-
PDF
- Split View
-
Views
-
Cite
Cite
Raphaëlle Luisier, Harri Lempiäinen, Nina Scherbichler, Albert Braeuning, Miriam Geissler, Valerie Dubost, Arne Müller, Nico Scheer, Salah-Dine Chibout, Hisanori Hara, Frank Picard, Diethilde Theil, Philippe Couttet, Antonio Vitobello, Olivier Grenet, Bettina Grasl-Kraupp, Heidrun Ellinger-Ziegelbauer, John P. Thomson, Richard R. Meehan, Clifford R. Elcombe, Colin J. Henderson, C. Roland Wolf, Michael Schwarz, Pierre Moulin, Rémi Terranova, Jonathan G. Moggs, Phenobarbital Induces Cell Cycle Transcriptional Responses in Mouse Liver Humanized for Constitutive Androstane and Pregnane X Receptors, Toxicological Sciences, Volume 139, Issue 2, June 2014, Pages 501–511, https://doi.org/10.1093/toxsci/kfu038
Close - Share Icon Share
Abstract
The constitutive androstane receptor (CAR) and the pregnane X receptor (PXR) are closely related nuclear receptors involved in drug metabolism and play important roles in the mechanism of phenobarbital (PB)-induced rodent nongenotoxic hepatocarcinogenesis. Here, we have used a humanized CAR/PXR mouse model to examine potential species differences in receptor-dependent mechanisms underlying liver tissue molecular responses to PB. Early and late transcriptomic responses to sustained PB exposure were investigated in liver tissue from double knock-out CAR and PXR (CARKO-PXRKO), double humanized CAR and PXR (CARh-PXRh), and wild-type C57BL/6 mice. Wild-type and CARh-PXRh mouse livers exhibited temporally and quantitatively similar transcriptional responses during 91 days of PB exposure including the sustained induction of the xenobiotic response gene Cyp2b10, the Wnt signaling inhibitor Wisp1, and noncoding RNA biomarkers from the Dlk1-Dio3 locus. Transient induction of DNA replication (Hells, Mcm6, and Esco2) and mitotic genes (Ccnb2, Cdc20, and Cdk1) and the proliferation-related nuclear antigen Mki67 were observed with peak expression occurring between 1 and 7 days PB exposure. All these transcriptional responses were absent in CARKO-PXRKO mouse livers and largely reversible in wild-type and CARh-PXRh mouse livers following 91 days of PB exposure and a subsequent 4-week recovery period. Furthermore, PB-mediated upregulation of the noncoding RNA Meg3, which has recently been associated with cellular pluripotency, exhibited a similar dose response and perivenous hepatocyte-specific localization in both wild-type and CARh-PXRh mice. Thus, mouse livers coexpressing human CAR and PXR support both the xenobiotic metabolizing and the proliferative transcriptional responses following exposure to PB.
Phenobarbital (PB), an anticonvulsant commonly used for treatment of epilepsy and other seizures, promotes both spontaneous and chemically induced liver tumors in rodents (Becker, 1982; Whysner et al., 1996) and has been widely used as a model compound for studying molecular mechanisms underlying rodent nongenotoxic hepatocarcinogenesis (Elcombe et al., 2014; Lempiainen et al., 2011; Phillips et al., 2009a,b; Ross et al., 2010; Thomson et al., 2013; Yamamoto et al., 2004). Murine liver tumor promotion by PB is dependent on the constitutive androstane receptor (CAR) and β-catenin (Huang et al., 2005; Rignall et al., 2011; Yamamoto et al., 2004), and prolonged PB treatment selects for Ctnnb1- (encoding β-catenin) mutated tumors (Aydinlik et al., 2001). CAR is required for the early PB-induced gene expression and DNA methylation changes that accompany murine hepatocyte hypertrophy and proliferative responses (Phillips et al., 2009a; Ross et al., 2010). PB regulates the nuclear localization of CAR (Kawamoto et al., 1999) through an indirect mechanism involving inhibition of epidermal growth factor receptor (EGFR) signaling (Mutoh et al., 2013). In addition to CAR, PB also activates the pregnane X receptor (PXR) (Lehmann et al., 1998), which has overlapping functions with CAR to regulate xenobiotic metabolism and detoxification in liver (Tolson and Wang, 2010), and whose coactivation may enhance CAR-mediated hepatocyte proliferation (Shizu et al., 2013).
An increased understanding of mechanisms underlying chemical carcinogenesis has raised doubts regarding the appropriateness of extrapolating some rodent tumor findings to humans (Holsapple et al., 2006). Based on epidemiological data in epileptics, there is no evidence of a specific role of PB in human liver cancer risk (IARC, 2001; Lamminpaa et al., 2002; La Vecchia and Negri, 2013; Whysner et al., 1996). Although prolonged treatment with PB does increase liver size in humans (Pirttiaho et al., 1982), human hepatocytes are resistant to the ability of PB to increase cell proliferation (Hasmall and Roberts, 1999; Parzefall et al., 1991) and inhibit apoptosis (Hasmall and Roberts, 1999).
The development of humanized mouse models provides a powerful approach for understanding pathways of human disease and to improve paradigms for the development of new drugs (Scheer et al., 2013; Scheer and Wolf, 2013). They also have very significant potential in drug safety testing particularly in the light of the significant species differences in metabolism and toxicological response. Because the process of rodent nongenotoxic carcinogenesis is often mediated by nuclear receptors including CAR and PXR, humanized mouse models in which the endogenous mouse CAR/PXR genes have been replaced with human CAR/PXR genes have been used to explore the species specificity of PB-mediated hepatocellular responses. Humanized C57BL/6 CAR/PXR mice displayed induction of cytochrome P450's and hepatocellular hypertrophy but did not show hepatocyte proliferation following acute exposure to PB (Ross et al., 2010). In contrast, PB-induced human CAR activation has been suggested to be associated with hepatocyte proliferation in an independent transgenic mouse model (Huang et al., 2005). In this study, we have used a humanized (CARh-PXRh) mouse model to examine potential species differences in receptor-dependent mechanisms underlying both early- and longer-term liver tissue transcriptomic responses to PB. We find that PB induces highly similar hepatic transcriptional programs in both wild-type and humanized CAR/PXR mice. This transcriptional response includes the upregulation of cell cycle genes, of the proliferative marker mKi67 and of Dlk1-Dio3 locus noncoding RNAs that have recently been associated with cellular pluripotency (Lempiainen et al., 2013; Stadtfeld et al., 2010). These findings are discussed in the context of using humanized nuclear receptor mouse models to explore the human relevance of rodent nongenotoxic carcinogens.
MATERIALS AND METHODS
Ethics statement
The wild-type, null, and humanized CAR/PXR mouse 13-week time course study was performed in conformity with the Swiss Animal Welfare Law (specifically under the Animal License No. 2345 by Kantonales Veterinäramt Basel-Stadt (Cantonal Veterinary Office, Basel). The wild-type and humanized CAR/PXR mouse 4-week dose response study (Figs. 3B and 3C) was performed following University of Tübingen institutional guidelines.
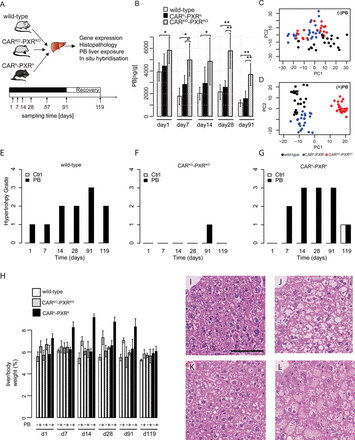
(A) Experimental design of the kinetic phenobarbital study for molecular and phenotypic profiling. Male C57BL6 wild-type, CARKO-PXRKO, and CARh-PXRh mice were given ad libitum access to PB or vehicle (drinking water). Mice were sacrificed at indicated time points, livers were sampled and aliquots used for genome-wide gene expression (mRNA and miRNA), phenobarbital exposure, histopathology, and localization studies. (B) Effect of CAR/PXR KO and humanization on PB liver concentration in wild-type (gray bars), CAR/PXR humanized (black bars), and CAR/PXR KO (striped bars) male mice as obtained by LC-MS/MS. PB liver concentration (ng/g) is shown as a mean ± SD (n = 5 animals per group). Statistical significance is indicated by * (p-value < 0.05; Student's t-test), and ** (p-value < 0.01; Student's t-test). (C) PCA analysis performed on expression data from nontreated control samples, which cannot discriminate CARKO-PXRKO from CARh-PXRh, whereas PC2 discriminates wild-type from humanized and knock-out animals. (D) PCA analysis performed on expression data from treated samples allowed to discriminate the samples from the three strains: as PC1 discriminates CARKO-PXRKO treated samples from wild-type and CARh-PXRh treated samples, PC2 discriminates CARh-PXRh treated samples from wild-type, and CARKO-PXRKO treated samples. (E–G) Effect of phenobarbital on hypertrophy grade in wild-type, CARKO-PXRKO, and CARh-PXRh respectively over time. Severity grades were on a 1–4 scale and are expressed as median (n = 5). (H) Liver per body weight ratios (%) following PB treatment in wild-type, CARKO-PXRKO, and CARh-PXRh over time. Liver per body weight ratios are shown as a mean ± SD (n = 5 animals per group). (I–L) Hematoxylin and eosin (H&E) staining on liver sections from vehicle-treated WT (I) and PB-treated WT (J), PXRKO-CARKO (K), and PXRh-CARh (L) mice at day 91. Black bar: 100 μm.
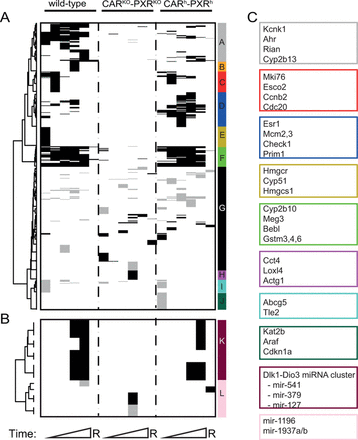
Summary of PB-mediated differential mRNA (A) and miRNA (B) expression analysis in wild-type, CAR/PXR knock-out, and human CAR/PXR knock-in animals over time. Black horizontal bars = significantly upregulated transcripts; gray horizontal bars = significantly downregulated transcripts; white color indicates unchanged transcripts. A transcript is considered significantly up- or downregulated if |$|\log _2 {\rm FC}| > 0.53$| (corresponding to a FC > 1.5) and B.H. corrected p-value <0.01. Genes were hierarchically clustered by (1) computing Euclidean distance between genes from decision matrix and (2) applying Ward clustering algorithm. Only transcripts that are differentially expressed in at least one time point and in at least one mouse strain were included in the clustering analysis. Representative transcripts from each cluster are shown alongside the vertical color-coded cluster bars (A–L).
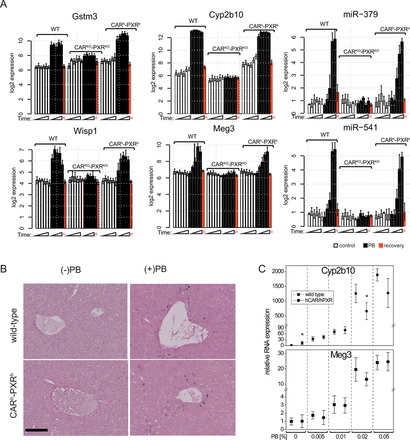
PB mediates similar transcriptional changes in wild-type, CAR/PXR knock-out, and human CAR/PXR knock-in animals. (A) Expression of Gstm3, Wisp1, Cyp2b10, Meg3, miR-541, and miR-379 transcripts in control (open bars), treated (black bars) and recovery time point (red bars) male mice as determined using microarrays. Gene expression (log2) is given as a mean ± SD (n = 3–5 animals per group). (B) Expression and localization of Meg3 in the liver. ISH of Meg3 transcript (blue staining) in control (28 days) and PB-treated (28 days, and 0.05% (wt/vol)) livers from wild-type and humanized mice. Black bar = 100 μm. (C) Expression of Cyp2b10 and Meg3 obtained by RT-PCR in CARh-PXRh mice and wild type controls, exposed to different concentrations of PB via the drinking water for 28 days. Mean ± SD (n = 4 mice per group) is shown relative to 18S rRNA expression. Statistically significant differences in PB-induced gene expression change between wild-type and CARh-PXRh genotypes at a given dose are indicated by asterisks (p < 0.05; Student's t-test).
Animal treatment and sample preparation
C57BL/6 male wild-type, knock-out CARKO-PXRKO and humanized CARh-PXRh mice (Scheer et al., 2008,2010) were obtained from TaconicArtemis (Germany). For the 13-week time course study, 9–11 week-old mice (age selected to avoid the confounding effect of liver maturation observed in younger animals) were allowed to acclimatize for 5 days prior to being randomly divided into two treatment groups (n = 5 per time point). Phenobarbital (PB; free acid, >99.0%, Sigma, St Louis, MO, no. 04710, 0.05% (wt/vol) in drinking water) was administered to one group through ad libitum access to drinking water, as previously reported (Phillips et al., 2009b; Lempiainen et al., 2013; Thomson et al., 2013). Mice were checked daily for activity and behavior and sacrificed on the indicated dates. For pharmacokinetics analysis, animals were anesthetized by inhalation of Isoflurane/O2 and blood was taken from the orbital plexus in tubes containing EDTA. Blood samples were centrifuged at 3000 × g, plasma removed carefully from the EDTA containing tubes, snap frozen and stored at −80°C. For liver histopathology, one middle section of the median lobe (∼4–5 mm) was sampled, fixed in 10% neutral-buffered formalin for 48 h, processed, embedded in paraffin, and stained with hematoxylin/eosin. For molecular profiling, all remaining parts of the liver (incl. left and caudal parts) as well as the remaining median part of the liver were sampled. To ensure sample homogeneity for different molecular profiling methods, frozen liver samples were reduced to powder with CovarisCryoprep (Covaris Inc., Woburn, MA) system and aliquoted on dry ice. For the 4-week dose response study, mice (n = 4 per group) were treated with PB starting at 8 weeks of age. PB was administered via the drinking water (PB solution prepared freshly every fourth day) at concentrations of 0.005, 0.01, 0.02, and 0.05% (wt/vol). Mice were kept on a 12 h dark/light cycle and had accessed to food and water ad libitum. All animals were sacrificed between 9 and 11 a.m. to avoid circadian influences.
Phenobarbital exposure analysis in blood and liver tissue
Measurement of PB concentrations in plasma and liver were performed by Liquid Chromatography-Mass Spectrometry/Mass Spectometry (LC-MS/MS). At each necropsy time point, ∼0.2 ml plasma was sampled. Each pulverized liver sample, ca. 100 mg/tube, was diluted by adding 900 μl saline and mixed thoroughly to generate a homogenate. Twenty microliters of the homogenate was subjected to protein precipitation with 200 μl of internal standard solution (methanol) and 200 μl of 1:1 of methanol and acetonitrile. The supernatant after centrifugation was diluted by 20% of methanol and subjected onto LC-MS/MS determination. The separation on C18 column (Venusil ASB C18) was achieved by a gradient with 100% of H2O and 100% of methanol with negative ion detection by turbo ion spray (API4000, Applied Biosystems). Data acquisition and peak integration were performed with software Analyst version 1.5.1 (Applied Biosystems). Student's t-test was used for statistical analysis of PB liver and plasma exposure. Differences were considered significant when p-value <0.05.
RNA isolation
Frozen liver samples were homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA) and subsequently purified on a silica-gel-based-membrane (RNeasy, Qiagen, Venlo, Netherlands) according to the manufacturer's instructions. RNA quality was assessed by measuring the RIN (RNA Integrity Number) using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). RNA was stored at −80°C. miRNA was quantified using the Rediplate Ribogreen RNA quantitation kit (Life Technologies).
Affymetrix mRNA and microRNA GeneChip processing and gene expression data analysis
Affymetrix GeneChip Mouse Genome 430 2.0 arrays were used to profile mRNA expression in liver tissue of wild-type and transgenic mice (+/− PB) exposure. Affymetrix GeneChip miRNA 2.0 arrays with coverage of miRBase version 15 were used to profile miRNA expression in liver tissue of same samples. The array was able to detect the expression of 722 mouse mature miRNAs and 690 mouse pre-miRNA. Five biological replicates were used for each treatment group. Processing of GeneChip experiments was conducted as recommended by the manufacturer of the GeneChip system (Affymetrix, Santa Clara, CA).
All the analyses were performed with the R statistical package version 2.13 (2005) and Bioconductor libraries version 1.4.7 (R Core Team, 2013). Affymetrix CEL files were normalized using the Robust Multichip Average (RMA) implementation of the algorithm available in R/Bioconductor (R Core Team, 2013). Quality metrics including scaling factors, average intensities, background intensities, RNA degradation, and raw Q values obtained from arrays prior and after normalization using Bioconductor's array QualityMetrics package (Kauffmann et al., 2009) were within acceptable limits except for six chips which were removed from subsequent analyses. Affymetrix microRNA chips were preprocessed and normalized according to the Affymetrix miRNA QCTool manual. Briefly, the background control probes of the chips were grouped into bins of same dinucleotide Guanine-Cytosine (GC) content. The median signal of the background bin that matches with the GC content of the probe was then subtracted from the probe signal. The background corrected probes (for all probes on the chip including those of other species) were quantile normalized across chips, log2 transformed and summarized into probe sets with the median polish method as in standard RMA (Bolstad et al., 2003). We floored all normalized signal values to 1.0.
Principal components analysis (PCA) was performed on the entire mRNA transcriptomic data set in order to characterize the overall structure of the data and identify major sources of gene expression variation. Independent PCA analyses were then performed on expression data from PB-treated and nontreated control samples in order to evaluate mouse genetic background effects (wild-type, CARKO-PXRKO, and CARh-PXRh) on PB treatment. Before statistical analysis, the transcriptomic data was filtered to remove 30% probes with lowest variation across samples and miRNA data was filtered to select for mouse probe-sets only. A two-way ANOVA model (treatment, time, and strain) was independently fitted to mRNA and miRNA data to assess statistical significance and linear contrasts at each time point using the Bioconductor's Limma package, which uses a Bayesian approach to better estimate the variance (Gentleman et al., 2004). The Benjamini-Hochberg method was applied to correct for multiple comparisons (Benjamini and Hochberg, 1995). Probe sets with B.H. (Benjamini and Hochberg) corrected p-values <0.01 and absolute log2 fold changes above 0.53 (fold change >1.5 or <0.69) were considered differentially expressed. For cluster analysis, Euclidean distance was used as a similarity measure and the Ward method for agglomerative hierarchical clustering.
Real-time RT-PCR analyses
Total RNA was reverse transcribed by avian myeloblastosis virus reverse transcriptase (Promega). Relative RNA expression levels were analyzed on a LightCycler system (Roche) using the Fast Start DNA MasterPLUS SYBR Green Kit (Roche) according to the manufacturer's instructions, with the following primers: 18S rRNA_forward 5′-CGGCTACCACATCCAAGGAA-3′, 18S rRNA_reverse 5′-GCTGGAATTACCGCGGCT-3′, Cyp2b10_forward 5′-TACTCCTATTCCATGTCTCCAAA-3′, Cyp2b10_reverse 5′-TCCAGAAGTCTCTTTTCACATGT-3′, Meg3_forward 5′-GTCTTCCTGTGCCATTTGCT-3′, Meg3_reverse 5′-TTCATCAGTCAGTAGGTGGGTCT-3′. Gene expression values were calculated based on the crossing point differences and PCR efficiencies using the Pfaffl method (Pfaffl, 2001). 18S rRNA expression was used for normalization.
In situ hybridization and immunohistochemistry
In situ hybridization (ISH) and immunohistochemistry (IHC) analyses were conducted as described in the Supplementary Materials and Methods.
RESULTS
Similar PB Exposure and Hepatocellular Hypertrophic Responses in Wild-Type and Humanized CAR/PXR Mice
To explore potential species differences in CAR and PXR receptor-dependent mechanisms underlying liver tissue molecular responses to PB, a kinetic study (1, 7, 14, 28, and 91 days of treatment and a 4-week recovery group) was run in C57BL/6 wild-type, CARKO-PXRKO, and CARh-PXRh mice with ad libitum PB (0.05% wt/vol in drinking water) administration (Fig. 1A). Phenobarbital concentrations in plasma and liver as determined by liquid chromatography-mass spectrometry are shown in Figure 1B and Supplementary table 1. A significantly elevated PB plasma concentration was observed in all CARKO-PXRKO mice compared with wild-type and CARh-PXRh strains that may reflect a deficiency of their livers to metabolize PB (Fig. 1B). Similar PB liver concentrations that decreased after day 1 were observed in both wild-type and CARh-PXRh mice indicating similar PB-induced metabolic activities in these two strains. The expression of mouse CAR and PXR transcripts was only detected in wild-type mouse livers (Supplementary fig. 4) consistent with the distinct nuclear receptor genotypes of CARKO-PXRKO and CARh-PXRh mice (Ross et al., 2010).
The most striking PB-induced histopathological change observed in both wild-type and humanized CAR/PXR mice was hepatocellular hypertrophy starting from 7 days of PB treatment and increased in severity at later time points (Figs. 1E–L). Hypertrophy was primarily detected at perivenous hepatocytes in the central zone of the lobule (zone III) (Figs. 1I–L). PB did not induce hepatocellular hypertrophy in CARKO-PXRKO mice (Figs. 1F and 1K) nor increase in liver to body weight ratio, consistent with previous reports (Huang et al., 2005; Ross et al., 2010). Very few mitotic figures were observed in any control or PB-treated animals. Hypertrophy evaluated by histopathology correlated well with increase in relative liver weight (Fig. 1H). Of note, PB induced higher grade of hepatocellular hypertrophy as well as higher relative liver weight in CARh-PXRh animals compared with wild-type animals.
Distinct Transcriptional Patterns are Observed in Wild-Type, Null CAR/PXR, and Humanized CAR/PXR Mouse Livers in the Absence of PB Exposure
In the absence of PB exposure, wild-type and CARh-PXRh mouse livers display some minor differences in their liver global transcriptome profiles including higher basal gene expression of Cyp2b10 in livers from humanized CAR/PXR mice compared with wild-type mice (Supplementary table 4). PCA analysis of transcriptomic data from nontreated control samples discriminates wild-type from CARh-PXRh and CARKO-PXRKO mouse livers (Fig. 1C). This was confirmed by differential gene expression analysis of genes in control wild-type, CARKO-PXRKO, and CARh-PXRh mouse livers (Supplementary fig. 1 and Supplementary table 4) and is presumably due to unique constitutive hCAR and hPXR interactions with endogenous mouse gene regulatory proteins and gene regulatory DNA sequences. Additional factors that may contribute to these observed differences include mouse substrain genetic differences (the PXR/CAR double knock-out line is ∼61% C57Bl/6J and 39% C57Bl/6N; the double humanized CAR/PXR line is ∼78% C57Bl/6J and 22% C57Bl/6N; the wild-type comparator control mice were ∼100% C57Bl/6J) (Scheer, TaconicArtemis, personal communication) and/or transgene-mediated perturbation of mouse hepatocyte chromatin architecture.
Mouse and Human CAR/PXR Mediate Similar and Reversible PB-Induced Mouse Liver Transcriptional Responses
PB-exposed wild-type and CARh-PXRh mouse livers display distinct liver global transcriptome profiles compared with CARKO-PXRKO mouse livers (Fig. 1D), consistent with PB predominantly inducing CAR- and PXR-mediated transcriptional responses (Phillips et al., 2009a; Ueda et al., 2002). Some degree of difference in PB-induced global liver transcriptional responses between wild-type and CARh-PXRh mice was indicated by principal component 2 (PC2) discrimination within the PCA analysis (Fig. 1D). To further explore potential similarities and differences between mouse and human CAR/PXR-mediated regulation of hepatic genes upon PB-treatment, we compared mRNA and miRNA expression levels in livers from PB-treated wild-type, CARKO-PXRKO, and CARh-PXRh mice with their respective time-matched control samples. The differential expression of mRNAs and miRNAs in control wild-type, CARKO-PXRKO and CARh-PXRh mouse livers is summarized in Figures 2A and 2B and a detailed gene list for the corresponding clusters (A–L) is provided in Supplementary table 5. The most significantly upregulated genes in wild-type C57BL/6 mice upon PB-treatment included Cyp2b10, Gstm3, Wisp1, Meig1, Abcc4, Cyp2b9, Cyp2c37, Prom1, and Gadd45b (see Fig. 3A and Supplementary table 5) consistent with previous observations in PB-treated B6C3F1 mice (Lempiainen et al., 2011, 2013; Phillips et al., 2009a,b; Thomson et al., 2013). Moreover, Meg3 noncoding RNA and miRNAs associated with the Dlk1-Dio3 imprinted cluster (miR-541 and miR-379), that have recently been associated with cellular pluripotency and proposed as novel candidate biomarkers for mouse liver nongenotoxic carcinogenesis (Lempiainen et al., 2013), were also progressively upregulated upon PB-treatment (Figs. 2B and 3A). Importantly, similar quantitative and temporal responses for these PB-mediated molecular changes were also observed in humanized CAR/PXR mice whilst being absent in CAR/PXR null mice (Figs. 2B and 3A). Humanized CAR/PXR mice akin to wild-type mice also supported the PB-mediated induction of Kcnk1, a male-specific CAR-dependent transcriptional response to PB that has been associated with the attenuation of hepatic hyperplasia Saito et al., 2013 . PB-induced Meg3 induction in wild-type and humanized CAR/PXR mice was also confirmed to exhibit a very similar localization to perivenous hepatocytes in the central zone of the lobule (Fig. 3B).
The quantitative similarity between PB-mediated hepatic induction of Cyp2b10 and Meg3 in wild-type and CARh-PXRh mice was further explored in a 4-week dose response study (Fig. 3C). Humanized CAR/PXR and wild-type mice of 8 weeks age were given four different concentrations of PB ranging from 0.005 to 0.05% (wt/vol) in drinking water. Both Meg3 and Cyp2b10 gene expression increased in a dose-dependent manner and appeared to reach a plateau at around 0.02% suggesting saturation (Fig. 3C).
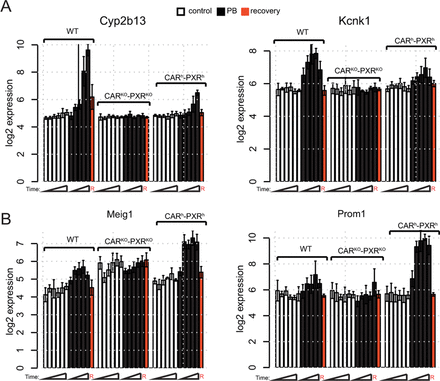
Some differences in the magnitude and timing of liver transcriptional responses were observed between humanized CAR/PXR and wild-type mice. One group of PB-induced gene expression changes was observed to be more prominent in wild-type than CARh-PXRh mice (see Fig. 4A for examples and Supplementary table 6 for a detailed list of genes which exhibit significant differences in PB-mediated gene induction between humanized and wild-type mice in at least one time point) and an additional distinct group of PB-induced gene expression changes was observed to be more prominent in CARh-PXRh than the wild-type mice (e.g., see Fig. 4B). To assess whether PB exposure differences might contribute to the observed differential PB-induced expression of these genes, we performed linear modeling between gene expression and liver exposure but did not observe any significant effects (Supplementary fig. 2).
Temporal and quantitative differences in PB-mediated transcriptional responses in wild-type versus humanized CAR/PXR mouse livers. (A) PB-mediated gene induction of Cyp2b13 and Kcnk1 is more prominent in wild-type than humanized animals. (B) PB-mediated gene induction of Meig1 and Prom1 is more prominent in humanized animals than wild-type. Expression (log2) in control (open bars), treated (black bars), and recovery time point (red bars) male animals is given as mean ± SD (n = 3–5 animals per group).
To determine the potential reversibility of molecular responses induced by 13 weeks of PB treatment, microarray experiments using liver samples from 13-week PB-treated mice followed by 4 weeks recovery (wild-type, CARKO-PXRKO, and CARh-PXRh) were compared with liver samples of nontreated mice from the same strains at the 119-day time point. Differential gene expression analyses revealed that only six genes (representing 1% of differentially expressed genes over time) maintained a qualitatively consistent residual differential expression in humanized and/or wild-type mice (Serpinb1a, Nebl, Cyp2b13, Gna14, Gm20265, and AI132709) after 4 weeks of recovery (see Supplementary table 7 for complete gene list) suggesting either residual CAR/PXR activity associated with positive gene regulation after removal of PB treatment, longer-term stability of this subset of PB-induced mRNAs in mouse liver or long-lasting epigenetic changes associated with their gene regulatory regions. Importantly, the 119-day expression levels for all of the above residual differentially expressed genes was significantly lower than expression levels observed following 91 days of PB treatment suggesting a slow return to normal basal gene expression levels. A small number of genes were uniquely differentially expressed at the recovery time point in humanized or knock-out mice (Supplementary table 7), including a number of inflammatory genes.
Human CAR and PXR Support Mouse Liver Transcriptional Upregulation of DNA Replication, Cell Cycle and Mitotic Genes upon PB Exposure
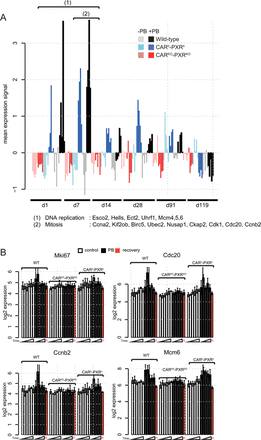
Further analysis of the different clusters associated with PB-mediated differential expression (Fig. 2A) revealed a transient CAR/PXR-dependent cell cycle response after 1 and 7 days of PB treatment characterized by upregulation of genes associated with DNA replication, cell cycle and mitosis (Supplementary tables 2 and 3; Figs. 5A and 5B). Importantly, this PB-mediated cell cycle transcriptional response was also observed in humanized CAR/PXR mice whilst being absent in CAR/PXR null mice. Further analysis of gene functions associated with this cluster by mapping to cell cycle phases suggests that PB supports all phases of cell cycle progression at the transcriptional level from S-phase entry to cytokinesis (Fig. 6).
PB mediates cell cycle transcriptional responses in wild-type and humanized CAR/PXR mouse livers. (A) Median expression per animal of a cluster of genes enriched for DNA replication and cell cycle functions that were differentially expressed upon PB treatment between day 1 and day 7 (cluster C from Fig. 2A). PB-mediated entry in S-phase after one day is supported by a subset of DNA replication genes significantly upregulated from day 1 (1) whereas mitosis and cytokinesis are supported by a subset of cell cycle regulatory genes significantly upregulated around day 7 (2). (B) Expression of Mki67, Cdc20, Ccn20, and Mcm6 determined using microarrays. Expression in control (open bars), treated (black bars) and recovery time point male mice is given as mean ± SD (n = 3–5 animals per group).
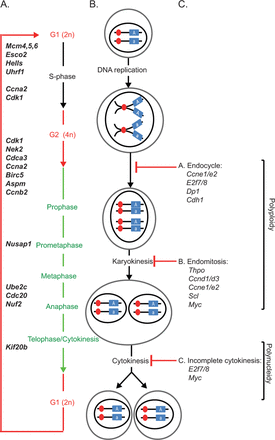
Mouse and human CAR/PXR mediate similar PB-induced upregulation of genes driving both entry into S-phase and progression to cytokinesis in wild-type and humanized mice. (A) Schematic representation of different cell cycle stages; a selection of genes which are both reported to regulate the different stages and are significantly upregulated upon PB treatment between day 1 and day 3 of PB treatment is shown. (B) Schematic representation of DNA content along the different cell cycle stages. (C) Polyploidy or polynucleidy can result from incomplete cytokinesis, endocycle, or endomitosis that are likely regulated by genes reviewed in Pandit et al. (2013). Genes in bold-italic in panel (A) are differentially upregulated between day 1 and day 7 upon PB treatment in wild-type animals and CARh-PXRh, but not in CARKO-PXRKO, consistent with a PB-induced hepatic proliferative transcriptional response. No PB-induced transcriptional responses were observed in either wild-type animals or CARh-PXRh for genes associated with polyploidy or polynucleidy in panel (C).
DISCUSSION
This study demonstrates for the first time that mouse livers expressing only the human versions of CAR and PXR can support PB-induced xenobiotic and proliferative responses at the transcriptional level. Wild-type and CARh-PXRh mouse livers exhibited temporally and quantitatively similar transcriptional responses during 91 days of PB exposure including the sustained induction of the xenobiotic response gene Cyp2b10, the Wnt signaling inhibitor Wisp1 and noncoding RNA biomarkers from the Dlk1-Dio3 locus (Lempiainen et al., 2013). Importantly, mouse livers expressing human CAR and PXR also supported PB-mediated transient DNA replication (Hells, Mcm6, and Esco2), cell cycle (Ccnb2, Cdc20, and Cdk1) and proliferation-related nuclear antigen Mki67 transcriptional responses consistent with hepatocyte proliferation. Our data are consistent with a previous report that PB (0.05%; 1 week) induced human CAR activation in a transgenic mouse model associated with hepatocyte DNA replication based on increased ploidy and PCNA protein expression (Huang et al., 2005). Another study in humanized C57BL/6 CAR/PXR mice concluded that PB-induced cytochrome P450's and hepatocellular hypertrophy but not hepatocyte DNA replication (based on BrdU incorporation) following acute exposure to PB (4 days; ip 80 mg/kg) (Ross et al., 2010). However, a more recent follow-up 7-day study using an alternate dietary route of administration to generate higher systemic exposure revealed that CARh-PXRh mice can support PB-mediated DNA replication (based on BrdU incorporation) although at higher systemic exposures relative to wild-type mice (Elcombe, personal communication). The measurement of PB-induced mouse liver proliferation is confounded by changes in a heterogenous liver parenchymal cell population that include both mononuclear and binucleated cells containing multiples of the diploid component of DNA (known as polyploidy) (Bohm and Noltemeyer, 1981; Bursch et al., 2004; Gonzales et al., 1998) that may result from incomplete cytokinesis, endoreplication, or a combination of both (Gentric et al., 2012a,b; Styles, 1993). Thus, increase in either Ki67 mRNA expression, Ki-67/PCNA IHC staining, or BrdU incorporation may reflect the increased DNA content of a polyploid hepatocyte rather than DNA replication associated with complete cell division cycles and hyperplasia. However, our observation of progressive PB-induction of genes that drive S-phase, mitosis, and cytokinesis in wild-type and humanized CAR/PXR mouse livers (Fig. 6) supports a proliferative response. Furthermore, we did not observe PB-mediated changes in the expression of genes implicated in driving polyploidization of hepatocytes such as E2f7/8 and c-Myc (see Supplementary fig. 3) (Pandit et al., 2012, 2013).
Species differences in CAR activation by direct ligands such as the human CAR-selective CITCO (Auerbach et al., 2005) and mouse CAR-selective TCPOBOP (Nims et al., 1993) are thought to be in part due to differences in ligand-binding domain protein structure. Murine and human CAR and PXR ligand binding domains share unusually low amino acid sequence conservation for nuclear receptor orthologs (Moore et al., 2002) which may account for differences in respective receptor activities upon PB exposure, as the ligand-binding domain indirectly affects other receptor functions such as dimerization, ligand binding, interaction with heat shock proteins, nuclear localization, and transactivation functions. Indeed, structural differences in human CAR versus mouse CAR translated into structure-activity differences in the ability of different ligands to activate or deactivate CAR in comparative assays (Moore et al., 2000). In contrast to the species differences observed for some CAR ligands, our data suggest that the indirect activation of both mouse and human CAR by PB leads to very similar hepatic xenobiotic and proliferative transcriptional responses in a C57BL/6 mouse genetic background. Although we did not find any compelling evidence for PB-mediated transcriptional responses that were specific to humanized CAR/PXR mice, multiple genes (e.g., Meig1, Prom1, Knck1, Cyp2b13) were observed to exhibit quantitatively distinct transcriptional responses to PB in CARh-PXRh versus wild-type mice and these did not appear to be driven by differences in liver PB exposure. These quantitative differences are thus likely to reflect target gene regulatory sequence and/or chromatin structural differences in mouse CAR- and human CAR-mediated DNA binding and recruitment of mouse liver transcriptional coregulatory proteins. Consistent with this notion, a precedent for species-specific transcription factor interactions with their target genes has recently been reported (Soccio et al., 2011).
Our data have important implications for the utility of humanized CAR/PXR mouse models in human nongenotoxic carcinogenesis risk assessment. Although the CARh-PXRh mice used in our studies express human CAR splice variants 1–3 in a ratio that closely resembles human liver CAR expression profile and is unchanged by exposure to PB (Ross et al., 2010), one caveat of this model is that human CAR and PXR receptors function in the context of mouse target gene regulatory elements and chromatin structure. Previous work has shown that PB induces extensive changes in DNA and histone modification patterns across the regulatory regions of CAR target genes in mouse liver (Lempiainen et al., 2011; Phillips and Goodman, 2009; Thomson et al., 2012, 2013), and species-specific differences in these epigenetic perturbations may also play an important role in determining susceptibility to PB-mediated hepatocarcinogenesis. Further species differences in CAR signaling might also be conferred via the recently described PB-EGFR signaling pathway interactions (Mutoh et al., 2013).
Based on a weight of evidence human relevance framework concept focusing on mode of action and key events, PB-induced rodent nongenotoxic hepatocarcinogenesis is not considered to be a relevant mechanism for humans (Holsapple et al., 2006) and there is no evidence of a specific role of PB in human liver cancer risk based on epidemiological data in epileptics (IARC, 2001; Lamminpaa et al., 2002; La Vecchia and Negri, 2013; Whysner et al., 1996). The fact that CAR activation is a key event for PB-induced rodent liver tumor formation (Elcombe et al., 2014) suggests that the use of humanized CAR mouse models may more closely reflect human transcriptional responses and should be more predictive of human risk. However, our data suggest that humanized nuclear receptor mice may not be a simple model for extrapolating the risk of rodent tumor findings to humans. Understanding and using these models will require the careful integration of quantitative exposure-response relationships with the temporal and spatial dynamics of human nuclear receptor expression, mechanism of modulation by coactivators and further evaluation of the relevance of heterologous mouse-human gene regulatory protein interactions.
FUNDING
Innovative Medicine Initiative Joint Undertaking (IMI JU) (115001; MARCAR project; URL: http://www.imi-marcar.eu/);Novartis and Bayer Pharma; Cancer Research UK Programme (C4639/A12330 to C.R.W); MRC UK Core Programme (632RMERA1892 to R.R.M.).
All IMI-MARCAR consortium partners had a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We would like to thank Ulrich Längle and Andreas Mahl for in vivo study support. V.D., A.M., S-D.C., H.H., F.P., D.T., P.C., O.G., P.M., R.T., and J.G.M. are full time employees of Novartis Pharma AG. H.L. and A.V. are recipients of Novartis Institutes for Biomedical Research Postdoctoral Fellowships. N.S. is a full time employee of Taconic Artemis. H.E-Z. is a full time employee of Bayer. C.R.E. is a full time employee of CXR Biosciences.
REFERENCES




Comments