL-22 (132), thus suggesting the possibility that several cytokines can initiate TLS establishment and their relative contribution is likely to be influenced by the site of inflammation and etiological agent.
To date, only one cytokine, IL-27, has been identified that directly inhibits TLS development by negatively regulating the differentiation of Th17 cells, a major driver of TLS development and RA pathogenesis (133).
In summary, multiple pathways and several cell types can act as initiators of TLS assembly and induce activation or priming of the resident fibroblasts in a way that leads to a lymphocyte permissive tissue state (Figure 1). The capacity of leukocytes, other than T and B cells, to provide cytokines for stromal cell activation demonstrates a critical uncoupling between stromal cell priming and lymphocyte accumulation in TLS, establishing a model whereby stromal cell priming might occur prior and largely independently from a significant lymphocyte migration into the tissue.
Maturation and Stabilization of Fibroblastic Stromal Cells Allowing TLS Formation
Transient activation of stromal cells that often occurs in acute phases of inflammation is not sufficient to support complete lymphoid-like fibroblast maturation associated with TLS formation. Upon resolution of inflammation, the “primed state” of fibroblasts is likely to be lost; alternatively, these activated cells may disappear. Only selected circumstances, such as antigen persistence or severity and length of the inflammatory response, may drive the development of lymphoid tissue-like mesenchyme. Part of this complex phenomenon is also reliant on the dramatic changes that both stromal cells and leukocytes induce in the lymphatic and blood vasculature and that occurs as an integral part TLS development (57, 134). In this context, the ability of TLS-associated fibroblasts to secrete pro-angiogenic factors, including VEGF-C and VEGF-D, should also be highlighted (27).
Interestingly, this two-step process of mesenchymal cell priming and maturation is reminiscent of the early phases in the process of lymphoid neogenesis, whereby the early production of CXCL13 and the upregulation of ICAM-1 and VCAM-1 on local mesenchymal cells occur independently of lymphotoxin and LTi. In SLOs, this first phase is followed by the LTα1β2-dependent interaction of LTi cells with the primed mesenchymal cells leading to their specialization as LTo cells capable of inducing HEV development, lymphocyte recruitment, and stromal cell specialization in various subsets (28, 29, 135). Interestingly, in SLOs, the resident mesenchyme is unable to maintain the durable production of survival factors and chemokines if TNFR or LTβR engagement is missing and only a few disorganized LN form in LTα−/− or LTβR−/− mice (38, 50). Accordingly, the prolonged treatment of adult wild-type mice with LTβR-Fc leads to dedifferentiation of FDC, HEV, and partially fibroblastic reticular cells (FRC), and as a consequence to reduced lymphocyte recruitment, retention, and compartmentalization (136).
Similarly, in TLS, full differentiation of the lymphoid tissue-like fibroblasts also requires the presence of lymphocytes and LTβR signaling. TLS can form in LTα−/− mice but display a disorganized pattern of lymphocyte aggregation, in absence of clear B/T cell segregation or HEV differentiation (90). LTβR-Fc treatment of established TLS leads to the same outcome, suggesting a continuous need for these signals in order to maintain differentiated HEV and chemokine-expressing fibroblast networks (95, 137, 138). Continuous LTβR and TNFR1 signaling is also required for sustained expression of VCAM-1, CXCL13, and CCL21 in TLS that form in the aorta and in the brain (64, 76, 77). This finding is reminiscent of the requirement for LTα2β1 in order to maintain normal numbers and compartmentalization of lymphocytes in the spleen, PP, and ILFs (47, 53, 137, 139, 140). The combined activation of TNFRI and LTβR is required for the formation of TLS in both inducible and spontaneous models of atherosclerosis (76, 77). However, blockade of LTβR is sufficient to reduce insulitis and diabetes (138) in a NOD mouse model characterized by the presence of podoplanin+ FRC networks and HEV differentiation (63).
All together, these data are consistent with a model of TLS formation in which there is an initial phase of stromal cell priming that occurs independently of LT and precedes tissue infiltration by adaptive immune cells. In the second step, the maturation of resident fibroblasts to a full LTo phenotype appears to be dependent in most settings on LT and TNF, presumably needed to enable dual activation of the NF-κB cascade, with the alternative pathway being maintained over time. Of note, continuous and strong expression of TNF and possibly other NF-kB activating cytokines may bypass this LTα1β2 requirement and still lead to TLS development, though the precise mechanism by which this phenomenon occurs has not been fully clarified. In the context of ectopic lymphoneogenesis, one may therefore propose an extension of the term “lymphoid tissue inducer” to different leukocyte cell types that express sufficient levels of LTα1β2 to induce full differentiation of resident mesenchymal cells into a lymphoid tissue phenotype (96, 108, 141–145). Naïve B cells and DC may qualify for this term, as these cell types on a wild type but not on a LT-deficient background can induce formation of lymphoid tissue structures in vivo (47, 146). Evidence is less strong for a LTi like role for T cells although they can express LTα1β2 upon cytokine exposure or activation (147).
Downstream the activation of LTβ receptor is the production of lymphoid tissue chemokines, critically required for lymphocyte recruitment and TLS development. Accordingly, ectopic CCL19 expression under the rat-insulin promoter alone is able to form small infiltrates rich in T cells and dendritic cells; while ectopic CCL21 expression is able to induce the formation of large and better organized infiltrates, characterized by the specific development of T and B zone stroma and HEV differentiation. Similarly, ectopic CXCL13 expression is known to regulate lymphoid tissue neogenesis with T and B cell segregation and complete specialization of the stromal compartment, including FDCs and HEVs (63, 137). These findings in TLS formation are in keeping with the critical role played by CXCL13 and CCL21 in lymphoid organ development during embryogenesis (95).
Interestingly, different functional phenotypes, in terms of chemokine expression, have been observed in fibroblasts isolated from different anatomical sites. In the skin, podoplanin+ inflamed fibroblasts express modest levels of IL-7 and CXCL13, but high quantities of CXCL12 and VEGF-C (27). In contrast, podoplanin+ cells isolated from gut and tumors significantly upregulate the lymphoid chemokines CXCL13, CCL19, CXCL12, and various cytokines, including VEGF-C, connective tissue growth factors, including fibroblast growth factors (27). Stromal cells isolated from iBALT are characterized by CXCL12 expression (92), while the network of podoplanin+ CD31− cells isolated from salivary gland TLS express CXCL13 and CCL19 but not CXCL12 (69). Differences in terms of TLS organization and chemokine expression can also be observed at different sites or even in TLS at the same anatomical site when they are induced in response to different antigens. For example, intranasal administration of the poxvirus modified vaccinia virus Ankara (MVA) in mice is able to induce highly organized iBALT with B cell follicles containing a network of CXCL13-expressing FDCs and CXCL12-producing follicular stromal cells. However, mice treated with P. aeruginosa developed iBALT with B cell follicles that consisted of CXCL12+ follicular stromal cells but not CXCL13+ FDC (92). The signals responsible for these phenotypic and functional differences are unclear and most likely result from an integrated response to different anatomical environments, antigenic stimulations, and inflammatory milieus. While this review focuses on fibroblasts, the ability of hematopoietic cells, including macrophages, dendritic cells, and Th17 cells, to ectopically express CXCL13 should also be acknowledged, suggesting the possibility to consider the cytokine/chemokine, rather than its cellular origin as a therapeutic target (148–152).
Stromal Fibroblast Organization in SLOs and TLSs
Mature SLOs are characterized by the anatomical organization of lymphocytes in distinct compartments, which is due to the segregated chemokine expression of CCL19/21 in T zones and CXCL13 in B zones. The source of these chemokines are specialized subsets of resident FRC and FDC, which attract and retain specific leukocyte populations, besides providing survival factors.
Fibroblastic reticular cell subsets, including FDC, are defined by their phenotype, anatomical location, and function (2, 24, 153, 154) (Figure 2). T zone FRCs or TRC classically inhabit the T cell cortex in LNs and are characterized by the expression of podoplanin and lack of the vascular marker CD31. TRCs are responsible for the recruitment, retention, and movement of naïve T lymphocytes and DCs via their expression of CCL19 and CCL21. Besides regulating immune cell trafficking, TRCs produce extracellular matrix, forming a system of microchannels (conduits) that connect the subcapsular sinus with the paracortex and HEVs. TRCs also provide a significant source of IL-7, which in combination with CCL19, sustains naïve T cell survival within the LN T zone, regulating T cell homeostasis (22, 155).
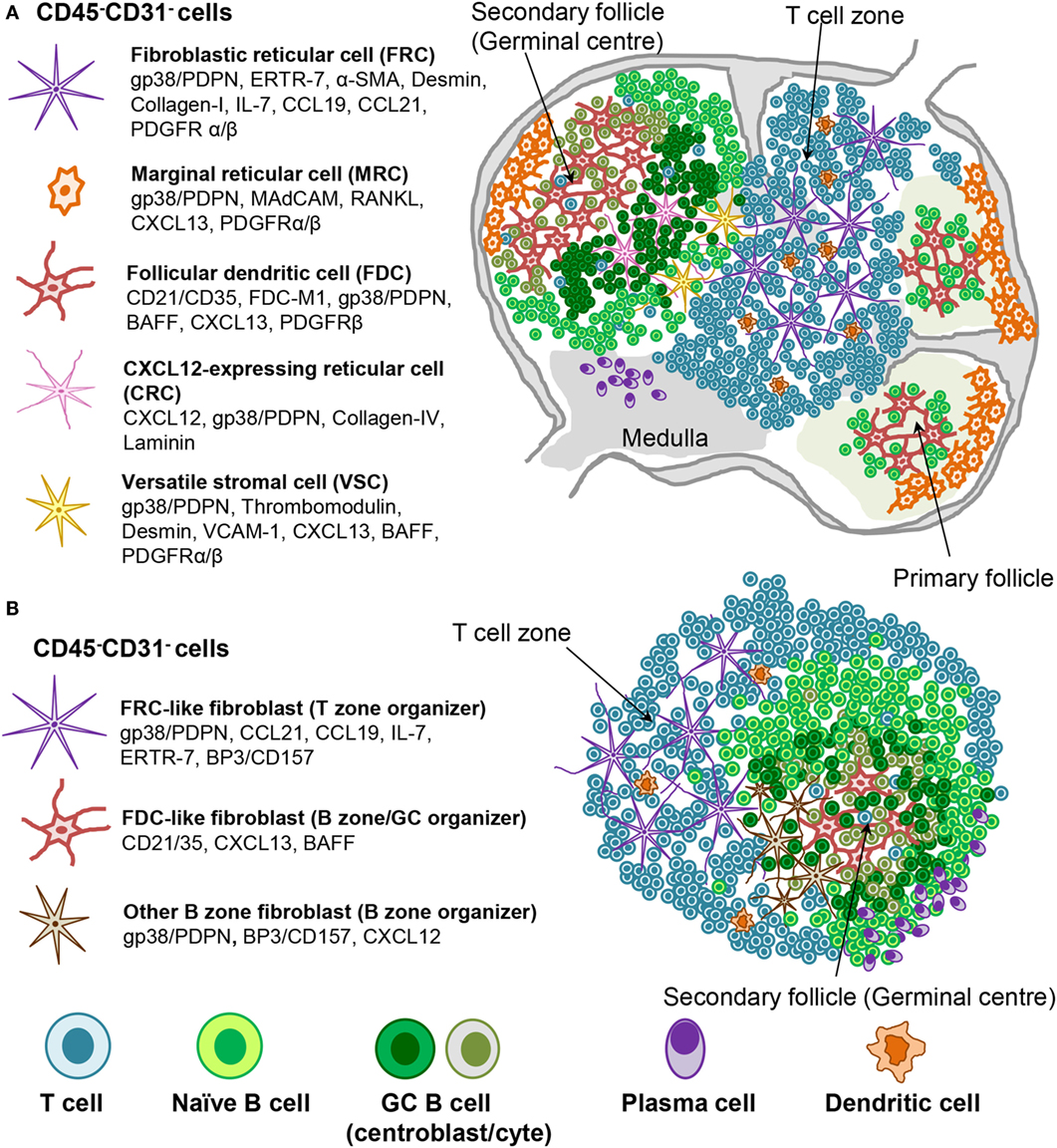
Figure 2. Stromal fibroblast populations in SLO and TLS. (A) Fibroblast populations in the lymph node control the organization and survival of lymphocytes in distinct areas. Fibroblastic reticular cells (FRC) produce CCL19 and CCL21 along with the survival factor IL-7 to attract and maintain T cell populations and provide a niche in which their interaction with dendritic cells (DC) can occur. Follicular dendritic cells (FDC) produce CXCL13, which attracts CXCR5+ cells to the B cell follicles. Other stromal cells are thought to play a role during the germinal center reaction (i.e., CRCs) or in antigen delivery (MRC). (B) Mature TLS are characterized by segregation into distinct T cell and B cell areas including the presence of germinal center like structures and areas rich in plasma cells. Stromal cell populations that perform comparable functions to those found within SLO can be identified, which underlie this T/B cell segregation within the larger TLS.
Fibroblastic reticular cells that inhabit the outer B zone, also termed B cell zone FRCs (BRC), are characterized by high levels of BP-3 and podoplanin expression but lack FDC markers like CD21/35. BRC represent an important source of oxysterol chemoattractants, BAFF, CXCL13, and the Notch ligand DL4 (156–158). Marginal reticular cells (MRCs) represent another B zone FRC population that sits underneath the subcapsular sinus and is characterized by RANKL and CXCL13 expression (35, 159). BRC and possibly MRC form conduit networks able to deliver lymph-drained information, including antigens, from the subcapsular sinus to the B cell area and eventually to the FDCs present within the B cell follicles (2, 153, 156–162). Due to their phenotypic similarities, MRCs were proposed to represent the adult equivalent of embryonic LTo cells (159). In support of this concept, MRCs were shown to be the precursors of LN FDCs during immune reactions (162).
Follicular dendritic cells are the prominent stromal cell subset found within the central part of primary B cell follicles and localize specifically to the light zone of the GCs in secondary follicles. FDCs are defined by their dendritic appearance and their capacity to retain opsonized antigen via Fc and complement receptors. FDCs serve as long-term reservoir of native antigen for the positive selection of affinity matured GC B cells or centrocytes (163). In addition, FDCs are a source of CXCL13 and BAFF, responsible for migration and survival of naïve B cells. TNFR and LTβR signaling are critically required for the maintenance of the FDC network and in general to support the CXCL13 producing stroma in the follicles (53, 164, 165), as evidenced by their rapid disappearance upon TNFR or LT blockade (45, 166–169).
Recently, a population of CXCL12-expressing reticular cells (CRCs) has been described in the dark zone of the primary follicles and polarized GCs, where they are required for the recruitment of CXCR4+ centroblasts and effective GC responses (170). This subset is fate mapped by both the Cd21-cre and Ccl19-cre mouse lines with the fate mapping indicating past and/or present expression (171). It lacks most of the classical markers of FDCs [such as CD21/35, VCAM-1, FDC-M2, FDC-M1, M-2, and CD16/32 (FcγRII/III)] or FRCs (such as laminin and type IV collagen association). Moreover, CXCL12 expression on CRCs is independent of LT or TNFα thus establishing critical phenotypic and developmental differences between CRC and FDCs (171). Finally, a population of versatile stromal cells (VSC) has been described at the T zone edges of the B cells follicle, able to respond to inflammatory stimuli, B cell contact, and LTα1β2 stimulation by upregulating CXCL13 expression (172).
Many of the FRC subtypes are fate mapped by the same Ccl19-Cre transgenic mouse, suggesting a common precursor in embryonic life for the majority of FRC subsets, at least in the LN (171). In future, mice allowing inducible fate mapping are needed to gather more direct evidence for such a precursor–progeny relationship, both in embryonic and postnatal LN. Currently, very little is known on the specific differentiation and survival signals leading to the complex stromal cell organization observed in adult life.
To study signals regulating FRC function, various FRC lines have been generated from adult murine and human SLO. Murine cell lines constitutively produce CXCL12 and sometimes BAFF but require stimulation of the TNF receptor to upregulate various inflammatory chemokines (e.g., CXCL10, CCL4/5) as well as IL-7 (33, 35). Stimulation of LTβ- or TNF-receptors strongly upregulates matrix production, with the combination of both signals showing synergistic effects. Those signals could be mimicked by CD4+ T cells, physiological neighbors of FRC in vivo. Similarly, stimulation of a MAdCAM+ MRC line via both LTβ- and TNF-receptors was also able to induce further expression of CXCL13 and CCL19 (33, 35). Interestingly, fluid flow was shown able to induce CCL21 expression in a FRC line (173); while human LN FRC responded to TNFα, IL-6, IL-4, and IL-13 by upregulating various cytokines, adhesion molecules, and metalloproteinases (174). These in vitro data support an ability of lymphoid tissue fibroblasts to adopt different functions depending on the signals received from the surrounding environment, and thereby influence neighboring immune or stromal cells.
Our knowledge about stromal fibroblasts within murine and human TLS is still very limited. Current evidence suggests the presence of TRC-like cells within the T cell rich zones, and of FDC-like cells that inhabit B cell rich areas in more organized TLS. MRCs, BRCs, CRCs, or VSCs have not yet been described in TLS. Depending on the size and type of TLS, fibroblasts display a variable degree of the phenotypic and functional features described for their SLO counterparts (Figure 2). It is debatable the extent to which TLS stroma reaches a comparable level of polarization and differentiation compared to SLO stroma. The two subsets identified so far in TLS, namely CCL21+ TRC and CXCL13+ FDC, can associate and probably form distinct compartments, such as T and B zones but also allow the formation of functional conduits and GCs (63). These niches appear to be functional in generating a specific adaptive immune response, but it is less clear whether fibroblasts in TLS allow a comparable regulation of immune processes. It is likely that in TLS, similar to SLOs, the predominant lymphocytic population that accumulate in each area imprint locally activated mesenchyme to support an increased requirement for specific survival and chemo-attractive factors. Lymphoid stromal cell differentiation would therefore be programed by anatomical location through contact with neighboring cells. Any polarization toward an FRC-like or FDC-like phenotype may be reenforced as lymphocyte segregation arises within mature TLS by the expression of one or two chemokines, such as CXCL13 and CCL21 (61, 66, 67, 72), thereby restricting stromal cell contact to either T or B cells. It is known that both CXCL13 and CCL21 can induce LTα1β2 expression in the responding lymphocytes, further enhancing chemokine expression by the neighboring fibroblasts (95, 142). The additional factors needed besides LTα1β2 to drive fibroblasts into a differentiation program specific for the B zone or the T zone are currently unknown.
In murine TLS, TRC-like cells expressing podoplanin and other markers (Figure 2; Table 1) are found throughout T cell rich zones as three-dimensional reticular networks and partly co-localize with dendritic cells thereby forming an environment where cognate T cell stimulation is possible. They associate with matrix fibers that can form functional conduits and connect with HEVs. Often they express CCL21, which is key to the T zone formation. CCL19 and IL-7 expressed by FRC may contribute to local T cell accumulation and possibly to random T cell migration along the TRC network (2, 153, 155, 175).
Follicular dendritic cell networks form only within B zones of large TLSs in mice and are then associated with functional GCs allowing B cell differentiation to affinity matured plasma cells (63, 88, 92, 94, 176, 177). Interestingly, not all tissues, and only a minority of TLSs form fully mature FDC networks, suggesting that while the stimulus for FDC differentiation might be the same as that observed in SLO (TNFα and LT), the threshold required for full FDC differentiation in peripheral tissue is higher. An alternative possibility is that FDC precursors in the periphery are scarce and differentially distributed in different organs. Whether the phenotype, origin, and function of FDCs in peripheral TLSs differ from those of SLO is not known, neither is their differential dependency on LT or LT-inducing pathways. It is important to highlight that even in SLOs, at least two different precursors have been identified for FDCs, which differ between spleen and LNs (162, 178, 179), thus potentially increasing the complexity of signals and progenitors required for FDC differentiation in TLS.
While it is likely that in TLS a single lymphoid tissue-like progenitor gives rise to both FRC and FDC-like cells, it cannot be excluded that FDC might differentiate from FRC-like cells or another precursor later in TLS development. Interestingly, the absence of MRC-like cells and the lack of any anatomical capsule within the TLS appears to exclude the involvement of an “MRC-like progenitor” in FDC differentiation in TLS. Data obtained from parabiosis experiments and fate mapping experiments by Peduto and colleagues suggest that local, rather than circulating, precursors are responsible for the expansion of the stromal network responsible for TLS establishment (27). Proliferation of the resident stroma has been also observed in an inducible model of TLS, both in the vascular and stromal compartments (134) (Nayar et al., manuscript in preparation), which mimics the expansion of the stromal compartment during immunization in SLOs (22, 180–182). Therefore, the most likely scenario is that local differentiation and expansion of tissue-resident mesenchymal cell/s accounts for TLS development.
Even less is known about fibroblasts found in TLS within human pathology, in part due to the lack of markers identifying fibroblasts and distinguishing different cell subsets. Most efforts have concentrated on reporting the presence or absence of CD21+ FDCs or lymphoid tissue chemokines, such as CXCL13, CCL21, CCL19, and CXCL12 (7, 8, 183). Discrete expression of CXCL13, CXCL12, and CCL21 has been described in salivary glands of patients with SS, RA, multiple sclerosis, primary sclerosing cholangitis, atherosclerosis, inflammatory bowel disease, chronic lung diseases, Helicobacter pylori-induced gastritis, and lymphoma [reviewed in Ref. (7)]. However, this classical work largely preceded the phenotypic characterization of fibroblast subsets in mice and therefore lacks in-depth characterization of the stromal cell compartment. Nonetheless, the association between the level of organization of TLS with lymphoid chemokine expression (7), together with identification of the source of lymphoid chemokines in the αSMA+ or desmin+ stromal compartment (63), suggests that TLS fibroblast subsets exist in humans. More recently, our laboratories have demonstrated the presence of podoplanin+ FRC-like cells in tonsil, RA synovium, and SS salivary glands, as based on the expression of podoplanin and CCL21 and the association with T cells, DC, and matrix fibers (63). These FRC-like cells were distinct from the CD21+ FDC that organized B cell rich zones, as described previously by several laboratories and summarized in Table 1.
While the use of podoplanin to identify “lymphoid tissue stromal cells” in the periphery remains a reasonable approach, podoplanin expression has also been observed on epithelium, lymphatic vessels, Th17 cells, and myeloid cells (184). Its use therefore requires careful analysis of cell morphology and double labeling with cellular markers (absent on fibroblasts) to validate the specificity of the population of cells detected. In future, it will be important to use further markers to discriminate human fibroblast subsets in TLS, in order to improve stromal cell characterization in human TLS histology samples.
Effector Functions of TLS
Tertiary lymphoid structures are classically defined as lymphoid aggregates forming in organs whose main function is other than the initiation of an adaptive immune response. TLS appear to form there because of an abundance of antigen, either “self” in autoimmune diseases, “self” or “altered self” in cancer, or foreign antigen/s during infections and transplant rejection. At those ectopic sites, TLS can contribute to the generation of an antigen-specific immune response with plasma cell and antibody generation, often maintained by the persistence of the antigen and/or inflammatory signals (6, 8). The local stromal structures needed for naïve cell recruitment and affinity maturation of the B cell compartment are mainly observed in the most organized TLS (66, 185). Interestingly, TLS do not develop in all forms of chronic inflammation and only arise in certain permissive tissues. A classical association with mucosal epithelium has been observed. However, TLS can form in the synovium, a tissue devoid of epithelial structures (7). The factors involved in tissue permissiveness are not clear, and while it is intuitive to suggest the need for proximity to antigen and antigen presenting cells, this is not sufficient as exemplified by the case of the skin, classically considered a “hostile site” for TLS formation.
Aggregation of lymphocytes in small TLS is commonly observed in transient infections where it is considered a positive development, aimed at containing local infections. The development of lung TLS in response to influenza supports the further development of a strong antigen-driven T cell response contributing to viral clearance (186, 187). In these cases, TLS disappear shortly after pathogen clearance leaving the tissue intact (68, 188).
However, in chronic inflammation, the presence of TLS has been associated with poor clinical outcome and disease progression rather than resolution (94, 189). TLS formation correlates with serum autoantibody levels, disease severity, tissue damage, and decreased organ function in several diseases including SS [reviewed in Ref. (7)]. In RA, the formation of subchondral bone TLS supports osteoclast activation and tissue damage (190), and the presence of synovial TLS associates with anti citrullinated antibody production and poor response to anti-TNF antibodies (74, 191). Accordingly, levels of TLS-associated CXCL13 expression correlates with disease severity and persistence of subclinical synovitis (190, 192, 193).
It is arguable that TLS represent a response to and are not per se a cause of inflammation. However, a combination of factors, among which excessive cell recruitment, poor lymphatic drainage, disorganized cellular interaction, and excessive survival factors can contribute to TLS persistence in tissue, favoring a pathogenic role in the context of diseases. Interestingly, not all patients with autoimmune disease develop TLS, despite the presence of factors associated with its development, for example, Th17 cells in RA (133). This suggests that the biological relationship between disease progression, clinical features, and TLS formation is more complex. In this context, the detection of GC within salivary glands of patients with SS has been classically associated with increased risk of lymphoma (194), suggesting that chronic antigen stimulation and excess of survival factors might favor the development of malignant B cell clones. However, this proposal is controversial and a strong positive correlation between the two pathogenic entities cannot be identified, leaving the relationship between TLS formation and lymphoma development uncertain (194, 195).
The role of TLS in the context of cancerous growth is also debated. TLS are believed to sustain the antitumor response in solid malignancies that arise in the colon, breast and ovaries as the presence of TLS in the context of cancer has been associated with a favorable prognosis (196). Nonetheless the ability of tumor cells to induce T regulatory cells (Treg) and suppress the host immune response is well known and there is the possibility that cancer cells highjack TLS to exert this immunosuppressive function (197–200).
Classically, the key effector function associated with TLS development has been the formation of GCs and the production of autoantibodies [reviewed in Ref. (7)]. However, more recently a more complex role for TLS in the context of T cell activation and maturation of pathogenic T cell response has been proposed.
While the production of CXCL13 represents a sensitive and powerful readout of stromal cell activation in TLS (69), this is unlikely, in the early phases of TLS establishment, to be restricted to the rather complex events of follicular B cell differentiation, an event that occurs later in TLS (69). It is likely that early CXCL13 production in the context of TLS assembly is aimed at driving the recruitment of CXCR5+ B cells and T follicular helper (Tfh) cells (201). Accordingly, interfering with Tfh infiltration by ICOS-L blockade results in reduced TLS assembly and progression of vascular disease in a model of TLS associated with atherosclerosis (202). The origin of Tfh that arise within TLS is currently not clear. As mentioned, Tfh populations have been identified within the circulation in both mice and humans that could be recruited into TLS by newly established CXCL13 gradients (203–205). There is however the possibility that TLS provide a site for local Tfh differentiation. In support to this hypothesis, naïve T cell recruitment and priming has been reported within TLS that form in pancreatic tissue in NOD mice (93). However, naïve T cell recruitment requires the upregulation of CCR7 and L-selectin ligands on the vascular endothelium, typically HEVs (59, 206), which is another hallmark of mature TLS. Effector T cells are more likely to be recruited in the earliest phases of TLS assembly, thus suggesting that in TLS, as opposed to SLO, T cell differentiation into Tfh might occur from previously activated peripheral T cell populations that already display effector functions. In support of this hypothesis, Th17 cells isolated from TLS of EAE affected mice display features of Tfh, including the upregulation of expression of CXCR5, ICOS and Bcl6 (109). Within TLS the activated fibroblast compartment may provide key signals for T cell differentiation, such as IL-6, required for the induction and maintenance of the Tfh phenotype (207, 208), thus suggesting an additional role for stromal cells in the context of TLS development.
The presence and function of Treg populations in the context of TLS has been less studied. Treg recruitment into TLS might directly interfere with the activity of TLS associated Tfh cells, similarly to what has been described in SLOs (209, 210). Accordingly, in TLS that form in association with atherosclerosis, disruption of CD8+ Treg activity is known to induce an expanded GC B cell response (202). Additionally, in a mouse model of lung adenocarcinoma, Treg that inhabit local TLS, are known to interfere with the antitumor T cell response. In addition to directly inhibiting T cell responses within the TLS, Treg appear to impact upon the recruitment of additional lymphocytes to the TLS by affecting the formation or maintenance of PNAd+ HEVs (211, 212). Interestingly, the tumor microenvironment is conducive to the recruitment, generation, and maintenance of Treg populations (197) and, indeed, Treg infiltration in solid tumors is considered a negative prognostic factor. Whether Treg are less active or abundant in TLS associated with chronic inflammation, as compared to cancer, remains to be answered.
T regulatory and Th17 cell differentiation is promoted by TGF-β; however, Th17 development occurs on a background of proinflammatory cytokines, such IL-6, IL-21, and IL-23 (213–215). It is possible that in TLS that arise in chronic autoimmunity, the inflammatory milieu, partly established by locally activated fibroblasts, positively enforce Th17 over Treg differentiation, favoring the upregulation of the transcription factor RORγ while inhibiting FoxP3 expression (216). Additionally, an environment that is rich in IL-1 and IL-6 and deficient in TGFβ is sufficient to reprogram Treg toward a Th17 phenotype (217, 218), thus suggesting that the inflammatory microenvironment associated with TLS in chronic inflammation may not be able to maintain Treg phenotype cells. Indeed, Th17 differentiation has been observed in RA TLS (133). The differences between diverse T effector populations and the balance between T effector and Treg cells in the different lymphoid niches may therefore explain some of the discrepancies observed between immune responses that occur within TLS associated with cancer and chronic inflammation.
It is not clear to what extent the stromal cell compartment contributes to TLS pathogenicity. In SLOs, FRC are increasingly recognized as active modulators of the immune response. FRCs can interfere with the T cell response through several mechanisms, releasing soluble modulators that negatively regulate T cell proliferation or providing negative co-stimulatory molecules such as PD-L1 (219–221). The ability of FRC to acquire peptide–major histocompatibility (MHC) II complexes from professional antigen presenting cells or to upregulate MHC I and II molecules has also been shown. Moreover, FRC are able to display peripheral tissue antigens mediating the deletion of peripheral autoreactive CD8+ T cells (19, 222, 223) and maintaining the homeostasis of Treg cells (222, 224). Taken together, these studies demonstrate that, while FRCs provide survival niches for lymphocyte homeostasis, they also govern the magnitude of the immune response (19, 221).
The extent to which the regulation of immune responses by stromal cell populations translates from SLOs to TLS has not been explored. Interestingly, fibroblasts isolated from non-inflamed peripheral tissues display a strong propensity to inhibit T cell responses, possibly to protect non-lymphoid tissues from the harmful effects of inflammation (221). In cancer-associated TLS, also characterized by a chronic inflammatory response, stromal fibroblasts are believed to contribute to immune evasion, preventing lymphocyte effector functions and immune cell access to the cancer site (12). However, in contrast with these, most recent findings and the immunosuppressive role described for stromal cells in SLOs and cancer, current evidence strongly suggests a pathogenic role for TLS in autoimmune conditions, sustaining lymphocyte survival and supporting lymphocyte persistence in the tissue (7, 8). The pathogenic, non-immunosuppressive role of lymphoid-like fibroblasts that inhabit TLS found in chronic inflammatory conditions appears therefore unique and requires further characterization. There is the possibility that functional differences exist among fibroblasts that bear a similar phenotype in TLS and SLOs or that functional differences are acquired during disease progression. The different origin of SLO and TLS fibroblasts and the cytokine milieu driving mesenchymal differentiation at ectopic peripheral sites are likely to contribute to this complex phenomenon. A more detailed characterization of the stromal compartment in TLS associated with chronic inflammation in comparison with SLOs might provide key elements to unravel these discrepancies.
Stromal Fibroblast Deletion: A Novel Strategy to Manipulate the Immune Response
Interesting insights into the function of SLO fibroblasts have been derived from cell deletion experiments. Taking advantage of the mouse lines expressing diphtheria toxin receptor (DTR) selectively in fibroblasts by the use of the fibroblast-specific promoters of FAP (fibroblast activation protein α) or CCL19, stromal cell deletion could be obtained in adult LNs. FAP–DTR mice treated with DTX showed disrupted LN homeostasis with strongly reduced numbers of T and B lymphocytes and DCs. Upon influenza infection, mice lacking FAP+ cells mounted a diminished immune response characterized by reduced numbers of GC B cells, plasma B cells, and Tfh cells (225). By using a different inducible transgenic model [Ccl19-Cre × Rosa26-diphtheria toxin receptor (iDTR) mice] Turley, Ludewig, and colleagues demonstrated that selective depletion of FRCs resulted in aberrant localization of T lymphocytes within the LN cortex as well as a reduction in both CD4 and CD8 T cell numbers via a mechanism dependent on IL-7. Antigen-specific T cells isolated from these immunized, FRC depleted mice failed to undergo priming and proliferation (157, 226). Humoral responses and B cell homeostasis were also impaired in the absence of FRC, with disorganized B cell accumulation within the GCs and significant reduction of virus-specific antibody production (157). While this defect was largely attributed to the inability of B cells to access homeostatic levels of BAFF once FRC were depleted (157), Acton et al. recently demonstrated that the engagement of CLEC-2 (expressed on DC) by its ligand podoplanin (expressed on FRC) is necessary for DCs to spread, migrate, and provide appropriate Ag presentation to T cells in LNs (227, 228). This suggests that the inability of the DCs to migrate into LNs in FRC-depleted LNs could contribute to the defect in T and B activation observed in this model. It has been reported that ablation of FDC achieved in Cd21-Cre × Rosa26-iDTR mice results in loss of primary B cell follicles. This effect, partially mediated by BAFF and CXCL13 depletion, is also supported by the decreased levels of IL-6 and integrins present in SLO of transgenic mice treated with DTX (229). All together, these studies indicate that alterations in SLO stromal compartments can alter lymphocyte survival, compartmentalization, and immunological competency, often sequentially linked, which profoundly impact on SLO function.
The impact of stromal cell deletion on TLS has not been addressed as yet and might provide critical clues on the relative immunosuppressive or proinflammatory role of TLS-associated stromal cells, ultimately unveiling whether the role of TLS in different diseases at various anatomic sites is beneficial or detrimental. Whether this approach, using antibody-based therapeutic agents will be feasible in humans is of interest. Deletion of specific subsets of fibroblasts might be problematic due to the anatomical impact related to the apparent overlap of markers between lymphoid stroma in SLOs, TLSs, and non-lymphoid tissues. However, a series of compounds able to interfere with stromal cell activation and functions are available and will present an interesting avenue to target stromal cell activation, alone or in combination with current immunomodulatory agents, such as anti-TNFα, anti-IL-6, anti-CTLA4 and anti-PDL1.
Conclusion
The main function of TLS is to maintain lymphocytes populations and provide a level of structural organization that enables the development and regulation of an adaptive immune response within peripheral tissues. This role is largely played by distinct populations of activated mesenchymal cells that acquire features similar to those of the lymphoid fibroblasts that inhabit SLOs. However, fibroblast maturation in TLS is an event, which is highly influenced by the anatomical site, the danger signals, and the inflammatory microenvironment, thus profoundly differing from the stereotypic mesenchyme specification observed during SLO development. Moreover, the organization and size of TLS does not equal that of their highly regulated SLO counterparts. The likely inability to precisely control leukocyte recirculation and cross talk within diseased tissue together with the absence of finely regulated chemokine gradients for lymphocyte positioning and the abundance of lymphocyte survival factors might explain why TLS fail to resolve and thereby contribute to immune-mediated inflammatory disease and its persistence.
Author Contributions
All the authors contributed in writing the review.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
FB and CB are part of RACE (Arthritis Research UK Rheumatoid Arthritis Pathogenesis Centre of Excellence). RACE was part-funded by Arthritis Research UK (20298); this Centre is a collaboration between the Universities of Glasgow, Newcastle, and Birmingham. FB is an ARUK Senior Fellow.
References
1. Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol (2003) 3(4):292–303. doi: 10.1038/nri1054
2. Roozendaal R, Mebius RE. Stromal cell-immune cell interactions. Annu Rev Immunol (2011) 29:23–43. doi:10.1146/annurev-immunol-031210-101357
3. Randall TD, Carragher DM, Rangel-Moreno J. Development of secondary lymphoid organs. Annu Rev Immunol (2008) 26:627–50. doi:10.1146/annurev.immunol.26.021607.090257
4. van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol (2010) 10(9):664–74. doi:10.1038/nri2832
5. Eberl G. From induced to programmed lymphoid tissues: the long road to preempt pathogens. Trends Immunol (2007) 28(10):423–8. doi:10.1016/j.it.2007.07.009
6. Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol (2012) 33(6):297–305. doi:10.1016/j.it.2012.04.006
7. Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol (2014) 14(7):447–62. doi:10.1038/nri3700
8. Buckley CD, Barone F, Nayar S, Benezech C, Caamano J. Stromal cells in chronic inflammation and tertiary lymphoid organ formation. Annu Rev Immunol (2015) 33:715–45. doi:10.1146/annurev-immunol-032713-120252
9. Noss EH, Brenner MB. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol Rev (2008) 223:252–70. doi:10.1111/j.1600-065X.2008.00648.x
10. Dieu-Nosjean MC, Goc J, Giraldo NA, Sautes-Fridman C, Fridman WH. Tertiary lymphoid structures in cancer and beyond. Trends Immunol (2014) 35(11):571–80. doi:10.1016/j.it.2014.09.006
11. Ohlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med (2014) 211(8):1503–23. doi:10.1084/jem.20140692
12. Turley SJ, Cremasco V, Astarita JL. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol (2015) 15(11):669–82. doi:10.1038/nri3902
13. Nishikawa SI, Hashi H, Honda K, Fraser S, Yoshida H. Inflammation, a prototype for organogenesis of the lymphopoietic/hematopoietic system. Curr Opin Immunol (2000) 12(3):342–5. doi:10.1016/S0952-7915(00)00097-2
14. Boehm T, Hess I, Swann JB. Evolution of lymphoid tissues. Trends Immunol (2012) 33(6):315–21. doi:10.1016/j.it.2012.02.005
15. Kweon MN, Yamamoto M, Rennert PD, Park EJ, Lee AY, Chang SY, et al. Prenatal blockage of lymphotoxin beta receptor and TNF receptor p55 signaling cascade resulted in the acceleration of tissue genesis for isolated lymphoid follicles in the large intestine. J Immunol (2005) 174(7):4365–72. doi:10.4049/jimmunol.174.7.4365
16. Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature (2008) 456(7221):507–10. doi:10.1038/nature07450
17. Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A (2002) 99(20):12877–82. doi:10.1073/pnas.162488599
18. Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell (2007) 129(7):1311–23. doi:10.1016/j.cell.2007.05.022
19. Fletcher AL, Lukacs-Kornek V, Reynoso ED, Pinner SE, Bellemare-Pelletier A, Curry MS, et al. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med (2010) 207(4):689–97. doi:10.1084/jem.20092642
20. Buckley CD. Why does chronic inflammation persist: an unexpected role for fibroblasts. Immunol Lett (2011) 138(1):12–4. doi:10.1016/j.imlet.2011.02.010
21. Malhotra D, Fletcher AL, Astarita J, Lukacs-Kornek V, Tayalia P, Gonzalez SF, et al. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat Immunol (2012) 13(5):499–510. doi:10.1038/ni.2262
22. Yang CY, Vogt TK, Favre S, Scarpellino L, Huang HY, Tacchini-Cottier F, et al. Trapping of naive lymphocytes triggers rapid growth and remodeling of the fibroblast network in reactive murine lymph nodes. Proc Natl Acad Sci U S A (2014) 111(1):E109–18. doi:10.1073/pnas.1312585111
23. Siegert S, Luther SA. Positive and negative regulation of T cell responses by fibroblastic reticular cells within paracortical regions of lymph nodes. Front Immunol (2012) 3:285. doi:10.3389/fimmu.2012.00285
24. Chang JE, Turley SJ. Stromal infrastructure of the lymph node and coordination of immunity. Trends Immunol (2015) 36(1):30–9. doi:10.1016/j.it.2014.11.003
25. Fletcher AL, Acton SE, Knoblich K. Lymph node fibroblastic reticular cells in health and disease. Nat Rev Immunol (2015) 15(6):350–61. doi:10.1038/nri3846
26. Onder L, Danuser R, Scandella E, Firner S, Chai Q, Hehlgans T, et al. Endothelial cell-specific lymphotoxin-beta receptor signaling is critical for lymph node and high endothelial venule formation. J Exp Med (2013) 210(3):465–73. doi:10.1084/jem.20121462
27. Peduto L, Dulauroy S, Lochner M, Spath GF, Morales MA, Cumano A, et al. Inflammation recapitulates the ontogeny of lymphoid stromal cells. J Immunol (2009) 182(9):5789–99. doi:10.4049/jimmunol.0803974
28. Benezech C, White A, Mader E, Serre K, Parnell S, Pfeffer K, et al. Ontogeny of stromal organizer cells during lymph node development. J Immunol (2010) 184(8):4521–30. doi:10.4049/jimmunol.0903113
29. van de Pavert SA, Olivier BJ, Goverse G, Vondenhoff MF, Greuter M, Beke P, et al. Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nat Immunol (2009) 10(11):1193–9. doi:10.1038/ni.1789
30. van de Pavert SA, Ferreira M, Domingues RG, Ribeiro H, Molenaar R, Moreira-Santos L, et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature (2014) 508(7494):123–7. doi:10.1038/nature13158
31. Blum KS, Pabst R. Keystones in lymph node development. J Anat (2006) 209(5):585–95. doi:10.1111/j.1469-7580.2006.00650.x
32. Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, et al. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity (2002) 17(4):525–35. doi:10.1016/S1074-7613(02)00423-5
33. Katakai T, Hara T, Sugai M, Gonda H, Shimizu A. Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J Exp Med (2004) 200(6):783–95. doi:10.1084/jem.20040254
34. Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol (2006) 7(4):344–53. doi:10.1038/ni1330
35. Katakai T, Suto H, Sugai M, Gonda H, Togawa A, Suematsu S, et al. Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J Immunol (2008) 181(9):6189–200. doi:10.4049/jimmunol.181.9.6189
36. Cupedo T, Mebius RE. Cellular interactions in lymph node development. J Immunol (2005) 174(1):21–5. doi:10.4049/jimmunol.174.1.21
37. White A, Carragher D, Parnell S, Msaki A, Perkins N, Lane P, et al. Lymphotoxin a-dependent and -independent signals regulate stromal organizer cell homeostasis during lymph node organogenesis. Blood (2007) 110(6):1950–9. doi:10.1182/blood-2007-01-070003
38. Chai Q, Onder L, Scandella E, Gil-Cruz C, Perez-Shibayama C, Cupovic J, et al. Maturation of lymph node fibroblastic reticular cells from myofibroblastic precursors is critical for antiviral immunity. Immunity (2013) 38(5):1013–24. doi:10.1016/j.immuni.2013.03.012
39. Vondenhoff MF, Greuter M, Goverse G, Elewaut D, Dewint P, Ware CF, et al. LTbetaR signaling induces cytokine expression and up-regulates lymphangiogenic factors in lymph node anlagen. J Immunol (2009) 182(9):5439–45. doi:10.4049/jimmunol.0801165
40. Yoshida H, Naito A, Inoue J, Satoh M, Santee-Cooper SM, Ware CF, et al. Different cytokines induce surface lymphotoxin-alphabeta on IL-7 receptor-alpha cells that differentially engender lymph nodes and Peyer’s patches. Immunity (2002) 17(6):823–33. doi:10.1016/S1074-7613(02)00479-X
41. Cupedo T, Vondenhoff MF, Heeregrave EJ, De Weerd AE, Jansen W, Jackson DG, et al. Presumptive lymph node organizers are differentially represented in developing mesenteric and peripheral nodes. J Immunol (2004) 173(5):2968–75. doi:10.4049/jimmunol.173.5.2968
42. Vondenhoff MF, van de Pavert SA, Dillard ME, Greuter M, Goverse G, Oliver G, et al. Lymph sacs are not required for the initiation of lymph node formation. Development (2008) 136(1):29–34. doi:10.1242/dev.028456
43. Benezech C, Nayar S, Finney BA, Withers DR, Lowe K, Desanti GE, et al. CLEC-2 is required for development and maintenance of lymph nodes. Blood (2014) 123(20):3200–7. doi:10.1182/blood-2013-03-489286
44. Brendolan A, Rosado MM, Carsetti R, Selleri L, Dear TN. Development and function of the mammalian spleen. Bioessays (2007) 29(2):166–77. doi:10.1002/bies.20528
45. Fu YX, Huang G, Wang Y, Chaplin DD. B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin alpha-dependent fashion. J Exp Med (1998) 187(7):1009–18. doi:10.1084/jem.187.7.1009
46. Endres R, Alimzhanov MB, Plitz T, Futterer A, Kosco-Vilbois MH, Nedospasov SA, et al. Mature follicular dendritic cell networks depend on expression of lymphotoxin beta receptor by radioresistant stromal cells and of lymphotoxin beta and tumor necrosis factor by B cells. J Exp Med (1999) 189(1):159–68. doi:10.1084/jem.189.1.159
47. Ngo VN, Cornall RJ, Cyster JG. Splenic T zone development is B cell dependent. J Exp Med (2001) 194(11):1649–60. doi:10.1084/jem.194.11.1649
48. Vondenhoff MF, Desanti GE, Cupedo T, Bertrand JY, Cumano A, Kraal G, et al. Separation of splenic red and white pulp occurs before birth in a LTalphabeta-independent manner. J Leukoc Biol (2008) 84(1):152–61. doi:10.1189/jlb.0907659
49. Timens W, Rozeboom T, Poppema S. Fetal and neonatal development of human spleen: an immunohistological study. Immunology (1987) 60(4):603–9.
50. Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity (1998) 9(1):59–70. doi:10.1016/S1074-7613(00)80588-9
51. Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science (2000) 288(5475):2369–73. doi:10.1126/science.288.5475.2369
52. Cook MC, Korner H, Riminton DS, Lemckert FA, Hasbold J, Amesbury M, et al. Generation of splenic follicular structure and B cell movement in tumor necrosis factor-deficient mice. J Exp Med (1998) 188(8):1503–10. doi:10.1084/jem.188.8.1503
53. Ngo VN, Korner H, Gunn MD, Schmidt KN, Riminton DS, Cooper MD, et al. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med (1999) 189(2):403–12. doi:10.1084/jem.189.2.403
54. Cyster JG, Ansel KM, Reif K, Ekland EH, Hyman PL, Tang HL, et al. Follicular stromal cells and lymphocyte homing to follicles. Immunol Rev (2000) 176:181–93. doi:10.1034/j.1600-065X.2000.00618.x
55. Coles M, Kioussis D, Veiga-Fernandes H. Cellular and molecular requirements in lymph node and Peyer’s patch development. Prog Mol Biol Transl Sci (2010) 92:177–205. doi:10.1016/s1877-1173(10)92008-5
56. Coles M, Veiga-Fernandes H. Insight into lymphoid tissue morphogenesis. Immunol Lett (2013) 156(1–2):46–53. doi:10.1016/j.imlet.2013.08.001
57. Ruddle NH. Lymphatic vessels and tertiary lymphoid organs. J Clin Invest (2014) 124(3):953–9. doi:10.1172/JCI71611
58. Drayton DL, Ying X, Lee J, Lesslauer W, Ruddle NH. Ectopic LT directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med (2003) 197(9):1153–63. doi:10.1084/jem.20021761
59. Stranford S, Ruddle NH. Follicular dendritic cells, conduits, lymphatic vessels, and high endothelial venules in tertiary lymphoid organs: parallels with lymph node stroma. Front Immunol (2012) 3:350. doi:10.3389/fimmu.2012.00350
60. Jones GW, Jones SA. Ectopic lymphoid follicles: inducible centres for generating antigen-specific immune responses within tissues. Immunology (2016) 147(2):141–51. doi:10.1111/imm.12554
61. Manzo A, Bugatti S, Caporali R, Prevo R, Jackson DG, Uguccioni M, et al. CCL21 expression pattern of human secondary lymphoid organ stroma is conserved in inflammatory lesions with lymphoid neogenesis. Am J Pathol (2007) 171(5):1549–62. doi:10.2353/ajpath.2007.061275
62. Dutertre CA, Clement M, Morvan M, Schakel K, Castier Y, Alsac JM, et al. Deciphering the stromal and hematopoietic cell network of the adventitia from non-aneurysmal and aneurysmal human aorta. PLoS One (2014) 9(2):e89983. doi:10.1371/journal.pone.0089983
63. Link A, Hardie DL, Favre S, Britschgi MR, Adams DH, Sixt M, et al. Association of T-Zone reticular networks and conduits with ectopic lymphoid tissues in mice and humans. Am J Pathol (2011) 178(4):1662–75. doi:10.1016/j.ajpath.2010.12.039
64. Pikor NB, Astarita JL, Summers-Deluca L, Galicia G, Qu J, Ward LA, et al. Integration of Th17- and lymphotoxin-derived signals initiates meningeal-resident stromal cell remodeling to propagate neuroinflammation. Immunity (2015) 43(6):1160–73. doi:10.1016/j.immuni.2015.11.010
65. Cupovic J, Onder L, Gil-Cruz C, Weiler E, Caviezel-Firner S, Perez-Shibayama C, et al. Central nervous system stromal cells control local CD8(+) T cell responses during virus-induced neuroinflammation. Immunity (2016) 44(3):622–33. doi:10.1016/j.immuni.2015.12.022
66. Barone F, Bombardieri M, Manzo A, Blades MC, Morgan PR, Challacombe SJ, et al. Association of CXCL13 and CCL21 expression with the progressive organization of lymphoid-like structures in Sjogren’s syndrome. Arthritis Rheum (2005) 52(6):1773–84. doi:10.1002/art.21062
67. Barone F, Bombardieri M, Rosado MM, Morgan PR, Challacombe SJ, De Vita S, et al. CXCL13, CCL21, and CXCL12 expression in salivary glands of patients with Sjogren’s syndrome and MALT lymphoma: association with reactive and malignant areas of lymphoid organization. J Immunol (2008) 180(7):5130–40. doi:10.4049/jimmunol.180.7.5130
68. Bombardieri M, Barone F, Lucchesi D, Nayar S, van den Berg WB, Proctor G, et al. Inducible tertiary lymphoid structures, autoimmunity, and exocrine dysfunction in a novel model of salivary gland inflammation in C57BL/6 mice. J Immunol (2012) 189(7):3767–76. doi:10.4049/jimmunol.1201216
69. Barone F, Nayar S, Campos J, Cloake T, Withers DR, Toellner KM, et al. IL-22 regulates lymphoid chemokine production and assembly of tertiary lymphoid organs. Proc Natl Acad Sci U S A (2015) 112(35):11024–9. doi:10.1073/pnas.1503315112
70. Grant AJ, Lalor PF, Hubscher SG, Briskin M, Adams DH. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease). Hepatology (2001) 33(5):1065–72. doi:10.1053/jhep.2001.24231
71. Grant AJ, Goddard S, Ahmed-Choudhury J, Reynolds G, Jackson DG, Briskin M, et al. Hepatic expression of secondary lymphoid chemokine (CCL21) promotes the development of portal-associated lymphoid tissue in chronic inflammatory liver disease. Am J Pathol (2002) 160(4):1445–55. doi:10.1016/S0002-9440(10)62570-9
72. Manzo A, Paoletti S, Carulli M, Blades MC, Barone F, Yanni G, et al. Systematic microanatomical analysis of CXCL13 and CCL21in situ production and progressive lymphoid organization in rheumatoid synovitis. Eur J Immunol (2005) 35(5):1347–59. doi:10.1002/eji.200425830
73. Bauer S, Jendro MC, Wadle A, Kleber S, Stenner F, Dinser R, et al. Fibroblast activation protein is expressed by rheumatoid myofibroblast-like synoviocytes. Arthritis Res Ther (2006) 8(6):R171. doi:10.1186/ar2080
74. Humby F, Bombardieri M, Manzo A, Kelly S, Blades MC, Kirkham B, et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med (2009) 6(1):e1. doi:10.1371/journal.pmed.0060001
75. Park YE, Woo YJ, Park SH, Moon YM, Oh HJ, Kim JI, et al. IL-17 increases cadherin-11 expression in a model of autoimmune experimental arthritis and in rheumatoid arthritis. Immunol Lett (2011) 140(1–2):97–103. doi:10.1016/j.imlet.2011.07.003
76. Grabner R, Lotzer K, Dopping S, Hildner M, Radke D, Beer M, et al. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE-/- mice. J Exp Med (2009) 206(1):233–48. doi:10.1084/jem.20080752
77. Lotzer K, Dopping S, Connert S, Grabner R, Spanbroek R, Lemser B, et al. Mouse aorta smooth muscle cells differentiate into lymphoid tissue organizer-like cells on combined tumor necrosis factor receptor-1/lymphotoxin beta-receptor NF-kappaB signaling. Arterioscler Thromb Vasc Biol (2010) 30(3):395–402. doi:10.1161/ATVBAHA.109.191395
78. Weih F, Gräbner R, Hu D, Beer M, Habenicht AJ. Control of dichotomic innate and adaptive immune responses by artery tertiary lymphoid organs in atherosclerosis. Front Physiol (2012) 3:226. doi:10.3389/fphys.2012.00226
79. Hu D, Mohanta SK, Yin C, Peng L, Ma Z, Srikakulapu P, et al. Artery tertiary lymphoid organs control aorta immunity and protect against atherosclerosis via vascular smooth muscle cell lymphotoxin beta receptors. Immunity (2015) 42(6):1100–15. doi:10.1016/j.immuni.2015.05.015
80. Magliozzi R, Columba-Cabezas S, Serafini B, Aloisi F. Intracerebral expression of CXCL13 and BAFF is accompanied by formation of lymphoid follicle-like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol (2004) 148(1–2):11–23. doi:10.1016/j.jneuroim.2003.10.056
81. Powell DW, Pinchuk IV, Saada JI, Chen X, Mifflin RC. Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol (2011) 73:213–37. doi:10.1146/annurev.physiol.70.113006.100646
82. Owens BM, Simmons A. Intestinal stromal cells in mucosal immunity and homeostasis. Mucosal Immunol (2013) 6(2):224–34. doi:10.1038/mi.2012.125
83. Owens BM, Steevels TA, Dudek M, Walcott D, Sun MY, Mayer A, et al. CD90(+) stromal cells are non-professional innate immune effectors of the human colonic mucosa. Front Immunol (2013) 4:307. doi:10.3389/fimmu.2013.00307
84. Beswick EJ, Johnson JR, Saada JI, Humen M, House J, Dann S, et al. TLR4 activation enhances the PD-L1-mediated tolerogenic capacity of colonic CD90+ stromal cells. J Immunol (2014) 193(5):2218–29. doi:10.4049/jimmunol.1203441
85. Owens BM. Inflammation, innate immunity, and the intestinal stromal cell niche: opportunities and challenges. Front Immunol (2015) 6:319. doi:10.3389/fimmu.2015.00319
86. Winter S, Loddenkemper C, Aebischer A, Rabel K, Hoffmann K, Meyer TF, et al. The chemokine receptor CXCR5 is pivotal for ectopic mucosa-associated lymphoid tissue neogenesis in chronic Helicobacter pylori-induced inflammation. J Mol Med (Berl) (2010) 88(11):1169–80. doi:10.1007/00109-010-0658-6
87. Mazzucchelli L, Blaser A, Kappeler A, Scharli P, Laissue JA, Baggiolini M, et al. BCA-1 is highly expressed in Helicobacter pylori-induced mucosa-associated lymphoid tissue and gastric lymphoma. J Clin Invest (1999) 104(10):R49–54. doi:10.1172/JCI7830
88. Wengner AM, Hopken UE, Petrow PK, Hartmann S, Schurigt U, Brauer R, et al. CXCR5- and CCR7-dependent lymphoid neogenesis in a murine model of chronic antigen-induced arthritis. Arthritis Rheum (2007) 56(10):3271–83. doi:10.1002/art.22939
89. Kahnert A, Hopken UE, Stein M, Bandermann S, Lipp M, Kaufmann SH. Mycobacterium tuberculosis triggers formation of lymphoid structure in murine lungs. J Infect Dis (2007) 195(1):46–54. doi:10.1086/508894
90. Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, Hwang JY, Kusser K, Hartson L, et al. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol (2011) 12(7):639–46. doi:10.1038/ni.2053
91. Perros F, Dorfmuller P, Montani D, Hammad H, Waelput W, Girerd B, et al. Pulmonary lymphoid neogenesis in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med (2012) 185(3):311–21. doi:10.1164/rccm.201105-0927OC
92. Fleige H, Ravens S, Moschovakis GL, Bolter J, Willenzon S, Sutter G, et al. IL-17-induced CXCL12 recruits B cells and induces follicle formation in BALT in the absence of differentiated FDCs. J Exp Med (2014) 211(4):643–51. doi:10.1084/jem.20131737
93. Lee Y, Chin RK, Christiansen P, Sun Y, Tumanov AV, Wang J, et al. Recruitment and activation of naive T cells in the islets by lymphotoxin beta receptor-dependent tertiary lymphoid structure. Immunity (2006) 25(3):499–509. doi:10.1016/j.immuni.2006.06.016
94. Astorri E, Bombardieri M, Gabba S, Peakman M, Pozzilli P, Pitzalis C. Evolution of ectopic lymphoid neogenesis and in situ autoantibody production in autoimmune nonobese diabetic mice: cellular and molecular characterization of tertiary lymphoid structures in pancreatic islets. J Immunol (2010) 185(6):3359–68. doi:10.4049/jimmunol.1001836
95. Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol (2002) 169(1):424–33. doi:10.4049/jimmunol.169.1.424
96. Marinkovic T, Garin A, Yokota Y, Fu YX, Ruddle NH, Furtado GC, et al. Interaction of mature CD3+CD4+ T cells with dendritic cells triggers the development of tertiary lymphoid structures in the thyroid. J Clin Invest (2006) 116(10):2622–32. doi:10.1172/jci28993
97. Nagatake T, Fukuyama S, Kim DY, Goda K, Igarashi O, Sato S, et al. Id2-, RORgammat-, and LTbetaR-independent initiation of lymphoid organogenesis in ocular immunity. J Exp Med (2009) 206(11):2351–64. doi:10.1084/jem.20091436
98. Furtado GC, Pacer ME, Bongers G, Benezech C, He Z, Chen L, et al. TNFalpha-dependent development of lymphoid tissue in the absence of RORgammat(+) lymphoid tissue inducer cells. Mucosal Immunol (2014) 7(3):602–14. doi:10.1038/mi.2013.79
99. De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science (1994) 264(5159):703–7. doi:10.1126/science.8171322
100. Banks TA, Rouse BT, Kerley MK, Blair PJ, Godfrey VL, Kuklin NA, et al. Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol (1995) 155(4):1685–93.
101. Alimzhanov MB, Kuprash DV, Kosco-Vilbois MH, Luz A, Turetskaya RL, Tarakhovsky A, et al. Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice. Proc Natl Acad Sci U S A (1997) 94(17):9302–7. doi:10.1073/pnas.94.17.9302
102. Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity (1997) 6(4):491–500. doi:10.1016/S1074-7613(00)80292-7
103. Rennert PD, Browning JL, Mebius R, Mackay F, Hochman PS. Surface lymphotoxin alpha/beta complex is required for the development of peripheral lymphoid organs. J Exp Med (1996) 184(5):1999–2006. doi:10.1084/jem.184.5.1999
104. Rennert PD, Browning JL, Hochman PS. Selective disruption of lymphotoxin ligands reveals a novel set of mucosal lymph nodes and unique effects on lymph node cellular organization. Int Immunol (1997) 9(11):1627–39. doi:10.1093/intimm/9.11.1627
105. Rennert PD, James D, Mackay F, Browning JL, Hochman PS. Lymph node genesis is induced by signaling through the lymphotoxin beta receptor. Immunity (1998) 9(1):71–9. doi:10.1016/S1074-7613(00)80589-0
106. Kratz A, Campos-Neto A, Hanson MS, Ruddle NH. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med (1996) 183(4):1461–72. doi:10.1084/jem.183.4.1461
107. Rangel-Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, Randall TD. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest (2006) 116(12):3183–94. doi:10.1172/JCI28756
108. Khader SA, Guglani L, Rangel-Moreno J, Gopal R, Junecko BA, Fountain JJ, et al. IL-23 is required for long-term control of Mycobacterium tuberculosis and B cell follicle formation in the infected lung. J Immunol (2011) 187(10):5402–7. doi:10.4049/jimmunol.1101377
109. Peters A, Pitcher LA, Sullivan JM, Mitsdoerffer M, Acton SE, Franz B, et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity (2011) 35(6):986–96. doi:10.1016/j.immuni.2011.10.015
110. Husson H, Lugli SM, Ghia P, Cardoso A, Roth A, Brohmi K, et al. Functional effects of TNF and lymphotoxin alpha1beta2 on FDC-like cells. Cell Immunol (2000) 203(2):134–43. doi:10.1006/cimm.2000.1688
111. Hardy R, Juarez M, Naylor A, Tu J, Rabbitt EH, Filer A, et al. Synovial DKK1 expression is regulated by local glucocorticoid metabolism in inflammatory arthritis. Arthritis Res Ther (2012) 14(5):R226. doi:10.1186/ar4065
112. Corneth OB, Reijmers RM, Mus AM, Asmawidjaja PS, van Hamburg JP, Papazian N, et al. Loss of IL-22 inhibits autoantibody formation in collagen-induced arthritis in mice. Eur J Immunol (2016) 46(6):1404–14. doi:10.1002/eji.201546241
113. Brembilla NC, Dufour AM, Alvarez M, Hugues S, Montanari E, Truchetet ME, et al. IL-22 capacitates dermal fibroblast responses to TNF in scleroderma. Ann Rheum Dis (2016) 75(9):1697–705. doi:10.1136/annrheumdis-2015-207477
114. Schrama D, Thor Straten P, Fischer WH, McLellan AD, Brocker EB, Reisfeld RA, et al. Targeting of lymphotoxin-alpha to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid-like tissue. Immunity (2001) 14(2):111–21. doi:10.1016/S1074-7613(01)00094-2
115. Yu P, Lee Y, Liu W, Chin RK, Wang J, Wang Y, et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol (2004) 5(2):141–9. doi:10.1038/ni1029
116. Hess E, Duheron V, Decossas M, Lezot F, Berdal A, Chea S, et al. RANKL induces organized lymph node growth by stromal cell proliferation. J Immunol (2012) 188(3):1245–54. doi:10.4049/jimmunol.1101513
117. Mueller CG, Hess E. Emerging functions of RANKL in lymphoid tissues. Front Immunol (2012) 3:261. doi:10.3389/fimmu.2012.00261
118. Cordeiro OG, Chypre M, Brouard N, Rauber S, Alloush F, Romera-Hernandez M, et al. Integrin-alpha IIb identifies murine lymph node lymphatic endothelial cells responsive to RANKL. PLoS One (2016) 11(3):e0151848. doi:10.1371/journal.pone.0151848
119. Goya S, Matsuoka H, Mori M, Morishita H, Kida H, Kobashi Y, et al. Sustained interleukin-6 signalling leads to the development of lymphoid organ-like structures in the lung. J Pathol (2003) 200(1):82–7. doi:10.1002/path.1321
120. Ammirante M, Shalapour S, Kang Y, Jamieson CA, Karin M. Tissue injury and hypoxia promote malignant progression of prostate cancer by inducing CXCL13 expression in tumor myofibroblasts. Proc Natl Acad Sci U S A (2014) 111(41):14776–81. doi:10.1073/pnas.1416498111
121. Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med (1997) 185(12):2143–56. doi:10.1084/jem.185.12.2143
122. Doucet C, Brouty-Boye D, Pottin-Clemenceau C, Canonica GW, Jasmin C, Azzarone B. Interleukin (IL) 4 and IL-13 act on human lung fibroblasts. Implication in asthma. J Clin Invest (1998) 101(10):2129–39. doi:10.1172/JCI741
123. Luther SA, Ansel KM, Cyster JG. Overlapping roles of CXCL13, interleukin 7 receptor alpha, and CCR7 ligands in lymph node development. J Exp Med (2003) 197(9):1191–8. doi:10.1084/jem.20021294
124. Canete JD, Celis R, Yeremenko N, Sanmarti R, van Duivenvoorde L, Ramirez J, et al. Ectopic lymphoid neogenesis is strongly associated with activation of the IL-23 pathway in rheumatoid synovitis. Arthritis Res Ther (2015) 17:173. doi:10.1186/s13075-015-0688-0
125. Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell (2006) 126(6):1121–33. doi:10.1016/j.cell.2006.07.035
126. Cai Y, Shen X, Ding C, Qi C, Li K, Li X, et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity (2011) 35(4):596–610. doi:10.1016/j.immuni.2011.08.001
127. Mabuchi T, Takekoshi T, Hwang ST. Epidermal CCR6+ gammadelta T cells are major producers of IL-22 and IL-17 in a murine model of psoriasiform dermatitis. J Immunol (2011) 187(10):5026–31. doi:10.4049/jimmunol.1101817
128. Pantelyushin S, Haak S, Ingold B, Kulig P, Heppner FL, Navarini AA, et al. Rorgammat+ innate lymphocytes and gammadelta T cells initiate psoriasiform plaque formation in mice. J Clin Invest (2012) 122(6):2252–6. doi:10.1172/JCI61862
129. Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science (2015) 348(6237):aaa6566. doi:10.1126/science.aaa6566
130. Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF, Cayatte C, Chen Y, et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity (2015) 43(4):727–38. doi:10.1016/j.immuni.2015.09.003
131. Crome SQ, Wang AY, Levings MK. Translational mini-review series on Th17 cells: function and regulation of human T helper 17 cells in health and disease. Clin Exp Immunol (2010) 159(2):109–19. doi:10.1111/j.1365-2249.2009.04037
132. Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med (2010) 207(6):1293–305. doi:10.1084/jem.20092054
133. Jones GW, Bombardieri M, Greenhill CJ, McLeod L, Nerviani A, Rocher-Ros V, et al. Interleukin-27 inhibits ectopic lymphoid-like structure development in early inflammatory arthritis. J Exp Med (2015) 212(11):1793–802. doi:10.1084/jem.20132307
134. Nayar S, Campos J, Chung MM, Navarro-Nunez L, Chachlani M, Steinthal N, et al. Bimodal expansion of the lymphatic vessels is regulated by the sequential expression of IL-7 and lymphotoxin alpha1beta2 in newly formed tertiary lymphoid structures. J Immunol (2016) 197(5):1957–67. doi:10.4049/jimmunol.1500686
135. Brendolan A, Caamano JH. Mesenchymal cell differentiation during lymph node organogenesis. Front Immunol (2012) 3:381. doi:10.3389/fimmu.2012.00381
136. Browning JL, Allaire N, Ngam-Ek A, Notidis E, Hunt J, Perrin S, et al. Lymphotoxin-beta receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity (2005) 23(5):539–50. doi:10.1016/j.immuni.2005.10.002
137. Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity (2000) 12(5):471–81. doi:10.1016/S1074-7613(00)80199-5
138. Wu Q, Salomon B, Chen M, Wang Y, Hoffman LM, Bluestone JA, et al. Reversal of spontaneous autoimmune insulitis in nonobese diabetic mice by soluble lymphotoxin receptor. J Exp Med (2001) 193(11):1327–32. doi:10.1084/jem.193.11.1327
139. Tumanov A, Kuprash D, Lagarkova M, Grivennikov S, Abe K, Shakhov A, et al. Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity (2002) 17(3):239–50. doi:10.1016/S1074-7613(02)00397-7
140. Lochner M, Ohnmacht C, Presley L, Bruhns P, Si-Tahar M, Sawa S, et al. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORgamma t and LTi cells. J Exp Med (2011) 208(1):125–34. doi:10.1084/jem.20100052
141. Ware CF, Crowe PD, Grayson MH, Androlewicz MJ, Browning JL. Expression of surface lymphotoxin and tumor necrosis factor on activated T, B, and natural killer cells. J Immunol (1992) 149(12):3881–8.
142. Ansel KM, Ngo VN, Hyman PL, Luther SA, Forster R, Sedgwick JD, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature (2000) 406(6793):309–14. doi:10.1038/35018581
143. Suematsu S, Watanabe T. Generation of a synthetic lymphoid tissue-like organoid in mice. Nat Biotechnol (2004) 22(12):1539–45. doi:10.1038/nbt1039
144. Furtado GC, Marinkovic T, Martin AP, Garin A, Hoch B, Hubner W, et al. Lymphotoxin beta receptor signaling is required for inflammatory lymphangiogenesis in the thyroid. Proc Natl Acad Sci U S A (2007) 104(12):5026–31. doi:10.1073/pnas.0606697104
145. GeurtsvanKessel CH, Willart MA, Bergen IM, van Rijt LS, Muskens F, Elewaut D, et al. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med (2009) 206(11):2339–49. doi:10.1084/jem.20090410
146. Moussion C, Girard JP. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature (2011) 479(7374):542–6. doi:10.1038/nature10540
147. Muniz LR, Pacer ME, Lira SA, Furtado GC. A critical role for dendritic cells in the formation of lymphatic vessels within tertiary lymphoid structures. J Immunol (2011) 187(2):828–34. doi:10.4049/jimmunol.1004233
148. Carlsen HS, Baekkevold ES, Morton HC, Haraldsen G, Brandtzaeg P. Monocyte-like and mature macrophages produce CXCL13 (B cell-attracting chemokine 1) in inflammatory lesions with lymphoid neogenesis. Blood (2004) 104(10):3021–7. doi:10.1182/blood-2004-02-0701
149. Perrier P, Martinez FO, Locati M, Bianchi G, Nebuloni M, Vago G, et al. Distinct transcriptional programs activated by interleukin-10 with or without lipopolysaccharide in dendritic cells: induction of the B cell-activating chemokine, CXC chemokine ligand 13. J Immunol (2004) 172(11):7031–42. doi:10.4049/jimmunol.172.11.7031
150. Vermi W, Facchetti F, Riboldi E, Heine H, Scutera S, Stornello S, et al. Role of dendritic cell-derived CXCL13 in the pathogenesis of Bartonella henselae B-rich granuloma. Blood (2006) 107(2):454–62. doi:10.1182/blood-2005-04-1342
151. Manzo A, Vitolo B, Humby F, Caporali R, Jarrossay D, Dell’accio F, et al. Mature antigen-experienced T helper cells synthesize and secrete the B cell chemoattractant CXCL13 in the inflammatory environment of the rheumatoid joint. Arthritis Rheum (2008) 58(11):3377–87. doi:10.1002/art.23966
152. McDonald KG, McDonough JS, Dieckgraefe BK, Newberry RD. Dendritic cells produce CXCL13 and participate in the development of murine small intestine lymphoid tissues. Am J Pathol (2010) 176(5):2367–77. doi:10.2353/ajpath.2010.090723
153. Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol (2009) 9(9):618–29. doi:10.1038/nri2588
154. Brown FD, Turley SJ. Fibroblastic reticular cells: organization and regulation of the T lymphocyte life cycle. J Immunol (2015) 194(4):1389–94. doi:10.4049/jimmunol.1402520
155. Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol (2007) 8(11):1255–65. doi:10.1038/ni1513
156. Yi T, Wang X, Kelly LM, An J, Xu Y, Sailer AW, et al. Oxysterol gradient generation by lymphoid stromal cells guides activated B cell movement during humoral responses. Immunity (2012) 37(3):535–48. doi:10.1016/j.immuni.2012.06.015
157. Cremasco V, Woodruff MC, Onder L, Cupovic J, Nieves-Bonilla JM, Schildberg FA, et al. B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nat Immunol (2014) 15(10):973–81. doi:10.1038/ni.2965
158. Fasnacht N, Huang HY, Koch U, Favre S, Auderset F, Chai Q, et al. Specific fibroblastic niches in secondary lymphoid organs orchestrate distinct Notch-regulated immune responses. J Exp Med (2014) 211(11):2265–79. doi:10.1084/jem.20132528
159. Katakai T. Marginal reticular cells: a stromal subset directly descended from the lymphoid tissue organizer. Front Immunol (2012) 3:200. doi:10.3389/fimmu.2012.00200
160. Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature (2007) 450(7166):110–4. doi:10.1038/nature06287
161. Roozendaal R, Mempel TR, Pitcher LA, Gonzalez SF, Verschoor A, Mebius RE, et al. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity (2009) 30(2):264–76. doi:10.1016/j.immuni.2008.12.014
162. Jarjour M, Jorquera A, Mondor I, Wienert S, Narang P, Coles MC, et al. Fate mapping reveals origin and dynamics of lymph node follicular dendritic cells. J Exp Med (2014) 211(6):1109–22. doi:10.1084/jem.20132409
163. Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin Immunol (2008) 20(1):14–25. doi:10.1016/j.smim.2007.12.001
164. Fu YX, Chaplin DD. Development and maturation of secondary lymphoid tissues. Annu Rev Immunol (1999) 17:399–433. doi:10.1146/annurev.immunol.17.1.399
165. Lu TT, Browning JL. Role of the lymphotoxin/LIGHT system in the development and maintenance of reticular networks and vasculature in lymphoid tissues. Front Immunol (2014) 5:47. doi:10.3389/fimmu.2014.00047
166. Le Hir M, Bluethmann H, Kosco-Vilbois MH, Muller M, di Padova F, Moore M, et al. Differentiation of follicular dendritic cells and full antibody responses require tumor necrosis factor receptor-1 signaling. J Exp Med (1996) 183(5):2367–72. doi:10.1084/jem.183.5.2367
167. Matsumoto M, Mariathasan S, Nahm MH, Baranyay F, Peschon JJ, Chaplin DD. Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science (1996) 271(5253):1289–91. doi:10.1126/science.271.5253.1289
168. Fu YX, Molina H, Matsumoto M, Huang G, Min J, Chaplin DD. Lymphotoxin-alpha (LTalpha) supports development of splenic follicular structure that is required for IgG responses. J Exp Med (1997) 185(12):2111–20. doi:10.1084/jem.185.12.2111
169. Lorenz RG, Chaplin DD, McDonald KG, McDonough JS, Newberry RD. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin beta receptor, and TNF receptor I function. J Immunol (2003) 170(11):5475–82. doi:10.4049/jimmunol.170.11.5475
170. Bannard O, Horton RM, Allen CD, An J, Nagasawa T, Cyster JG. Germinal center centroblasts transition to a centrocyte phenotype according to a timed program and depend on the dark zone for effective selection. Immunity (2013) 39(5):912–24. doi:10.1016/j.immuni.2013.08.038
171. Rodda LB, Bannard O, Ludewig B, Nagasawa T, Cyster JG. Phenotypic and morphological properties of germinal center dark zone Cxcl12-expressing reticular cells. J Immunol (2015) 195(10):4781–91. doi:10.4049/jimmunol.1501191
172. Mionnet C, Mondor I, Jorquera A, Loosveld M, Maurizio J, Arcangeli ML, et al. Identification of a new stromal cell type involved in the regulation of inflamed B cell follicles. PLoS Biol (2013) 11(10):e1001672. doi:10.1371/journal.pbio.1001672
173. Tomei AA, Siegert S, Britschgi MR, Luther SA, Swartz MA. Fluid flow regulates stromal cell organization and CCL21 expression in a tissue-engineered lymph node microenvironment. J Immunol (2009) 183(7):4273–83. doi:10.4049/jimmunol.0900835
174. Vega F, Coombes KR, Thomazy VA, Patel K, Lang W, Jones D. Tissue-specific function of lymph node fibroblastic reticulum cells. Pathobiology (2006) 73(2):71–81. doi:10.1159/000094491
175. Fletcher AL, Malhotra D, Acton SE, Lukacs-Kornek V, Bellemare-Pelletier A, Curry M, et al. Reproducible isolation of lymph node stromal cells reveals site-dependent differences in fibroblastic reticular cells. Front Immunol (2011) 2:35. doi:10.3389/fimmu.2011.00035
176. Rangel-Moreno J, Moyron-Quiroz JE, Hartson L, Kusser K, Randall TD. Pulmonary expression of CXC chemokine ligand 13, CC chemokine ligand 19, and CC chemokine ligand 21 is essential for local immunity to influenza. Proc Natl Acad Sci U S A (2007) 104(25):10577–82. doi:10.1073/pnas.0700591104
177. Rangel-Moreno J, Carragher DM, Misra RS, Kusser K, Hartson L, Moquin A, et al. B cells promote resistance to heterosubtypic strains of influenza via multiple mechanisms. J Immunol (2008) 180(1):454–63. doi:10.4049/jimmunol.180.1.454
178. Krautler NJ, Kana V, Kranich J, Tian Y, Perera D, Lemm D, et al. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell (2012) 150(1):194–206. doi:10.1016/j.cell.2012.05.032
179. Castagnaro L, Lenti E, Maruzzelli S, Spinardi L, Migliori E, Farinello D, et al. Nkx2-5+Islet1+ Mesenchymal precursors generate distinct spleen stromal cell subsets and participate in restoring stromal network integrity. Immunity (2013) 38(4):782–91. doi:10.1016/j.immuni.2012.12.005
180. Liao S, Ruddle NH. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J Immunol (2006) 177(5):3369–79. doi:10.4049/jimmunol.177.5.3369
181. Chyou S, Benahmed F, Chen J, Kumar V, Tian S, Lipp M, et al. Coordinated regulation of lymph node vascular-stromal growth first by CD11c+ cells and then by T and B cells. J Immunol (2011) 187(11):5558–67. doi:10.4049/jimmunol.1101724
182. Kumar V, Chyou S, Stein JV, Lu TT. Optical projection tomography reveals dynamics of HEV growth after immunization with protein plus CFA and features shared with HEVs in acute autoinflammatory lymphadenopathy. Front Immunol (2012) 3:282. doi:10.3389/fimmu.2012.00282
183. Barone F, Nayar S, Buckley CD. The role of non-hematopoietic stromal cells in the persistence of inflammation. Front Immunol (2012) 3:416. doi:10.3389/fimmu.2012.00416
184. Astarita JL, Acton SE, Turley SJ. Podoplanin: emerging functions in development, the immune system, and cancer. Front Immunol (2012) 3:283. doi:10.3389/fimmu.2012.00283
185. Bombardieri M, Barone F, Humby F, Kelly S, McGurk M, Morgan P, et al. Activation-induced cytidine deaminase expression in follicular dendritic cell networks and interfollicular large B cells supports functionality of ectopic lymphoid neogenesis in autoimmune sialoadenitis and MALT lymphoma in Sjogren’s syndrome. J Immunol (2007) 179(7):4929–38. doi:10.4049/jimmunol.179.7.4929
186. Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med (2004) 10(9):927–34. doi:10.1038/nm1091
187. Moyron-Quiroz JE, Rangel-Moreno J, Hartson L, Kusser K, Tighe MP, Klonowski KD, et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity (2006) 25(4):643–54. doi:10.1016/j.immuni.2006.08.022
188. Wohrer S, Troch M, Raderer M. Therapy of gastric mucosa-associated lymphoid tissue lymphoma. Expert Opin Pharmacother (2007) 8(9):1263–73. doi:10.1517/14656566.8.9.1263
189. Salomonsson S, Larsson P, Tengner P, Mellquist E, Hjelmstrom P, Wahren-Herlenius M. Expression of the B cell-attracting chemokine CXCL13 in the target organ and autoantibody production in ectopic lymphoid tissue in the chronic inflammatory disease Sjogren’s syndrome. Scand J Immunol (2002) 55(4):336–42. doi:10.1046/j.1365-3083.2002.01058.x
190. Bugatti S, Caporali R, Manzo A, Vitolo B, Pitzalis C, Montecucco C. Involvement of subchondral bone marrow in rheumatoid arthritis: lymphoid neogenesis and in situ relationship to subchondral bone marrow osteoclast recruitment. Arthritis Rheum (2005) 52(11):3448–59. doi:10.1002/art.21377
191. Canete JD, Celis R, Moll C, Izquierdo E, Marsal S, Sanmarti R, et al. Clinical significance of synovial lymphoid neogenesis and its reversal after anti-tumour necrosis factor alpha therapy in rheumatoid arthritis. Ann Rheum Dis (2009) 68(5):751–6. doi:10.1136/ard.2008.089284
192. Bugatti S, Caporali R, Manzo A, Sakellariou G, Rossi S, Montecucco C. Ultrasonographic and MRI characterisation of the palindromic phase of rheumatoid arthritis. Ann Rheum Dis (2012) 71(4):625–6. doi:10.1136/annrheumdis-2011-200077
193. Bugatti S, Manzo A, Benaglio F, Klersy C, Vitolo B, Todoerti M, et al. Serum levels of CXCL13 are associated with ultrasonographic synovitis and predict power Doppler persistence in early rheumatoid arthritis treated with non-biological disease-modifying anti-rheumatic drugs. Arthritis Res Ther (2012) 14(1):R34. doi:10.1186/ar3742
194. Theander E, Vasaitis L, Baecklund E, Nordmark G, Warfvinge G, Liedholm R, et al. Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjogren’s syndrome. Ann Rheum Dis (2011) 70(8):1363–8. doi:10.1136/ard.2010.144782
195. Theander E, Henriksson G, Ljungberg O, Mandl T, Manthorpe R, Jacobsson LT. Lymphoma and other malignancies in primary Sjogren’s syndrome: a cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis (2006) 65(6):796–803. doi:10.1136/ard.2005.041186
196. Hiraoka N, Ino Y, Yamazaki-Itoh R. Tertiary lymphoid organs in cancer tissues. Front Immunol (2016) 7:244. doi:10.3389/fimmu.2016.00244
197. Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, et al. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med (2005) 202(7):919–29. doi:10.1084/jem.20050463
198. Valzasina B, Piconese S, Guiducci C, Colombo MP. Tumor-induced expansion of regulatory T cells by conversion of CD4+CD25- lymphocytes is thymus and proliferation independent. Cancer Res (2006) 66(8):4488–95. doi:10.1158/0008-5472.CAN-05-4217
199. Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, et al. Tumor evasion of the immune system by converting CD4+CD25- T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-beta. J Immunol (2007) 178(5):2883–92. doi:10.4049/jimmunol.178.5.2883
200. Joshi NS, Akama-Garren EH, Lu Y, Lee DY, Chang GP, Li A, et al. Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity (2015) 43(3):579–90. doi:10.1016/j.immuni.2015.08.006
201. Slight SR, Rangel-Moreno J, Gopal R, Lin Y, Fallert Junecko BA, Mehra S, et al. CXCR5(+) T helper cells mediate protective immunity against tuberculosis. J Clin Invest (2013) 123(2):712–26. doi:10.1172/JCI65728
202. Clement M, Guedj K, Andreata F, Morvan M, Bey L, Khallou-Laschet J, et al. Control of the T follicular helper-germinal center B-cell axis by CD8(+) regulatory T cells limits atherosclerosis and tertiary lymphoid organ development. Circulation (2015) 131(6):560–70. doi:10.1161/CIRCULATIONAHA.114.010988
203. Chevalier N, Jarrossay D, Ho E, Avery DT, Ma CS, Yu D, et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J Immunol (2011) 186(10):5556–68. doi:10.4049/jimmunol.1002828
204. Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity (2011) 34(1):108–21. doi:10.1016/j.immuni.2010.12.012
205. Sage PT, Alvarez D, Godec J, von Andrian UH, Sharpe AH. Circulating T follicular regulatory and helper cells have memory-like properties. J Clin Invest (2014) 124(12):5191–204. doi:10.1172/JCI76861
206. Ying X, Chan K, Shenoy P, Hill M, Ruddle NH. Lymphotoxin plays a crucial role in the development and function of nasal-associated lymphoid tissue through regulation of chemokines and peripheral node addressin. Am J Pathol (2005) 166(1):135–46. doi:10.1016/S0002-9440(10)62239-0
207. Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity (2014) 41(4):529–42. doi:10.1016/j.immuni.2014.10.004
208. Noss EH, Nguyen HN, Chang SK, Watts GF, Brenner MB. Genetic polymorphism directs IL-6 expression in fibroblasts but not selected other cell types. Proc Natl Acad Sci U S A (2015) 112(48):14948–53. doi:10.1073/pnas.1520861112
209. Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med (2011) 17(8):975–82. doi:10.1038/nm.2425
210. Miles B, Miller SM, Folkvord JM, Kimball A, Chamanian M, Meditz AL, et al. Follicular regulatory T cells impair follicular T helper cells in HIV and SIV infection. Nat Commun (2015) 6:8608. doi:10.1038/ncomms9608
211. Quezada SA, Peggs KS, Simpson TR, Shen Y, Littman DR, Allison JP. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp Med (2008) 205(9):2125–38. doi:10.1084/jem.20080099
212. Hindley JP, Jones E, Smart K, Bridgeman H, Lauder SN, Ondondo B, et al. T-cell trafficking facilitated by high endothelial venules is required for tumor control after regulatory T-cell depletion. Cancer Res (2012) 72(21):5473–82. doi:10.1158/0008-5472.CAN-12-1912
213. Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem (2003) 278(3):1910–4. doi:10.1074/jbc.M207577200
214. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature (2006) 441(7090):235–8. doi:10.1038/nature04753
215. Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature (2007) 448(7152):484–7. doi:10.1038/nature05970
216. Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A (2008) 105(47):18460–5. doi:10.1073/pnas.0809850105
217. Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol (2007) 178(11):6725–9. doi:10.4049/jimmunol.178.11.6725
218. Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity (2008) 29(1):44–56. doi:10.1016/j.immuni.2008.05.007
219. Mueller SN, Matloubian M, Clemens DM, Sharpe AH, Freeman GJ, Gangappa S, et al. Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection. Proc Natl Acad Sci U S A (2007) 104(39):15430–5. doi:10.1073/pnas.0702579104
220. Lukacs-Kornek V, Malhotra D, Fletcher AL, Acton SE, Elpek KG, Tayalia P, et al. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol (2011) 12(11):1096–104. doi:10.1038/ni.2112
221. Siegert S, Huang HY, Yang CY, Scarpellino L, Carrie L, Essex S, et al. Fibroblastic reticular cells from lymph nodes attenuate T cell expansion by producing nitric oxide. PLoS One (2011) 6(11):e27618. doi:10.1371/journal.pone.0027618
222. Baptista AP, Roozendaal R, Reijmers RM, Koning JJ, Unger WW, Greuter M, et al. Lymph node stromal cells constrain immunity via MHC class II self-antigen presentation. Elife (2014) 3:3. doi:10.7554/eLife.04433
223. Dubrot J, Duraes FV, Potin L, Capotosti F, Brighouse D, Suter T, et al. Lymph node stromal cells acquire peptide-MHCII complexes from dendritic cells and induce antigen-specific CD4(+) T cell tolerance. J Exp Med (2014) 211(6):1153–66. doi:10.1084/jem.20132000
224. Cording S, Wahl B, Kulkarni D, Chopra H, Pezoldt J, Buettner M, et al. The intestinal micro-environment imprints stromal cells to promote efficient Treg induction in gut-draining lymph nodes. Mucosal Immunol (2014) 7(2):359–68. doi:10.1038/mi.2013.54
225. Denton AE, Roberts EW, Linterman MA, Fearon DT. Fibroblastic reticular cells of the lymph node are required for retention of resting but not activated CD8+ T cells. Proc Natl Acad Sci U S A (2014) 111(33):12139–44. doi:10.1073/pnas.1412910111
226. Novkovic M, Onder L, Cupovic J, Abe J, Bomze D, Cremasco V, et al. Topological small-world organization of the fibroblastic reticular cell network determines lymph node functionality. PLoS Biol (2016) 14(7):e1002515. doi:10.1371/journal.pbio.1002515
227. Acton SE, Astarita JL, Malhotra D, Lukacs-Kornek V, Franz B, Hess PR, et al. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2. Immunity (2012) 37(2):276–89. doi:10.1016/j.immuni.2012.05.022
228. Astarita JL, Cremasco V, Fu J, Darnell MC, Peck JR, Nieves-Bonilla JM, et al. The CLEC-2-podoplanin axis controls the contractility of fibroblastic reticular cells and lymph node microarchitecture. Nat Immunol (2015) 16(1):75–84. doi:10.1038/ni.3035
Keywords: tertiary lymphoid structures, chemokines, tumor necrosis factor-alpha, lymphotoxin alpha1, beta2 heterotrimer, fibroblasts
Citation: Barone F, Gardner DH, Nayar S, Steinthal N, Buckley CD and Luther SA (2016) Stromal Fibroblasts in Tertiary Lymphoid Structures: A Novel Target in Chronic Inflammation. Front. Immunol. 7:477. doi: 10.3389/fimmu.2016.00477
Received: 06 September 2016; Accepted: 20 October 2016;
Published: 08 November 2016
Edited by:
Andreas Habenicht, Ludwig Maximilian University of Munich, GermanyReviewed by:
Theresa T. Lu, Weill Cornell Medical College, USABurkhard Ludewig, Kantonal Hospital St. Gallen, Switzerland
Copyright: © 2016 Barone, Gardner, Nayar, Steinthal, Buckley and Luther. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca Barone, f.barone@bham.ac.uk
 Francesca Barone
Francesca Barone David H. Gardner1
David H. Gardner1 Christopher D. Buckley
Christopher D. Buckley Sanjiv A. Luther
Sanjiv A. Luther