The Role of the Extracellular Matrix in Cancer Stemness
- Department of Regenerative and Cancer Cell Biology, Albany Medical College, Albany, NY, United States
As our understanding of cancer cell biology progresses, it has become clear that tumors are a heterogenous mixture of different cell populations, some of which contain so called “cancer stem cells” (CSCs). Hallmarks of CSCs include self-renewing capability, tumor-initiating capacity and chemoresistance. The extracellular matrix (ECM), a major structural component of the tumor microenvironment, is a highly dynamic structure and increasing evidence suggests that ECM proteins establish a physical and biochemical niche for CSCs. In cancer, abnormal ECM dynamics occur due to disrupted balance between ECM synthesis and secretion and altered expression of matrix-remodeling enzymes. Tumor-derived ECM is biochemically distinct in its composition and is stiffer compared to normal ECM. In this review, we will provide a brief overview of how different components of the ECM modulate CSC properties then discuss how physical, mechanical, and biochemical cues from the ECM drive cancer stemness. Given the fact that current CSC targeting therapies face many challenges, a better understanding of CSC-ECM interactions will be crucial to identify more effective therapeutic strategies to eliminate CSCs.
Introduction: ECM as a CSC Niche
The extracellular matrix (ECM) is a major structural component of the tumor microenvironment and comprised of a network of biochemically distinct components, including fibrous proteins, glycoproteins, proteoglycans, and polysaccharides. The ECM is a highly dynamic structure, constantly undergoing a remodeling process where ECM components are deposited, degraded, or modified (Lu et al., 2012). Increasing evidence suggests that the ECM serves as a niche for normal and cancer stem cells (CSCs). CSCs, also called tumor-initiating cells, are a small population of cells within tumors that have capabilities of self-renewal properties, tumor initiation and chemoresistance (Kreso and Dick, 2014; Batlle and Clevers, 2017). As one of the CSC niches, the ECM provides both structural and biochemical support to regulate proliferation, self-renewal, and differentiation of CSCs. In this review, we will cover the current understanding of how different ECM components affect the cancer “stemness” phenotype.
Categories of ECM Proteins and Their Role in Cancer Stemness
Fibrous ECM Proteins
Collagens constitute the main structural element of the ECM and are the most copious type of fibrous proteins within the interstitial ECM. Collagens play a role in tissue development by providing mechanical strength, altering cell adhesion, promoting cell migration (Frantz et al., 2010). Studies have reported that several collagens (e.g., COL3A1, COL4A2, COL7A1, COL17A1) are overexpressed by CSCs (Table 1). Multiple collagen subtypes have been shown to increase epithelial-mesenchymal transition (EMT), tumor-initiating potential, drug resistance and self-renewal of CSCs (Table 1 and Figure 1).
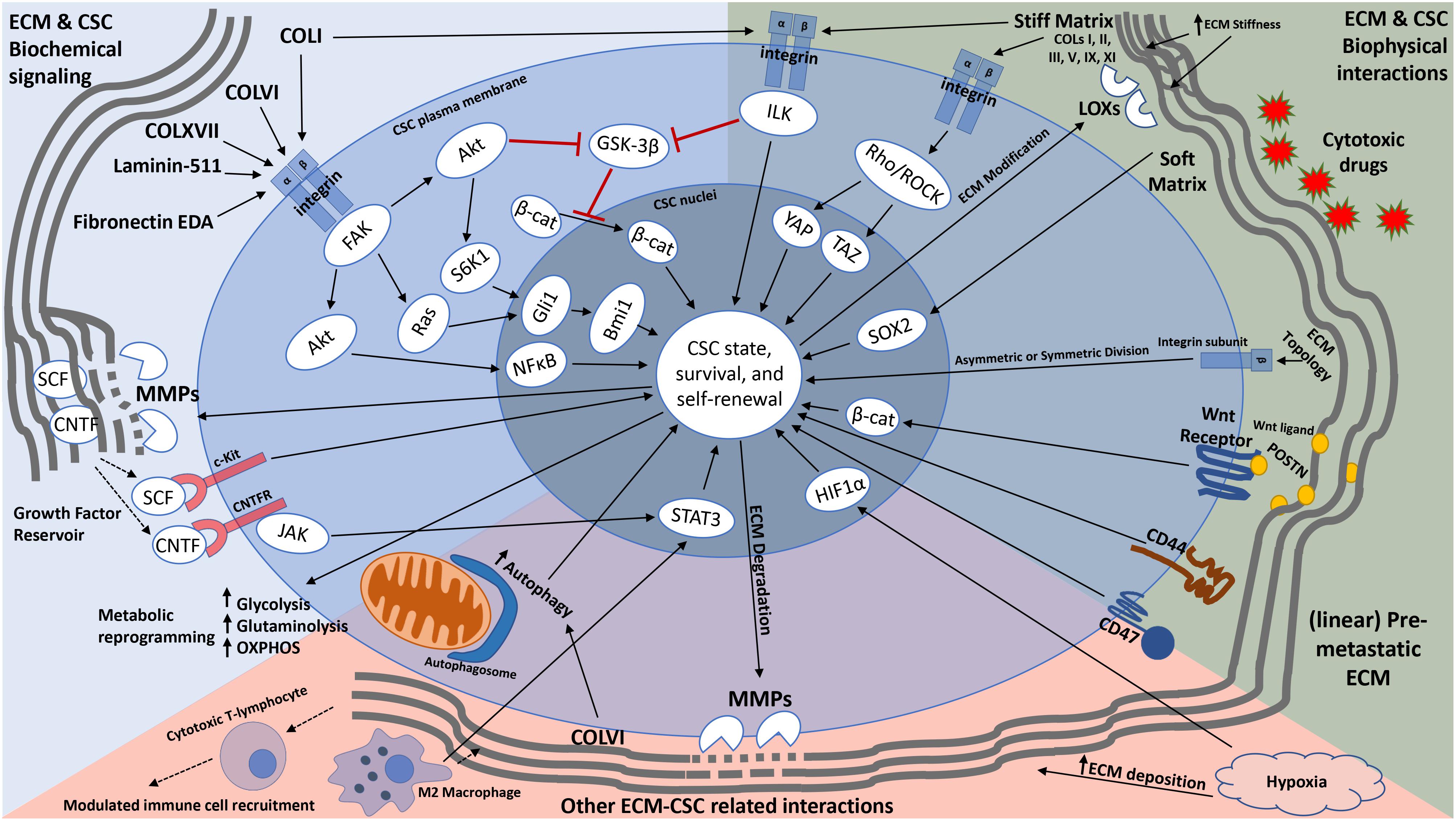
Figure 1. Schematic representation of how the ECM modulates cancer stemness. In addition to providing cues that transform non-CSCs into CSCs (through EMT) and maintain a stemness state, the ECM can modulate CSC metabolism, influence immune cell recruitment, and serve as a reservoir for growth factors and other signaling molecules that aid in CSC self-renewal and maintenance. Furthermore, the ECM provides not only a physical barrier to CSCs from cytotoxic drugs, but also anchorage sites for CSCs for cell division and metastatic colonization. CSCs are also able to modify their local ECM through upregulation of ECM degrading and modifying enzymes (such as MMPs and LOXs). Solid long arrows represent downstream signaling activation or event, solid short arrows represent elevated activity or expression, dotted arrows represent growth factor release or immune cell migration, red lines with flat heads represent inhibition.
Glycoproteins
Glycoproteins, which make the ECM a cohesive network of molecules by linking cells together with structural components, include fibulin, fibrillin, laminin, fibronectin, vitronectin, tenascin-C, Secreted Protein Acidic and Rich in Cysteine (SPARC), periostin (POSTN), thrombospondin, mucins (MUCs) and nidogen (Table 1). CSCs overexpress several glycoproteins (e.g., tenascin-C, POSTN, MUC1) and their receptors (e.g., integrins αVβ3 and α9β1, CD47). Adhesive glycoproteins bind to integrins, non-integrin receptors, growth factors, and other ECM components to activate downstream signaling pathways to regulate EMT, self-renewal, and drug resistance of CSCs (Table 1). For example, fibronectin, a major adhesive ECM glycoprotein that attaches cells to a variety of ECM components, has been shown to increase EMT, self-renewal, expression of CSC markers and drug resistance of CSCs. Laminins, another class of adhesive glycoproteins that constitute structural scaffolding of all basement membranes, support self-renewal of CSCs through their interaction with integrins. Some glycoproteins have dual roles in cancer stemness depending on the cancer type. For instance, fibulin-3, an ECM glycoprotein associated with basement membranes, inhibits self-renewal in lung and pancreatic CSCs while stimulating breast CSC self-renewal (Table 1).
Proteoglycans
Proteoglycans are glycosylated proteins composed of a core protein and one or several covalently attached sulfated glycosaminoglycan chains and are present in the ECM of connective tissues. Proteoglycans play a crucial role in ECM assembly and cell signaling. They bind to growth factors, cytokines and other ECM molecules and act as co-receptors to assist ligand and cell surface binding to modulate downstream signaling. Several proteoglycans (e.g., decorin, lumican, biglycan, versican, aggrecan) are highly expressed by CSCs and their roles in cancer stemness are summarized in Table 1.
Polysaccharides
Polysaccharides, a chain of monosaccharide repeats linked through glycosidic bonds, fill the interstitial space and buffer physical stress on the ECM. Hyaluronic acid (HA or “hyaluronan”) is a high-molecular-mass polysaccharide that constitutes a major component of interstitial gels, especially in soft connective tissues. In tumors, HA is produced by both tumor stroma and tumor cells, and its binding to the cellular receptor CD44 activates intracellular signaling (e.g., PI3K/Akt and Erk pathways, RhoA and Rac, Ras, NF-kB and Src signaling) to promote cell survival, cancer stemness, motility and invasion by cytoskeletal reorganization. High levels of HA are produced by CSCs and HA–CD44 interaction has been shown to promote acquisition of CSC characteristics and chemoresistance in breast, ovarian and head and neck CSCs (Table 1).
ECM Provides Physical and Mechanical Cues to Drive Cancer Stemness
Physical Properties
Physical properties of the ECM such as rigidity, porosity and topography impact various anchorage-dependent CSC functions. The interstitial ECM, mainly composed of collagens, proteoglycans and hyaluronan, provides a physical barrier that hinders the transport of solutes, water and chemotherapeutic drugs. In this regard, it has been shown that cisplatin, a chemotherapeutic drug frequently used to treat various solid tumors, extensively binds to collagen fibers in tumors (Chang et al., 2016). Binding of chemotherapeutic drugs to the ECM prevents drug penetration into tumors, thereby increasing CSC survival. The ECM also provides sites for adhesion of CSCs in the tumor microenvironment. ECM-CSC interaction via CSC receptors such as integrins (e.g., β1, α6, β3, β4), discoidin domain receptors (DDR1, DDR2), CD44 (HA receptor) and CD47 (thrombospondin 1 receptor) enhances CSC properties. For example, CSCs bind to HA through CD44 and this increases not only the expression of stemness factors NANOG and SOX2 but also MDR1 (Multi Drug Resistance 1) expression and drug resistance in breast and ovarian CSCs (Bourguignon et al., 2008). The ECM also provides anchorage and homing sites for CSCs in pre-metastatic niches, initiating metastatic colonization and organotropism of cancer cells. For instance, infiltrating breast tumor cells induce the expression of POSTN in the stroma of the secondary target organ (e.g., lung). By recruiting Wnt ligands and increasing Wnt signaling in CSCs, POSTN sustains CSC population in the secondary site and promotes metastatic colonization (Malanchi et al., 2011). Changes in the ECM topology also affects CSC self-renewal by controlling the balance between symmetric and asymmetric cell divisions. The spatial distribution of the ECM has been shown to guide the orientation of the cell division axis by controlling the location of actin polymerization at the membrane through focal adhesions and the segregation of cortical components in the interphase (Thery et al., 2005). The β1 sub-family of integrins also regulates stem cell self-renewal by controlling the balance between symmetric and asymmetric cell divisions (Lechler and Fuchs, 2005; Taddei et al., 2008). Furthermore, ECM distribution affects migration of cancer cells and immune cells. During tumor progression, wavy collagen fibers become straightened and align perpendicular to the tumor boundary (Provenzano et al., 2006). It has been shown that linear collagen fibers oriented perpendicular to the tumors facilitate high-speed migration of breast cancer cells and paired macrophages to promote metastasis to distant organs (Roussos et al., 2011).
Mechanical Properties
Tumor ECM is typically stiffer than normal tissue ECM due to overexpression of many ECM components (e.g., collagens I, II, III, V, IX, and XI, heparan sulfate proteoglycans) and ECM-modifying enzymes [e.g., lysyl oxidase (LOX)] (Levental et al., 2009). Mechanical properties conferred by ECM stiffness are transmitted to CSCs through the formation of focal adhesions and subsequent activation of mechanotransduction pathways (e.g., Rho/ROCK, YAP/TAZ). ECM stiffness plays a crucial role in regulating stem cell self-renewal and differentiation. Several studies have demonstrated that ECM stiffness directs human mesenchymal stem cells (MSCs) and neural stem cells to differentiate into different cell lineages (Engler et al., 2006; Saha et al., 2008; Winer et al., 2009). Human MSCs cultured on hydrogel with an elastic modulus very similar to bone marrow, exhibit enhanced self-renewal and multipotency (Winer et al., 2009). In the case of melanoma CSCs, three-dimensional (3D) soft fibrin matrices promote histone 3 lysine residue 9 (H3K9) demethylation and increase SOX2 expression and self-renewal, whereas stiff matrices exert the opposite effects (Liu et al., 2012; Tan et al., 2014). Conversely, breast CSCs increase CSC marker expression on stiff matrix through integrin linked kinase (ILK) signaling (Pang et al., 2016; You et al., 2016), suggesting that the effect of matrix stiffness on stemness is cancer type specific.
ECM Modulates Biochemical Cues to Drive Cancer Stemness
EMT/De-Differentiation
The ECM can provide external cues that induce EMT, one of the cellular transformation processes that has been shown to route some cancer cell types from a differentiated to a stem cell state (Mani et al., 2008). Collagen I has been shown to induce EMT through activation of ILK and subsequently NF-κB-dependent inactivation of GSK-3β (Medici and Nawshad, 2010), along with the nuclear translocation of β-catenin (Li et al., 2010). Collagen XVII and laminin-5 can also induce EMT-driven cancer stemness through the activation of FAK/Akt paired with inhibition of GSK-3β (Liu et al., 2018). The induction of EMT and CSC phenotypes by the ECM seems to be driven by a master regulator, Akt. Akt activation, which can be achieved via intracellular focal adhesion proteins such as FAK and ILK, subsequently modulates the activity of downstream effectors. For instance, Akt can activate NF-κB, which has been shown to upregulate the expression the stemness genes SOX2, NANOG and KLF4 in breast and prostate cancer cells (Liu et al., 2010; Moreira et al., 2015). Akt, as well as ILK, can also inactivate GSK-3β, which increases the nuclear translocation of β-catenin, a transcription factor that is associated with stemness and is also an activator of NOTCH and Wnt signaling (Vadlamudi et al., 2005; Fang et al., 2010). Therefore, ECM regulates the switch between CSC and non-CSC states by inducing EMT.
Self-Renewal/Maintenance
The ECM also promotes CSC self-renewal. In this regard, collagen I has been shown to preserve stemness in malignant and non-malignant stem cells by activating transcriptional programs that induce self-renewal (Kirkland, 2009; Suh and Han, 2011). Binding of collagen to α2β1 integrin results in the nuclear translocation of Bmi1, a stemness-inducing transcription factor downstream of Hedgehog signaling. Studies have shown that Bmi1 is a transcriptional target of Gli1, a stemness related gene, and that FAK/Ras signaling enhances the expression of Gli1 (Goel et al., 2013). Akt/p-S6K1 signaling has also been shown to play a regulatory role in activity of Gli1 (Wang et al., 2012). Laminin and fibronectin signaling also plays a crucial role in CSC self-renewal. Laminin 511 can sustain breast cancer stemness through activation of α6β1 integrin, in a TAZ-dependent manner (Chang et al., 2015). TAZ expression and nuclear localization induce the expression of the stemness transcription factors, OCT4, SOX2 and NANOG in non-malignant and malignant cells (Varelas et al., 2008; Chang et al., 2015; Xiao et al., 2015). Fibronectin’s extra domain A (EDA) has also been demonstrated to positively regulate CSC self-renewal through activation of α9β1 integrin/FAK/ERK/Akt/β-catenin pathway (Ou et al., 2013).
Growth Factor Reservoir and Release
The ECM might serve as a reservoir for factors that aid in the sustenance of CSCs. Embryonic stem cells (ESCs) have been shown to utilize matrix metalloproteases 1 (MMP1) to release ciliary neurotropic factor (CNTF) from an ESC-derived matrix, which enhances ESC self-renewal though JAK/STAT3 signaling (Przybyla et al., 2013), a pathway that has also been implicated in promoting self-renewal of breast CSCs (Wang et al., 2018). Hematopoietic stem cells (HSCs) also upregulate MMP-9 to release soluble kit-ligand, also known as stem cell factor (SCF), which promotes survival signaling and chemoresistance in many types of cancers (Foster et al., 2018). CSCs are thought to remodel their matrices more significantly than their non-cancer stem cell counterparts (Raja et al., 2015) as CSCs upregulate expression of different MMPs. This may enable them to effectively degrade and remodel ECM matrices (Inoue et al., 2010; Long et al., 2012) to release growth factors and cytokines to promote their survival.
Metabolic Reprogramming and Autophagy
The ECM serves as a functional repository for a plethora of factors that dynamically modulate the tumor microenvironment to promote CSC metabolism. Focal adhesion formations transduce ECM signaling into the tumor cells and activate the PI3K pathway which increases glycolysis, in addition to activating glutamine signaling in a Ras- and Myc- dependent manner. Furthermore, a stiff ECM acts as a driver of glycolysis in CSCs (Pickup et al., 2014). On the contrary, accumulating evidence suggests that CSCs also utilize OXPHOS, fatty acid oxidation and glutaminolysis (Sancho et al., 2016; Martinez-Outschoorn et al., 2017). In this regard, it has been demonstrated that CSCs with high telomerase activity upregulate glycolysis and OXPHOS in lung and ovarian cancers (Bonuccelli et al., 2017). Given the diversity of tumors and their microenvironments, it is possible that based on the availability of nutrients, CSCs can manipulate their metabolism. For example, while CSCs in a hypoxic microenvironment may survive by means of glycolysis, CSCs in a normoxic environment use oxidative metabolism. Furthermore, CSCs utilize metabolites secreted by cancer-associated fibroblasts such as lactate and ketone bodies to fuel OXPHOS (Nazio et al., 2019). Recycling of nutrients via autophagy is another way by which CSCs not only self-renew but also acquire drug resistance (Mowers et al., 2018). Autophagy impairment downregulates the expression of CSC markers and consequently the CSC self-renewal capacity in breast, liver, ovarian and pancreatic cancers, osteosarcoma and gliobastoma (Nazio et al., 2019). ECM-receptor ligation has been shown to induce autophagy (Neill et al., 2014; Kawano et al., 2017). Collagen VI, a promoter of tumorigenesis (Chen et al., 2013) and a supporter of stem cell niches (Urciuolo et al., 2013), also functions as an autophagy inducer in skeletal muscle stem cells by functionally interacting with decorin, a small leucin-rich proteoglycans (SLRP) that has been shown to induce stemness in glioblastoma (Farace et al., 2015). A growing number of studies indicate that collagen VI directly maintains CSCs by activating the Akt–GSK-3β–β-catenin–TCF/LEF axis, which is required for activation of autophagy (Fan et al., 2018). Decorin signaling, independent of Collagen VI, can also maintain stemness of trophoblasts and prevent their differentiation (Nandi et al., 2018).
Role of Hypoxia in ECM-Derived Cancer Stemness
Solid tumors frequently contain highly hypoxic regions and tumor hypoxia is positively associated with poor prognosis. Hypoxic tumor cells express stem cell markers, are highly undifferentiated and exhibit enhanced clonogenic potential in vitro and tumor initiating potential in vivo (Desplat et al., 2002; Jogi et al., 2002; Das et al., 2008; Kim et al., 2009). Furthermore, hypoxia can lead to increased ECM deposition and remodeling. Histological studies on clinical tumor samples have shown increased collagen deposition resulting in fibrosis in hypoxic regions of tumors (Shekhar et al., 2003). In addition to cancer cells, fibroblasts cultured under hypoxic conditions show increased type I procollagen α1 mRNA (Falanga et al., 1993; Tamamori et al., 1997; Norman et al., 2000). Abrogating HIF1α expression inhibits collagen deposition from both breast cancer cells and fibroblasts in vitro and in vivo (Gilkes et al., 2013a,b, 2014; Xiong et al., 2014). ECM remodeling enzymes such as LOX, LOX-like protein 2 (LOXL2), LOXL4, MMP2, MMP9 and MMP14 and growth factors inducing collagen deposition (e.g., VEGF) are HIF-regulated genes that are involved in tumor fibrosis (Gilkes et al., 2014). Since all these factors have been previously implicated cancer stemness, it is not surprising that the ECM acts a functional conduit for hypoxia-derived signals that foster cancer stemness.
ECM Modulates Immune Surveillance in CSC Microenvironment
Extracellular matrix can profoundly influence recruitment of immune cells into the tumor microenvironment. CSCs can evade immune surveillance by altering this microenvironment to favor their survival. For example, ECM drives the activation of pro-survival pathways such as PI3K/AKT, which has been shown to facilitate immune evasion in CSCs (Dituri et al., 2011). ECM proteins can recruit immunosuppressive cells such as tumor-associated macrophages (TAMs) (Stahl et al., 2013; Lu et al., 2014) and regulatory T cells (Bollyky et al., 2011) that have been known to promote CSC survival, while simultaneously blocking the recruitment of antitumorigenic immune cells such as cytotoxic T cells (O’Connor et al., 2012). In addition, the ECM composition can dramatically modulate the activation state of the tumor infiltrating immune cells. For instance, a stiff collagen-rich or POSTN-rich ECM allows macrophage polarization to a pro-tumorigenic M2 phenotype (Wesley et al., 1998; Zhou W.C. et al., 2015). Following recruitment, the M2 macrophages activate several CSC survival signaling pathways including Src, NF-κB (Lu et al., 2014), STAT3/SOX2 (Yang et al., 2013) and Hedgehog (Jinushi et al., 2011). ECM can also impair proliferation and activation of T cells, that are required for capturing and killing CSCs (Di Tomaso et al., 2010). A collagen-rich ECM can inhibit T-cell proliferation and activation through type I collagen-dependent fusion of LAIR receptors (Meyaard, 2008; Frantz et al., 2010) in addition to sequestering growth factors required for T cell proliferation (Meyaard, 2008; O’Connor et al., 2012). Furthermore, TAMs (Martinez and Gordon, 2014) and neutrophils (Yakubenko et al., 2018) that can selectively reorganize the ECM to promote malignant growth of cancers are preferentially recruited to the microenvironment.
CSC Targeting Therapies
Currently, there are several inhibitors targeting various aspects of ECM-induced cancer stemness that are undergoing clinical testing. For example, the CD47 blocking protein TTI-621 (Petrova et al., 2017) is currently being assessed in a number of phase I clinical trials (NCT03013218, NCT02663518, NCT02216409, NCT02678338) for various types of cancers. Other groups have targeted FAK with the inhibitor VS-6063 (Defactinib) (Lin et al., 2018), which has completed clinical phase I and II trials (NCT01778803, NCT01943292, NCT01951690) with one of those clinical trials assessing for CSCs as an endpoint (NCT01778803). Other inhibitors of stemness-related molecules further downstream of ECM signaling are also being tested in clinical trials, such as the STAT3 inhibitor BBI-608 (Sonbol et al., 2019) in a phase II trial that will test for presence of CSC as an endpoint (NCT02279719) and in a phase III clinical trial aimed at reducing CSCs by targeting phosphorylated Stat3 positive cancer cells (NCT02753127). The β-catenin pathway inhibitors PRI-724 and CWP232291 (Tai et al., 2015) are currently being tested in two phase I clinical trials (NCT01764477, NCT01398462). Inhibition of the Hedgehog pathway with the inhibitor GDC-0449 (Vismodegib) (Basset-Séguin et al., 2017), is also currently being clinically evaluated in a phase II trial which will test for the presence of pancreatic CSCs (NCT01088815).
Challenges and Conclusion
Although the above drugs may effectively reduce the number of CSCs, there are still many potential challenges that ECM components in a tumor microenvironment may set that could interfere with an otherwise successful treatment regimen. Firstly, ECM proteins have been shown to act as a physical barrier, making drug delivery to cancer cells more difficult. Secondly, ECM proteins can de-differentiate non-CSCs into CSCs, which makes eliminating all CSCs more challenging. Thirdly, ECM plays a role in modulating immune cell recruitment, hence, potential immunotherapeutic strategies could be hindered by dysregulated ECM components. Finally, the ECM has a very complex and dynamic nature: different ECM molecules are expressed in a time and tissue-specific manner where various isoforms of the same molecule can play opposing functions in cancer stemness in a context-dependent manner. Considering these concerns, it is crucial that future studies further elucidate the role of ECM components on cancer stemness in order to design therapies that effectively eradicate all CSCs.
Author Contributions
All authors conceptualized the content and wrote the manuscript.
Funding
D-JC was supported by the startup fund from Albany Medical College, the AACR Gertrude B. Elion Cancer Research Award (17-10-19-CHEO), and the Caring Together Research Fund.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Drs. Paula McKeown-Longo and Livingston Van De Water for their critical reading of the manuscript and Ms. Jessica Sage and Jennifer Cha for their assistance with manuscript preparation.
References
Barkho, B. Z., Song, H., Aimone, J. B., Smrt, R. D., Kuwabara, T., Nakashima, K., et al. (2006). Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 15, 407–421. doi: 10.1089/scd.2006.15.407
Basset-Séguin, N., Hauschild, A., Kunstfeld, R., Grob, J., Dréno, B., Mortier, L., et al. (2017). Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur. J. Cancer 86, 334–348. doi: 10.1016/j.ejca.2017.08.022
Batlle, E., and Clevers, H. (2017). Cancer stem cells revisited. Nat. Med. 23, 1124–1134. doi: 10.1038/nm.4409
Begum, A., Ewachiw, T., Jung, C., Huang, A., Norberg, K. J., Marchionni, L., et al. (2017). The extracellular matrix and focal adhesion kinase signaling regulate cancer stem cell function in pancreatic ductal adenocarcinoma. PLoS One 12:e0180181. doi: 10.1371/journal.pone.0180181
Bollyky, P. L., Wu, R. P., Falk, B. A., Lord, J. D., Long, S. A., Preisinger, A., et al. (2011). ECM components guide IL-10 producing regulatory T-cell (TR1) induction from effector memory T-cell precursors. Proc. Natl. Acad. Sci. U.S.A. 108, 7938–7943. doi: 10.1073/pnas.1017360108
Bonuccelli, G., Peiris-Pages, M., Ozsvari, B., Martinez-Outschoorn, U. E., Sotgia, F., and Lisanti, M. P. (2017). Targeting cancer stem cell propagation with palbociclib, a CDK4/6 inhibitor: telomerase drives tumor cell heterogeneity. Oncotarget 8, 9868–9884. doi: 10.18632/oncotarget.14196
Bourguignon, L. Y., Peyrollier, K., Xia, W., and Gilad, E. (2008). Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J. Biol. Chem. 283, 17635–17651. doi: 10.1074/jbc.M800109200
Bourguignon, L. Y., Wong, G., Earle, C., and Chen, L. (2012). Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. J. Biol. Chem. 287, 32800–32824. doi: 10.1074/jbc.M111.308528
Braam, S. R., Zeinstra, L., Litjens, S., Ward-van Oostwaard, D., van den Brink, S., van Laake, L., et al. (2008). Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alpha V beta 5 integrin. Stem Cells 26, 2257–2265. doi: 10.1634/stemcells.2008-0291
Cao, J., Ma, J., Sun, L., Li, J., Qin, T., Zhou, C., et al. (2018). Targeting glypican-4 overcomes 5-FU resistance and attenuates stem cell-like properties via suppression of Wnt/beta-catenin pathway in pancreatic cancer cells. J. Cell. Biochem. 119, 9498–9512. doi: 10.1002/jcb.27266
Chang, C., Goel, H. L., Gao, H. J., Pursell, B., Shultz, L. D., Greiner, D. L., et al. (2015). A laminin 511 matrix is regulated by TAZ and functions as the ligand for the alpha 6B beta 1 integrin to sustain breast cancer stem cells. Genes Dev. 29, 1–6. doi: 10.1101/gad.253682.114
Chang, Q., Ornatsky, O. I., Siddiqui, I., Straus, R., Baranov, V. I., and Hedley, D. W. (2016). Biodistribution of cisplatin revealed by imaging mass cytometry identifies extensive collagen binding in tumor and normal tissues. Sci. Rep. 6:36641. doi: 10.1038/srep36641
Chanmee, T., Ontong, P., Mochizuki, N., Kongtawelert, P., Konno, K., and Itano, N. (2014). Excessive hyaluronan production promotes acquisition of cancer stem cell signatures through the coordinated regulation of twist and the transforming growth factor beta (TGF-beta)-snail signaling axis. J. Biol. Chem. 289, 26038–26056. doi: 10.1074/jbc.M114.564120
Chen, P., Cescon, M., and Bonaldo, P. (2013). Collagen VI in cancer and its biological mechanisms. Trends Mol. Med. 19, 410–417. doi: 10.1016/j.molmed.2013.04.001
Cioffi, M., Trabulo, S., Hidalgo, M., Costello, E., Greenhalf, W., Erkan, M., et al. (2015). Inhibition of CD47 effectively targets pancreatic cancer stem cells via dual mechanisms. Clin. Cancer Res. 21, 2325–2337. doi: 10.1158/1078-0432.Ccr-14-1399
Curry, J. M., Thompson, K. J., Rao, S. G., Besmer, D. M., Murphy, A. M., Grdzelishvili, V. Z., et al. (2013). The use of a novel MUC1 antibody to identify cancer stem cells and circulating MUC1 in mice and patients with pancreatic cancer. J. Surg. Oncol. 107, 713–722. doi: 10.1002/jso.23316
Das, B., Tsuchida, R., Malkin, D., Koren, G., Baruchel, S., and Yeger, H. (2008). Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells 26, 1818–1830. doi: 10.1634/stemcells.2007-0724
Das, S., Rachagani, S., Torres-Gonzalez, M. P., Lakshmanan, I., Majhi, P. D., Smith, L. M., et al. (2015). Carboxyl-terminal domain of MUC16 imparts tumorigenic and metastatic functions through nuclear translocation of JAK2 to pancreatic cancer cells. Oncotarget 6, 5772–5787. doi: 10.18632/oncotarget.3308
Desplat, V., Faucher, J. L., Mahon, F. X., Dello Sbarba, P., Praloran, V., and Ivanovic, Z. (2002). Hypoxia modifies proliferation and differentiation of CD34(+) CML cells. Stem Cells 20, 347–354. doi: 10.1634/stemcells.20-4-347
Di Tomaso, T., Mazzoleni, S., Wang, E., Sovena, G., Clavenna, D., Franzin, A., et al. (2010). immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clin. Cancer Res. 16, 800–813. doi: 10.1158/1078-0432.Ccr-09-2730
Dituri, F., Mazzocca, A., Giannelli, G., and Antonaci, S. (2011). PI3K functions in cancer progression, anticancer immunity and immune evasion by tumors. Clin. Dev. Immunol. 2011:947858. doi: 10.1155/2011/947858
Domogatskaya, A., Rodin, S., Boutaud, A., and Tryggvason, K. (2008). Laminin-511 but Not-332,111, or-411 enables mouse embryonic stem cell self-renewal in vitro. Stem Cells 26, 2800–2809. doi: 10.1634/stemcells.2007-0389
Du, W. W., Fang, L., Yang, X. L., Sheng, W., Yang, B. L., Seth, A., et al. (2013). The role of versican in modulating breast cancer cell self-renewal. Mol. Cancer Res. 11, 443–455. doi: 10.1158/1541-7786.Mcr-12-0461
Ehninger, A., Boch, T., Medyouf, H., Mudder, K., Orend, G., and Trumpp, A. (2014). Loss of SPARC protects hematopoietic stem cells from chemotherapy toxicity by accelerating their return to quiescence. Blood 123, 4054–4063. doi: 10.1182/blood-2013-10-533711
Engelmann, K., Shen, H., and Finn, O. J. (2008). MCF7 side population cells with characteristics of cancer stem/progenitor cells express the tumor antigen MUC1. Cancer Res. 68, 2419–2426. doi: 10.1158/0008-5472.Can-07-2249
Engler, A. J., Sen, S., Sweeney, H. L., and Discher, D. E. (2006). Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689. doi: 10.1016/j.cell.2006.06.044
Falanga, V., Martin, T. A., Takagi, H., Kirsner, R. S., Helfman, T., Pardes, J., et al. (1993). Low oxygen tension increases mRNA levels of alpha 1 (I) procollagen in human dermal fibroblasts. J. Cell. Physiol. 157, 408–412. doi: 10.1002/jcp.1041570225
Fan, Q., Yang, L., Zhang, X., Ma, Y., Li, Y., Dong, L., et al. (2018). Autophagy promotes metastasis and glycolysis by upregulating MCT1 expression and Wnt/beta-catenin signaling pathway activation in hepatocellular carcinoma cells. J. Exp. Clin. Cancer Res. 37:9. doi: 10.1186/s13046-018-0673-y
Fang, D. D., Kim, Y. J., Lee, C. N., Aggarwal, S., McKinnon, K., Mesmer, D., et al. (2010). Expansion of CD133(+) colon cancer cultures retaining stem cell properties to enable cancer stem cell target discovery. Br. J. Cancer 102, 1265–1275. doi: 10.1038/sj.bjc.6605610
Farace, C., Oliver, J. A., Melguizo, C., Alvarez, P., Bandiera, P., Rama, A. R., et al. (2015). Microenvironmental modulation of decorin and lumican in temozolomide-resistant glioblastoma and neuroblastoma cancer stem-like cells. PLoS One 10:e0134111. doi: 10.1371/journal.pone.0134111
Fatrai, S., Schepers, H., Tadema, H., Vellenga, E., Daenen, S. M. G. J., and Schuringa, J. J. (2008). Mucin1 expression is enriched in the human stem cell fraction of cord blood and is upregulated in majority of the AML cases. Exp. Hematol. 36, 1254–1265. doi: 10.1016/j.exphem.2008.04.015
Foster, B. M., Zaidi, D., Young, T. R., Mobley, M. E., and Kerr, B. A. (2018). CD117/c-kit in cancer stem cell-mediated progression and therapeutic resistance. Biomedicines 6:31. doi: 10.3390/biomedicines6010031
Frantz, C., Stewart, K. M., and Weaver, V. M. (2010). The extracellular matrix at a glance. J. Cell Sci. 123, 4195–4200. doi: 10.1242/jcs.023820
Fukunaga-Kalabis, M., Martinez, G., Nguyen, T. K., Kim, D., Santiago-Walker, A., Roesch, A., et al. (2010). Tenascin-C promotes melanoma progression by maintaining the ABCB5-positive side population. Oncogene 29, 6115–6124. doi: 10.1038/onc.2010.350
Gilkes, D. M., Bajpai, S., Chaturvedi, P., Wirtz, D., and Semenza, G. L. (2013a). Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J. Biol. Chem. 288, 10819–10829. doi: 10.1074/jbc.M112.442939
Gilkes, D. M., Chaturvedi, P., Bajpai, S., Wong, C. C., Wei, H., Pitcairn, S., et al. (2013b). Collagen prolyl hydroxylases are essential for breast cancer metastasis. Cancer Res. 73, 3285–3296. doi: 10.1158/0008-5472.CAN-12-3963
Gilkes, D. M., Semenza, G. L., and Wirtz, D. (2014). Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat. Rev. Cancer 14, 430–439. doi: 10.1038/nrc3726
Goel, H. L., Pursell, B., Chang, C., Shaw, L. M., Mao, J. H., Simin, K., et al. (2013). GLI1 regulates a novel neuropilin-2/61 integrin based autocrine pathway that contributes to breast cancer initiation. Embo Mol. Med. 5, 488–508. doi: 10.1002/emmm.201202078
Govaere, O., Wouters, J., Petz, M., Vandewynckel, Y. P., Van den Eynde, K., Van den Broeck, A., et al. (2016). Laminin-332 sustains chemoresistance and quiescence as part of the human hepatic cancer stem cell niche. J. Hepatol. 64, 609–617. doi: 10.1016/j.jhep.2015.11.011
Grant, D. S., Yenisey, C., Rose, R. W., Tootell, M., Santra, M., and Iozzo, R. V. (2002). Decorin suppresses tumor cell-mediated angiogenesis. Oncogene 21, 4765–4777. doi: 10.1038/sj.onc.1205595
Hurt, E. M., Chan, K., Serrat, M. A. D., Thomas, S. B., Veenstra, T. D., and Farrar, W. L. (2010). Identification of vitronectin as an extrinsic inducer of cancer stem cell differentiation and tumor formation. Stem Cells 28, 390–398. doi: 10.1002/stem.271
Ibrahim, S. A., Hassan, H., Vilardo, L., Kumar, S. K., Kumar, A. V., Kelsch, R., et al. (2013). Syndecan-1 (CD138) modulates triple-negative breast cancer stem cell properties via regulation of LRP-6 and IL-6-mediated STAT3 signaling. PLoS One 8:e85737. doi: 10.1371/journal.pone.0085737
Ichii, M., Frank, M. B., Iozzo, R. V., and Kincade, P. W. (2012). The canonical Wnt pathway shapes niches supportive of hematopoietic stem/progenitor cells. Blood 119, 1683–1692. doi: 10.1182/blood-2011-07-369199
Inoue, A., Takahashi, H., Harada, H., Kohno, S., Ohue, S., Kobayashi, K., et al. (2010). Cancer stem-like cells of glioblastoma characteristically express MMP-13 and display highly invasive activity. Int. J. Oncol. 37,1121–1131.
Jaiswal, S., Jamieson, C. H. M., Pang, W. W., Park, C. Y., Chao, M. P., Majeti, R., et al. (2009). CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 138, 271–285. doi: 10.1016/j.cell.2009.05.046
Januchowski, R., Swierczewska, M., Sterzynska, K., Wojtowicz, K., Nowicki, M., and Zabel, M. (2016). Increased expression of several collagen genes is associated with drug resistance in ovarian cancer cell lines. J. Cancer 7, 1295–1310. doi: 10.7150/jca.15371
Jinushi, M., Chiba, S., Yoshiyama, H., Masutomi, K., Kinoshita, I., Dosaka-Akita, H., et al. (2011). Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc. Natl. Acad. Sci. U.S.A. 108, 12425–12430. doi: 10.1073/pnas.1106645108
Jogi, A., Ora, I., Nilsson, H., Lindeheim, A., Makino, Y., Poellinger, L., et al. (2002). Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc. Natl. Acad. Sci. U.S.A. 99, 7021–7026. doi: 10.1073/pnas.102660199
Kabos, P., Matundan, H., Zandian, M., Bertolotto, C., Robinson, M. L., Davy, B. E., et al. (2004). Neural precursors express multiple chondroitin sulfate proteoglycans, including the lectican family. Biochem. Biophys. Res. Commun. 318, 955–963. doi: 10.1016/j.bbrc.2004.04.114
Kato, M., Saunders, S., Nguyen, H., and Bernfield, M. (1995). Loss of cell-surface syndecan-1 causes epithelia to transform into anchorage-independent mesenchyme-like cells. Mol. Biol. Cell 6, 559–576.
Kaur, S., Elkahloun, A. G., Singh, S. P., Chen, Q. R., Meerzaman, D. M., Song, T., et al. (2016). A function-blocking CD47 antibody suppresses stem cell and EGF signaling in triple-negative breast cancer. Oncotarget 7, 10133–10152. doi: 10.18632/oncotarget.7100
Kaur, S., Soto-Pantoja, D. R., Stein, E. V., Liu, C. Y., Elkahloun, A. G., Pendrak, M. L., et al. (2013). Thrombospondin-1 signaling through CD47 inhibits self-renewal by regulating c-Myc and other stem cell transcription factors. Sci. Rep. 3:1673. doi: 10.1038/srep01673
Kawano, S., Torisu, T., Esaki, M., Torisu, K., Matsuno, Y., and Kitazono, T. (2017). Autophagy promotes degradation of internalized collagen and regulates distribution of focal adhesions to suppress cell adhesion. Biol. Open 6, 1644–1653. doi: 10.1242/bio.027458
Kim, I. G., Kim, S. Y., Choi, S. I., Lee, J. H., Kim, K. C., and Cho, E. W. (2014a). Fibulin-3-mediated inhibition of epithelial-to-mesenchymal transition and self-renewal of ALDH+ lung cancer stem cells through IGF1R signaling. Oncogene 33, 3908–3917. doi: 10.1038/onc.2013.373
Kim, I. G., Lee, J. H., Kim, S. Y., Kim, J. Y., and Cho, E. W. (2014b). Fibulin-3 negatively regulates ALDH1 via c-MET suppression and increases gamma-radiation-induced sensitivity in some pancreatic cancer cell lines. Biochem. Biophys. Res. Commun. 454, 369–375. doi: 10.1016/j.bbrc.2014.10.084
Kim, H. P., Han, S. W., Song, S. H., Jeong, E. G., Lee, M. Y., Hwang, D., et al. (2014c). Testican-1-mediated epithelial-mesenchymal transition signaling confers acquired resistance to lapatinib in HER2-positive gastric cancer. Oncogene 33, 3334–3341. doi: 10.1038/onc.2013.285
Kim, Y., Lin, Q., Zelterman, D., and Yun, Z. (2009). Hypoxia-regulated delta-like 1 homologue enhances cancer cell stemness and tumorigenicity. Cancer Res. 69, 9271–9280. doi: 10.1158/0008-5472.CAN-09-1605
Kirkland, S. C. (2009). Type I collagen inhibits differentiation and promotes a stem cell-like phenotype in human colorectal carcinoma cells. Br. J. Cancer 101, 320–326. doi: 10.1038/sj.bjc.6605143
Kreso, A., and Dick, J. E. (2014). Evolution of the cancer stem cell model. Cell Stem Cell 14, 275–291. doi: 10.1016/j.stem.2014.02.006
Kwak, J. H., Lee, N. H., Lee, H. Y., Hong, I. S., and Nam, J. S. (2016). HIF2alpha/EFEMP1 cascade mediates hypoxic effects on breast cancer stem cell hierarchy. Oncotarget 7, 43518–43533. doi: 10.18632/oncotarget.9846
Lambert, A. W., Wong, C. K., Ozturk, S., Papageorgis, P., Raghunathan, R., Alekseyev, Y., et al. (2016). Tumor cell-derived periostin regulates cytokines that maintain breast cancer stem cells. Mol. Cancer Res. 14, 103–113. doi: 10.1158/1541-7786.MCR-15-0079
Laperle, A., Hsiao, C., Lampe, M., Mortier, J., Saha, K., Palecek, S. P., et al. (2015). alpha-5 laminin synthesized by human pluripotent stem cells promotes self-renewal. Stem Cell Rep. 5, 195–206. doi: 10.1016/j.stemcr.2015.06.009
Lathia, J. D., Li, M., Hall, P. E., Gallagher, J., Hale, J. S., Wu, Q., et al. (2012). Laminin alpha 2 enables glioblastoma stem cell growth. Ann. Neurol. 72, 766–778. doi: 10.1002/ana.23674
Lechler, T., and Fuchs, E. (2005). Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437, 275–280. doi: 10.1038/nature03922
Levental, K. R., Yu, H., Kass, L., Lakins, J. N., Egeblad, M., Erler, J. T., et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906. doi: 10.1016/j.cell.2009.10.027
Li, A., Zhou, T., Guo, L., and Si, J. (2010). Collagen type I regulates beta-catenin tyrosine phosphorylation and nuclear translocation to promote migration and proliferation of gastric carcinoma cells. Oncol. Rep. 23, 1247–1255.
Li, C. L., Yang, D., Cao, X., Wang, F., Hong, D. Y., Wang, J., et al. (2017). Fibronectin induces epithelial-mesenchymal transition in human breast cancer MCF-7 cells via activation of calpain. Oncol. Lett. 13, 3889–3895. doi: 10.3892/ol.2017.5896
Lim, Y. C., Oh, S. Y., and Kim, H. (2012). Cellular characteristics of head and neck cancer stem cells in type IV collagen-coated adherent cultures. Exp. Cell Res. 318, 1104–1111. doi: 10.1016/j.yexcr.2012.02.038
Lin, H. M., Lee, B. Y., Castillo, L., Spielman, C., Grogan, J., Yeung, N. K., et al. (2018). Effect of FAK inhibitor VS-6063 (defactinib) on docetaxel efficacy in prostate cancer. Prostate 78, 308–317. doi: 10.1002/pros.23476
Liu, B., Xu, T., Xu, X., Cui, Y., and Xing, X. (2018). Biglycan promotes the chemotherapy resistance of colon cancer by activating NF-kappaB signal transduction. Mol. Cell. Biochem. 449, 285–294. doi: 10.1007/s11010-018-3365-1
Liu, C. C., Lin, J. H., Hsu, T. W., Hsu, J. W., Chang, J. W., and Su, K. (2016). Collagen XVII/laminin-5 activates epithelial-to-mesenchymal transition and is associated with poor prognosis in lung cancer. Oncotarget 9, 1656–1672. doi: 10.18632/oncotarget.11208
Liu, J., Tan, Y., Zhang, H., Zhang, Y., Xu, P., Chen, J., et al. (2012). Soft fibrin gels promote selection and growth of tumorigenic cells. Nat. Mater. 11, 734–741. doi: 10.1038/nmat3361
Liu, M., Sakamaki, T., Casimiro, M. C., Willmarth, N. E., Quong, A. A., Ju, X., et al. (2010). The canonical NF-kappaB pathway governs mammary tumorigenesis in transgenic mice and tumor stem cell expansion. Cancer Res. 70, 10464–10473. doi: 10.1158/0008-5472.CAN-10-0732
Long, H., Xie, R., Xiang, T., Zhao, Z., Lin, S., Liang, Z., et al. (2012). Autocrine CCL5 signaling promotes invasion and migration of CD133+ ovarian cancer stem-like cells via NF-kappaB-mediated MMP-9 upregulation. Stem Cells 30, 2309–2319. doi: 10.1002/stem.1194
Lu, H. H., Clauser, K. R., Tam, W. L., Frose, J., Ye, X., Eaton, E. N., et al. (2014). A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat. Cell Biol. 16, 1105–1117. doi: 10.1038/ncb3041
Lu, P., Weaver, V. M., and Werb, Z. (2012). The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 196, 395–406. doi: 10.1083/jcb.201102147
Majeti, R., Chao, M. P., Alizadeh, A. A., Pang, W. W., Jaiswal, S., Gibbs, K. D., et al. (2009). CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 138, 286–299. doi: 10.1016/j.cell.2009.05.045
Malanchi, I., Santamaria-Martínez, A., Susanto, E., Peng, H., Lehr, H. A., Delaloye, J. F., et al. (2011). Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481, 85–89. doi: 10.1038/nature10694
Mani, S. A., Guo, W., Liao, M. J., Eaton, E. N., Ayyanan, A., Zhou, A. Y., et al. (2008). The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715. doi: 10.1016/j.cell.2008.03.027
Maris, P., Blomme, A., Palacios, A. P., Costanza, B., Bellahcene, A., Bianchi, E., et al. (2015). Asporin is a fibroblast-derived TGF-beta1 inhibitor and a tumor suppressor associated with good prognosis in breast cancer. PLoS Med. 12:e1001871. doi: 10.1371/journal.pmed.1001871
Martinez, F. O., and Gordon, S. (2014). The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6:13. doi: 10.12703/P6-13
Martinez-Outschoorn, U. E., Peiris-Pages, M., Pestell, R. G., Sotgia, F., and Lisanti, M. P. (2017). Cancer metabolism: a therapeutic perspective. Nat. Rev. Clin. Oncol. 14:113. doi: 10.1038/nrclinonc.2017.1
Mateo, F., Meca-Cortes, O., Celia-Terrassa, T., Fernandez, Y., Abasolo, I., Sanchez-Cid, L., et al. (2014). SPARC mediates metastatic cooperation between CSC and non-CSC prostate cancer cell subpopulations. Mol. Cancer 13:237. doi: 10.1186/1476-4598-13-237
Medici, D., and Nawshad, A. (2010). Type I collagen promotes epithelial-mesenchymal transition through ILK-dependent activation of NF-kappaB and LEF-1. Matrix Biol. 29, 161–165. doi: 10.1016/j.matbio.2009.12.003
Meyaard, L. (2008). The inhibitory collagen receptor LAIR-1 (CD305). J. Leukoc. Biol. 83, 799–803. doi: 10.1189/jlb.0907609
Mikheev, A. M., Mikheeva, S. A., Trister, A. D., Tokita, M. J., Emerson, S. N., Parada, C. A., et al. (2015). Periostin is a novel therapeutic target that predicts and regulates glioma malignancy. Neuro Oncol. 17, 372–382. doi: 10.1093/neuonc/nou161
Mimeault, M., Johansson, S. L., Senapati, S., Momi, N., Chakraborty, S., and Batra, S. K. (2010). MUC4 down-regulation reverses chemoresistance of pancreatic cancer stem/progenitor cells and their progenies. Cancer Lett. 295, 69–84. doi: 10.1016/j.canlet.2010.02.015
Moreira, D., Zhang, Q., Hossain, D. M., Nechaev, S., Li, H., Kowolik, C. M., et al. (2015). TLR9 signaling through NF-kappaB/RELA and STAT3 promotes tumor-propagating potential of prostate cancer cells. Oncotarget 6, 17302–17313. doi: 10.18632/oncotarget.4029
Motegi, H., Kamoshima, Y., Terasaka, S., Kobayashi, H., and Houkin, K. (2014). Type 1 collagen as a potential niche component for CD133-positive glioblastoma cells. Neuropathology 34, 378–385. doi: 10.1111/neup.12117
Mowers, E. E., Sharifi, M. N., and Macleod, K. F. (2018). Functions of autophagy in the tumor microenvironment and cancer metastasis. FEBS J. 285, 1751–1766. doi: 10.1111/febs.14388
Nandi, P., Lim, H., Torres-Garcia, E. J., and Lala, P. K. (2018). Human trophoblast stem cell self-renewal and differentiation: role of decorin. Sci. Rep. 8:8977. doi: 10.1038/s41598-018-27119-4
Nazio, F., Bordi, M., Cianfanelli, V., Locatelli, F., and Cecconi, F. (2019). Autophagy and cancer stem cells: molecular mechanisms and therapeutic applications. Cell Death Differ. 26, 690–702. doi: 10.1038/s41418-019-0292-y
Neill, T., Schaefer, L., and Iozzo, R. V. (2014). Instructive roles of extracellular matrix on autophagy. Am. J. Pathol. 184, 2146–2153. doi: 10.1016/j.ajpath.2014.05.010
Nie, S., Gurrea, M., Zhu, J. H., Thakolwiboon, S., Heth, J. A., Muraszko, K. M., et al. (2015). Tenascin-C: a novel candidate marker for cancer stem cells in glioblastoma identified by tissue microarrays. J. Proteome Res. 14, 814–822. doi: 10.1021/pr5008653
Norman, J. T., Clark, I. M., and Garcia, P. L. (2000). Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int. 58, 2351–2366. doi: 10.1046/j.1523-1755.2000.00419.x
O’Connor, R. S., Hao, X. L., Shen, K. Y., Bashour, K., Akimova, T., Hancock, W. W., et al. (2012). Substrate rigidity regulates human t cell activation and proliferation. J. Immunol. 189, 1330–1339. doi: 10.4049/jimmunol.1102757
Oktem, G., Bilir, A., Uslu, R., Inan, S. V., Demiray, S. B., Atmaca, H., et al. (2014a). Expression profiling of stem cell signaling alters with spheroid formation in CD133(high)/CD44(high) prostate cancer stem cells. Oncol. Lett. 7, 2103–2109. doi: 10.3892/ol.2014.1992
Oktem, G., Sercan, O., Guven, U., Uslu, R., Uysal, A., Goksel, G., et al. (2014b). Cancer stem cell differentiation: TGF beta 1 and versican may trigger molecules for the organization of tumor spheroids. Oncol. Rep. 32, 641–649. doi: 10.3892/or.2014.3252
Okuda, H., Kobayashi, A., Xia, B., Watabe, M., Pai, S. K., Hirota, S., et al. (2012). Hyaluronan synthase HAS2 promotes tumor progression in bone by stimulating the interaction of breast cancer stem-like cells with macrophages and stromal cells. Cancer Res. 72, 537–547. doi: 10.1158/0008-5472.Can-11-1678
Oskarsson, T., Acharyya, S., Zhang, X. H. F., Vanharanta, S., Tavazoie, S. F., Morris, P. G., et al. (2011). Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat. Med. 17, 867–874. doi: 10.1038/nm.2379
Ou, J. J., Deng, J., Wei, X., Xie, G. F., Zhou, R. B., Yu, L. Q., et al. (2013). Fibronectin extra domain A (EDA) sustains CD133(+)/CD44(+) subpopulation of colorectal cancer cells. Stem Cell Res. 11, 820–833. doi: 10.1016/j.scr.2013.05.009
Pang, M. F., Siedlik, M. J., Han, S., Stallings-Mann, M., Radisky, D. C., and Nelson, C. M. (2016). Tissue stiffness and hypoxia modulate the integrin-linked kinase ilk to control breast cancer stem-like cells. Cancer Res. 76, 5277–5287. doi: 10.1158/0008-5472.CAN-16-0579
Petrova, P. S., Viller, N. N., Wong, M., Pang, X., Lin, G. H., Dodge, K., et al. (2017). TTI-621 (SIRPalphaFc): a CD47-blocking innate immune checkpoint inhibitor with broad antitumor activity and minimal erythrocyte binding. Clin. Cancer Res 23, 1068–1079. doi: 10.1158/1078-0432.CCR-16-1700
Pezzolo, A., Parodi, F., Marimpietri, D., Raffaghello, L., Cocco, C., Pistorio, A., et al. (2011). Oct-4(+)/Tenascin C+ neuroblastoma cells serve as progenitors of tumor-derived endothelial cells. Cell Res. 21, 1470–1486. doi: 10.1038/cr.2011.38
Pickup, M. W., Mouw, J. K., and Weaver, V. M. (2014). The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 15, 1243–1253. doi: 10.15252/embr.201439246
Ponnusamy, M. P., Seshacharyulu, P., Vaz, A., Dey, P., and Batra, S. K. (2011). MUC4 stabilizes HER2 expression and maintains the cancer stem cell population in ovarian cancer cells. J. Ovarian Res. 4:7. doi: 10.1186/1757-2215-4-7
Provenzano, P. P., Eliceiri, K. W., Campbell, J. M., Inman, D. R., White, J. G., and Keely, P. J. (2006). Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 4:38. doi: 10.1186/1741-7015-4-38
Przybyla, L. M., Theunissen, T. W., Jaenisch, R., and Voldman, J. (2013). Matrix remodeling maintains embryonic stem cell self-renewal by activating Stat3. Stem Cells 31, 1097–1106. doi: 10.1002/stem.1360
Pupa, S. M., Giuffre, S., Castiglioni, F., Bertola, L., Cantu, M., Bongarzone, I., et al. (2007). Regulation of breast cancer response to chemotherapy by fibulin-1. Cancer Res. 67, 4271–4277. doi: 10.1158/0008-5472.Can-06-4162
Rada, M., Nallanthighal, S., Cha, J., Ryan, K., Sage, J., Eldred, C., et al. (2018). Inhibitor of apoptosis proteins (IAPs) mediate collagen type XI alpha 1-driven cisplatin resistance in ovarian cancer. Oncogene 37, 4809–4820. doi: 10.1038/s41388-018-0297-x
Raja, A. M., Xu, S., Zhuo, S., Tai, D. C., Sun, W., So, P. T., et al. (2015). Differential remodeling of extracellular matrices by breast cancer initiating cells. J. Biophotonics 8, 804–815. doi: 10.1002/jbio.201400079
Reed, C. C., Waterhouse, A., Kirby, S., Kay, P., Owens, R. T., McQuillan, D. J., et al. (2005). Decorin prevents metastatic spreading of breast cancer. Oncogene 24, 1104–1110. doi: 10.1038/sj.onc.1208329
Roussos, E. T., Condeelis, J. S., and Patsialou, A. (2011). Chemotaxis in cancer. Nat. Rev. Cancer 11, 573–587. doi: 10.1038/nrc3078
Saha, K., Keung, A. J., Irwin, E. F., Li, Y., Little, L., Schaffer, D. V., et al. (2008). Substrate modulus directs neural stem cell behavior. Biophys. J. 95, 4426–4438. doi: 10.1529/biophysj.108.132217
Sancho, P., Barneda, D., and Heeschen, C. (2016). Hallmarks of cancer stem cell metabolism. Br. J. Cancer 114, 1305–1312. doi: 10.1038/bjc.2016.152
Sarkar, S., Mirzaei, R., Zemp, F. J., Wei, W., Senger, D. L., Robbins, S. M., et al. (2017). Activation of notch signaling by tenascin-C promotes growth of human brain tumor-initiating cells. Cancer Res. 77, 3231–3243. doi: 10.1158/0008-5472.Can-16-2171
Sharma, S., Xing, F., Liu, Y., Wu, K. R., Said, N., Pochampally, R., et al. (2016). Secreted protein acidic and rich in cysteine (SPARC) mediates metastatic dormancy of prostate cancer in bone. J. Biol. Chem. 291, 19351–19363. doi: 10.1074/jbc.M116.737379
Shekhar, M. P., Pauley, R., and Heppner, G. (2003). Host microenvironment in breast cancer development: extracellular matrix-stromal cell contribution to neoplastic phenotype of epithelial cells in the breast. Breast Cancer Res. 5, 130–135. doi: 10.1186/bcr580
Shiina, M., and Bourguignon, L. Y. (2015). Selective activation of cancer stem cells by size-specific hyaluronan in head and neck cancer. Int. J. Cell Biol. 2015:989070. doi: 10.1155/2015/989070
Shintani, K., Matsumine, A., Kusuzaki, K., Morikawa, J., Matsubara, T., Wakabayashi, T., et al. (2008). Decorin suppresses lung metastases of murine osteosarcoma. Oncol. Rep. 19, 1533–1539.
Smaldone, S., Bigarella, C. L., Del Solar, M., Ghaffari, S., and Ramirez, F. (2016a). Fibrillin-1 microfibrils influence adult bone marrow hematopoiesis. Matrix Biol. 5, 88–94. doi: 10.1016/j.matbio.2015.11.006
Smaldone, S., Clayton, N. P., del Solar, M., Pascual, G., Cheng, S. H., Wentworth, B. M., et al. (2016b). Fibrillin-1 regulates skeletal stem cell differentiation by modulating TGFbeta activity within the marrow niche. J. Bone. Miner. Res. 31, 86–97. doi: 10.1002/jbmr.2598
Sonbol, M. B., Ahn, D. H., and Bekaii-Saab, T. (2019). Therapeutic targeting strategies of cancer stem cells in gastrointestinal malignancies. Biomedicines 7:17. doi: 10.3390/biomedicines7010017
Soteriou, D., Iskender, B., Byron, A., Humphries, J. D., Borg-Bartolo, S., Haddock, M. C., et al. (2013). Comparative proteomic analysis of supportive and unsupportive extracellular matrix substrates for human embryonic stem cell maintenance. J. Biol. Chem. 288, 18716–18731. doi: 10.1074/jbc.M113.463372
Stahl, M., Schupp, J., Jager, B., Schmid, M., Zissel, G., Muller-Quernheim, J., et al. (2013). Lung collagens perpetuate pulmonary fibrosis via CD204 and M2 macrophage activation. PLoS One 8:e81382. doi: 10.1371/journal.pone.0081382
Stock, C., Jungmann, O., and Seidler, D. G. (2011). Decorin and chondroitin-6 sulfate inhibit B16V melanoma cell migration and invasion by cellular acidification. J. Cell. Physiol. 226, 2641–2650. doi: 10.1002/jcp.22612
Stroopinsky, D., Rosenblatt, J., Ito, K., Mills, H., Yin, L., Rajabi, H., et al. (2013). MUC1 is a potential target for the treatment of acute myeloid leukemia stem cells. Cancer Res. 73, 5569–5579. doi: 10.1158/0008-5472.Can-13-0677
Suh, H. N., and Han, H. J. (2011). Collagen I regulates the self-renewal of mouse embryonic stem cells through alpha 2 beta 1 integrin- and DDR1-dependent Bmi-1. J. Cell. Physiol. 226, 3422–3432. doi: 10.1002/jcp.22697
Sun, B., Huang, Z., Wang, B., Yu, Y., Lin, S., Luo, L., et al. (2017). Significance of glypican-3 (GPC3) expression in hepatocellular cancer diagnosis. Med. Sci. Monit. 23, 850–855.
Sun, Y., Kim, H. S., Saw, P. E., Jon, S., and Moon, W. K. (2015). Targeted therapy for breast cancer stem cells by liposomal delivery of siRNA against fibronectin EDB. Adv. Healthc. Mater. 4, 1675–1680. doi: 10.1002/adhm.201500190
Taddei, I., Deugnier, M. A., Faraldo, M. M., Petit, V., Bouvard, D., Medina, D., et al. (2008). Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat. Cell Biol. 10, 716–722. doi: 10.1038/ncb1734
Tai, D., Wells, K., Arcaroli, J., Vanderbilt, C., Aisner, D. L., Messersmith, W. A., et al. (2015). Targeting the WNT signaling pathway in cancer therapeutics. Oncologist 20, 1189–1198. doi: 10.1634/theoncologist.2015-0057
Tamamori, M., Ito, H., Hiroe, M., Marumo, F., and Hata, R. I. (1997). Stimulation of collagen synthesis in rat cardiac fibroblasts by exposure to hypoxic culture conditions and suppression of the effect by natriuretic peptides. Cell Biol. Int. 21, 175–180. doi: 10.1006/cbir.1997.0130
Tan, Y., Tajik, A., Chen, J., Jia, Q., Chowdhury, F., Wang, L., et al. (2014). Matrix softness regulates plasticity of tumour-repopulating cells via H3K9 demethylation and Sox2 expression. Nat. Commun. 5:4619. doi: 10.1038/ncomms5619
Thery, M., Racine, V., Pepin, A., Piel, M., Chen, Y., Sibarita, J. B., et al. (2005). The extracellular matrix guides the orientation of the cell division axis. Nat. Cell Biol. 7, 947–953. doi: 10.1038/ncb1307
Urciuolo, A., Quarta, M., Morbidoni, V., Gattazzo, F., Molon, S., Grumati, P., et al. (2013). Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat. Commun. 4:1964. doi: 10.1038/ncomms2964
Vadlamudi, U., Espinoza, H. M., Ganga, M., Martin, D. M., Liu, X., Engelhardt, J. F., et al. (2005). PITX2, beta-catenin and LEF-1 interact to synergistically regulate the LEF-1 promoter. J. Cell Sci. 118, 1129–1137. doi: 10.1242/jcs.01706
Varelas, X., Sakuma, R., Samavarchi-Tehrani, P., Peerani, R., Rao, B. M., Dembowy, J., et al. (2008). TAZ controls smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat. Cell Biol. 10, 837–848. doi: 10.1038/ncb1748
Vesuna, F., Lisok, A., Kimble, B., and Raman, V. (2009). Twist modulates breast cancer stem cells by transcriptional regulation of CD24 expression. Neoplasia 11, 1318–1328. doi: 10.1593/neo.91084
Wang, T., Fahrmann, J. F., Lee, H., Li, Y.-J., Tripathi, S. C., Yue, C., et al. (2018). JAK/STAT3-Regulated fatty Acid β-Oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab. 27, 136.e5–150.e5. doi: 10.1016/j.cmet.2017.11.001
Wang, X., Zuo, D., Chen, Y., Li, W., Liu, R., He, Y., et al. (2014). Shed Syndecan-1 is involved in chemotherapy resistance via the EGFR pathway in colorectal cancer. Br. J. Cancer 111, 1965–1976. doi: 10.1038/bjc.2014.493
Wang, X. W., Liu, J., Wang, Z., Huang, Y. M., Liu, W. P., Zhu, X., et al. (2013). Periostin contributes to the acquisition of multipotent stem cell-like properties in human mammary epithelial cells and breast cancer cells. PLoS One 8:e72962. doi: 10.1371/journal.pone.0072962
Wang, Y., Xia, W. Y., Lang, J. Y., Hsu, J., Wang, H. M., Ajani, J., et al. (2012). The crosstalk of mTOR/S6K1 and hedgehog pathways. Cancer Res. 72, 374–387. doi: 10.1158/1538-7445.Am2012-989
Wesley, R. B., Meng, X. P., Godin, D., and Galis, Z. S. (1998). Extracellular matrix modulates macrophage functions characteristic to atheroma - collagen type I enhances acquisition of resident macrophage traits by human peripheral blood monocytes in vitro. Arterioscler. Thromb. Vasc. Biol. 18, 432–440. doi: 10.1161/01.Atv.18.3.432
Winer, J. P., Janmey, P. A., McCormick, M. E., and Funaki, M. (2009). Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng. Part A 15, 147–154. doi: 10.1089/ten.tea.2007.0388
Wu, Y. H., Chang, T. H., Huang, Y. F., Chen, C. C., and Chou, C. Y. (2015). COL11A1 confers chemoresistance on ovarian cancer cells through the activation of Akt/c/EBPβ pathway and PDK1 stabilization. Oncotarget 6, 23748–23763.
Wu, Y. H., Huang, Y. F., Chang, T. H., and Chou, C. Y. (2017). Activation of TWIST1 by COL11A1 promotes chemoresistance and inhibits apoptosis in ovarian cancer cells by modulating NF-kappaB-mediated IKKbeta expression. Int. J. Cancer 141, 2305–2317. doi: 10.1002/ijc.30932
Xiao, H., Jiang, N., Zhou, B. Y., Liu, Q., and Du, C. Y. (2015). TAZ regulates cell proliferation and epithelial-mesenchymal transition of human hepatocellular carcinoma. Cancer Sci. 106, 151–159. doi: 10.1111/cas.12587
Xiong, G. F., Deng, L., Zhu, J. Q., Rychahou, P. G., and Xu, R. (2014). Prolyl-4-hydroxylase a subunit 2 promotes breast cancer progression and metastasis by regulating collagen deposition. BMC Cancer 14:1. doi: 10.1186/1471-2407-14-1
Yakubenko, V. P., Cui, K., Ardell, C. L., Brown, K. E., West, X. Z., Gao, D., et al. (2018). Oxidative modifications of extracellular matrix promote the second wave of inflammation via beta2 integrins. Blood 132, 78–88. doi: 10.1182/blood-2017-10-810176
Yang, J., Liao, D. B., Chen, C., Liu, Y., Chuang, T. H., Xiang, R., et al. (2013). Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem Cells 31, 248–258. doi: 10.1002/stem.1281
You, Y., Zheng, Q., Dong, Y., Xie, X., Wang, Y., Wu, S., et al. (2016). Matrix stiffness-mediated effects on stemness characteristics occurring in HCC cells. Oncotarget 7, 32221–32231. doi: 10.18632/oncotarget.8515
Yu, Q., Xue, Y., Liu, J., Xi, Z., Li, Z., and Liu, Y. (2018). Fibronectin promotes the malignancy of glioma stem-like cells via modulation of cell adhesion. differentiation, proliferation and chemoresistance. Front. Mol. Neurosci. 11:130. doi: 10.3389/fnmol.2018.00130
Yusuf, N., Inagaki, T., Kusunoki, S., Okabe, H., Yamada, I., Matsumoto, A., et al. (2014). SPARC was overexpressed in human endometrial cancer stem-like cells and promoted migration activity. Gynecol. Oncol. 134, 356–363. doi: 10.1016/j.ygyno.2014.04.009
Zhang, H., Lu, H. Q., Xiang, L. S., Bullen, J. W., Zhang, C. Z., Samanta, D., et al. (2015a). HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc. Natl. Acad. Sci. U.S.A. 112, E6215–E6223. doi: 10.1073/pnas.1520032112
Zhang, H., Yang, Y. A., Wang, Y. F., Gao, X. P., Wang, W. M., Liu, H., et al. (2015b). Relationship of tumor marker CA125 and ovarian tumor stem cells: preliminary identification. J. Ovarian Res. 8:19. doi: 10.1186/s13048-015-0132-8
Zheng, Y. H., Zou, F. Y., Wang, J. J., Yin, G. F., Le, V., Fei, Z. W., et al. (2015). Photodynamic therapy-mediated cancer vaccination enhances stem-like phenotype and immune escape. Which can be blocked by thrombospondin-1 signaling through CD47 receptor protein. J. Biol. Chem. 290, 8975–8986. doi: 10.1074/jbc.M114.624965
Zhou, N., Wang, R., Zhang, Y. Z., Lei, Z., Zhang, X. H., Hu, R. B., et al. (2015). Staurosporine induced apoptosis may activate cancer stem-like cells (CD44(+)/CD24(-)) in MCF-7 by upregulating mucin1 and EpCAM. J. Cancer 6, 1049–1057. doi: 10.7150/jca.12501
Zhou, W. C., Ke, S. Q., Huang, Z., Flavahan, W., Fang, X. G., Paul, J., et al. (2015). Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat. Cell Biol. 17, 170–182. doi: 10.1038/ncb3090
Keywords: extracellular matrix, cancer stem cells, self-renewal, chemoresistance, integrin
Citation: Nallanthighal S, Heiserman JP and Cheon D-J (2019) The Role of the Extracellular Matrix in Cancer Stemness. Front. Cell Dev. Biol. 7:86. doi: 10.3389/fcell.2019.00086
Received: 16 December 2018; Accepted: 03 May 2019;
Published: 05 July 2019.
Edited by:
Hasan Korkaya, Augusta University, United StatesReviewed by:
Christophe Ginestier, INSERM U1068 Centre de Recherche en Cancérologie de Marseille, FranceRuby Yun-Ju Huang, National Taiwan University, Taiwan
Copyright © 2019 Nallanthighal, Heiserman and Cheon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong-Joo Cheon, cheond@mail.amc.edu
 Sameera Nallanthighal
Sameera Nallanthighal  Dong-Joo Cheon
Dong-Joo Cheon