-
PDF
- Split View
-
Views
-
Cite
Cite
Izumi C. Mori, Reinhard Pinontoan, Tomonori Kawano, Shoshi Muto, Involvement of Superoxide Generation in Salicylic Acid-Induced Stomatal Closure in Vicia faba, Plant and Cell Physiology, Volume 42, Issue 12, 15 December 2001, Pages 1383–1388, https://doi.org/10.1093/pcp/pce176
Close - Share Icon Share
Abstract
Salicylic acid (SA), the known mediator of systemic acquired resistance, induced stomatal closure of Vicia faba L. Application of SA to the epidermal peels evoked an elevation of chemiluminescence of Cripridina lucigenin-derived chemiluminescent reagent (CLA) which is sensitive to superoxide anion (O2·–). The SA-induced generation of chemiluminescence was suppressed by O2·–-specific scavengers superoxide dismutase (SOD) and 4,5-dihydroxy-1,3-benzenedisulfonic acid (Tiron). These results suggest that O2·– was generated in epidermal peels by SA-treatment. A peroxidase inhibitor salicylhydroxamic acid (SHAM) inhibited guaiacol peroxidase activity and suppressed the SA-induced CLA chemiluminescence in the epidermal peels, suggesting that O2·– generation occurred by the peroxidase-catalyzed reaction as proposed for SA-treated tobacco cell suspension culture [Kawano et al. (1998)Plant Cell Physiol. 39: 721]. SOD, Tiron or SHAM suppressed the SA-induced stomatal closure. Moreover, application of superoxide-generating system also induced stomatal closure. These results support the concept of involvement of reactive oxygen species in signal transduction in SA-induced stomatal closure.
(Received April 23, 2001; Accepted October 5, 2001).
Introduction
Salicylic acid (SA) is a compound which mediates the induction of systemic acquired resistance (SAR) (Gaffney et al. 1993, Delaney et al. 1994). SAR is a series of defense responses elicited by a viral or fungal infection in tissues distal to the infection site. The local infection stimulus is converted to a signal(s) leading to an increase of SA in uninfected parts of the plant. The elevated concentration of SA activates expression of pathogenesis-related genes which results in a long lasting, broad specific resistance (Ward et al. 1991).
It has been well established that the action of SA is mediated through the production of reactive oxygen species (ROS). Chen et al. (1993) first proposed that the earliest event in SA signaling is inhibition of catalase by binding of SA and subsequent accumulation of H2O2 in tobacco. Recently we have reported that SA induces both extracellular generation of superoxide anion (O2·–) and an elevation in cytosolic free calcium ion concentration ([Ca2+]cyt) in tobacco cell suspension culture, and proposed that production of O2·– catalyzed by extracellular peroxidases, and the following increase in [Ca2+]cyt triggered by O2·–, may mediate the SA signal transduction (Kawano et al. 1998, Kawano and Muto 2000).
Pathogen attacks also cause stomatal closure, by which plants may limit further fungal invasion through stomata by restricting access to internal tissues. It has been reported that stomata close after application of SA (Manthe et al. 1992, Lee 1998) or elicitors (Lee et al. 1999) to epidermal peels. The mechanism responding to elicitors may be involved in resistance at the infection site, while the mechanism responding to SA may be important for preventing expanded infection in uninfected regions.
Stomata regulate the rates of transpiration and gas exchange in harmony with various environmental conditions. Numerous studies on transduction of cellular signals in stomatal guard cells have been reported for various stimuli including light, fusicoccin, and CO2 gas. However, little is known about the mechanism for recognition of such stimuli in guard cells. Biochemical studies showed the fusicoccin-receptor to be a 14-3-3 protein (Korthout and de Boer 1994, Marra et al. 1994) and a genetic study in Arabidopsis thaliana suggested that the blue light receptor may be zeaxanthin (Frechilla et al. 1999). Perception mechanisms for phytohormones, gases and other signals in guard cells have not been well documented.
In the present report, we show evidence for the involvement of peroxidase-mediated O2·– generation in the early step of signal transduction pathway in SA-induced stomatal closure in Vicia faba.
Results
SA induced stomatal closure
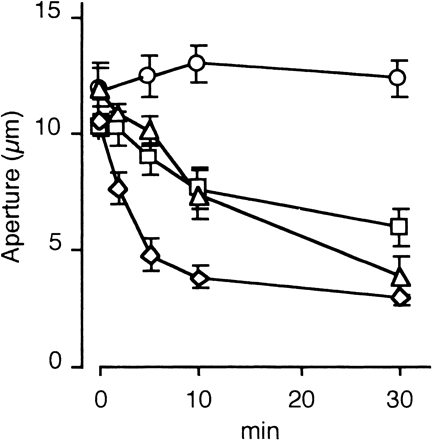
Effects of SA on fully opened stomata were examined (Fig. 1). Application of 2 mM SA to the epidermal peels caused stomatal closure by 54%, 64% and 82%, during incubation for 5, 10 and 30 min, respectively. Apertures were also reduced by 15%, 38% and 63% by incubating with 500 µM SA for 5, 10 and 30, and by 14%, 26% and 43% by 200 µM SA for 5, 10 and 30 min, respectively. Solvent control (0.5% ethanol) did not cause the reduction of stomatal aperture. These observations are essentially consistent with the findings in V. faba (Manthe et al. 1992) and Commelina communis (Lee 1998), showing SA-induced reduction of stomatal aperture.
SA induced biphasic O2·– production
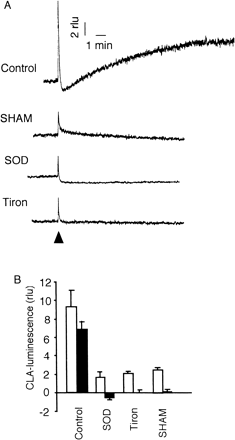
Immediately after application of 2 mM SA to epidermis, a sharp chemiluminescence spike of CLA (Cripridina lucigenin-derived chemiluminescent agent; 2-methyl-6-phenyl-3,7-dihydroimidazo[1,2-a]pyrazin-3-one) followed by a gradual prolonged increase was observed, suggesting that O2·– was generated biphasically (Fig. 2A). The first chemiluminescence spike peaked within 1 s. The chemiluminescence once decreased to a level below the resting level, and then increased gradually reaching a plateau at ~10 min. It is interesting that the time course of the increase of O2·– level seemed to parallel with the time course of the reduction of stomatal aperture. The increased level of luminescence was sustained for 30 min in typical traces (data not shown).
To confirm that the CLA-chemiluminescence reflects O2·– level, the effects of O2·–-specific scavengers on the SA-induced elevation of CLA-chemiluminescence were examined. When 330 units CuZn-superoxide dismutase (SOD) ml–1 was added to the medium, the first spike of the SA-induced CLA-luminescence was reduced by 80%, and the second prolonged phase was completely abolished. The height of the spike was reduced by 80% by treating with 5 mM 4,5-dihydroxy-1,3-benzenedisulfonic acid (Tiron), and second phase was not detected (Fig. 2B). These results strongly suggest that the prolonged phase is reflecting the increase in O2·– generation. The first spike is also likely to represent O2·– generation, since it was suppressed by 80% with the scavengers. We suppose that the O2·– generation rate of the first spike may be too quick to be completely suppressed by the scavengers.
Salicylhydroxyamic acid (SHAM, 5 mM), a peroxidase inhibitor also reduced the O2·– generation induced by SA to the same extent as reduced by Tiron and SOD (Fig. 2B), however, diphenylene iodonium (DPI, 5 µM), an inhibitor of NADPH oxidase had no effect (data not shown). This suggests that peroxidase but not NADPH oxidase is involved in the SA-induced O2·– generation. As SOD is not accessible to the intracellular space, the effect of SOD implies generation of O2·– in the extracellular space and involvement of cell wall-bound peroxidase(s).
Effects of O2·– scavengers, a peroxidase inhibitor and a Ca2+ chelator on SA-induced stomatal closure
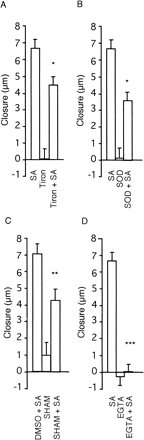
To evaluate the involvement of O2·– generation in SA-induced stomatal closure, the effects of scavengers on SA-induced stomatal closure were examined (Fig. 3A, B). The SA-induced stomatal closure was partially suppressed by both 5 mM Tiron (32%) and 330 units SOD ml–1 (45%), whereas the same concentrations of these scavengers did not affect the fully opened apertures in the absence of SA. This suggests the involvement of O2·– in the SA-induced stomatal closure.
The SA-induced stomatal closure was suppressed by 40% with SHAM (Fig. 3C), while DPI (5 µM) had no effect (data not shown), suggesting the involvement of peroxidase but not NADPH oxidase.
Addition of ethylene glycol-bis(β-aminoethyl ether) N,N,N′,N′-tetraacetic acid (EGTA), a Ca2+ chelator, to the incubation medium had no effect on the fully opened stomatal apertures, and completely suppressed the SA-induced stomatal closure (Fig. 3D). These results suggest that an influx of extracellular Ca2+ to guard cells is required for stomatal closure.
O2·– induced stomatal closure
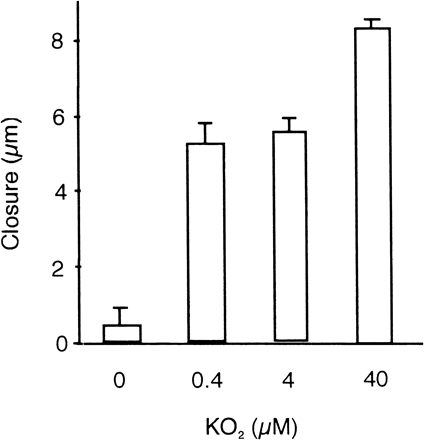
Stomatal aperture was reduced by the application of KO2 in a dose-dependent manner, whereas the solvent control dimethylsulfoxide (DMSO) did not cause a reduction of aperture (Fig. 4). Since KO2 immediately produces O2·– in water and its lifetime is very short, KO2 was dissolved in DMSO, dropped into the medium and stirred immediately, resulting in a rapid dissociation into O2·– and K+. This result implies that O2·– is a potent inducer of stomatal closure. Another artificial O2·–-generating system xanthine/xanthine oxidase was also examined, but unexpectedly, xanthine itself induced stomatal closure (data not shown) and thus this system is not suitable for this experiment.
GPX activity in epidermal peels
To confirm the existence of SHAM-sensitive peroxidase, guaiacol peroxidase (GPX) activity in the epidermal peels was examined. The GPX activities were 3.6±0.03 and 0.32±0.002 nmol min–1 (cm2 epidermal peel)–1 (n = 5) in the absence and presence of 5 mM SHAM, respectively, indicating that as much as 90% activity is sensitive to SHAM. After incubating for 10 min, both epidermal cells and guard cells of peels were stained brown color (not shown). The staining was prevented with 5 mM SHAM. These results indicate that SHAM-sensitive GPX is localized in both epidermal and guard cells, and thus O2·– is potentially generated in guard cells and peripheral region.
Discussion
Using tobacco cell suspension culture, we have proposed a model for the earliest events in SA signal transduction in plants, i.e. SA induces O2·– generation mediated by extracellular peroxidase, and then O2·– induces [Ca2+]cyt elevation (Kawano et al. 1998, Kawano and Muto 2000). The proposed mechanism of extracellular peroxidase-mediated O2·– generation is as follows:
GPX + H2O2 → Compound I + H2O (1)
Compound I + SA → SA· + Compound II (2)
Compound II + SA → SA· + GPX (3)
2 SA· + 2 O2 → 2 SA+ + 2 O2·– (4)
where GPX is the extracellularly secreted guaiacol peroxidase, and SA· and SA+ are the radical and the two-electron oxidized product of SA, respectively. The culture contains very low concentration of H2O2 produced by and leaked from cells (Kawano et al. 1998). The compound I of GPX can be reduced by SA to compound II. During the GPX-catalyzed reaction, SA and H2O2 may act as the donor and acceptor of electron exchange, respectively, finally yielding SA· which reduces O2 dissolved in water yielding O2·–. In addition, SA inhibits catalase activity in tobacco cell suspension culture and accumulates H2O2. Thus SA has a dual function, O2·– generation and H2O2 accumulation, in tobacco culture.
In the present study, we provided the evidence for generation of O2·– in SA signaling pathway leading to stomatal closure according to the similar reactions to tobacco suspension culture. Previously, Lee (1998) has suggested the generation of ROS, based on the observation that SA inhibits catalase, and its involvement in SA-induced stomatal closure in Commelina communis. He did not directly show the elevation of ROS, whereas we provided the evidence for O2·– generation by means of a combination of O2·–-sensitive chemiluminescent reagent and O2·–-specific scavengers. In our scheme, O2·– is generated in the extracellular space and the signal of O2·– leading to stomatal closure is transduced into the cells. As GPX exists in the epidermal and guard cells, O2·– could be generated in both cells when SA was supplied. We should note that ROS may also be provided by diffusion in a form of H2O2 from the cell walls of adjacent mesophyll and epidermal cells as well as those of guard cells. Allan and Fluhr (1997) have clearly shown that ROS elevation can migrate from one cell to the other cells via apoplastic route.
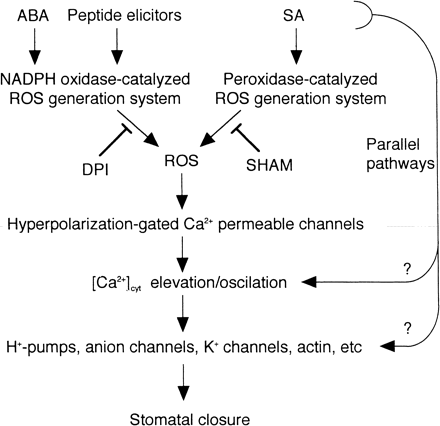
Pei et al. (2000) and Zhang et al. (2001) showed that ABA induced H2O2 production and the exogenously added H2O2 led stomatal closure in Arabidopsis and Vicia, respectively. The ABA-induced H2O2 production was inhibited by DPI, suggesting that it is NADPH oxidase-catalyzed O2·– generation followed by SOD-catalyzed dismutation of O2·– to H2O2. Pei et al. (2000) further showed the activation of a hyperpolarization-gated Ca2+ permeable channel by patch clamp technique. In V. faba, SA-induced [Ca2+]cyt elevation could not be examined due to technical difficulties in introducing aequorin into the cells. However, requirement of extracellular Ca2+ (Fig. 3D) suggests that [Ca2+]cyt elevation accompanies O2·– generation. By the analogy with tobacco cell suspension culture, SA signal transduction pathway leading to stomatal closure in Vicia epidermal peels and guard cells may be depicted as in Fig. 5. SA induces firstly O2·– generation mediated by SHAM-sensitive peroxidase, and subsequently the [Ca2+]cyt elevation achieved by activation of Ca2+ channels on the plasma membrane.
Similar to ROS generation systems in stomata, Allan and Fluhr (1997) have identified two distinguishable ROS generation mechanisms in tobacco epidermis in response to elicitors, i.e. intracellular DPI-sensitive (possibly NADPH oxidase-catalyzed) system for cryptogein, a fungal peptide elicitor, and cell wall-located enzyme (peroxidase and/or amine oxidase)-catalyzed system for amine elicitors.
SA-induced ROS generation was not detected by dichlorofluorescin, the conventional fluorescent ROS indicator (Allan and Fluhr 1997, Lee et al. 1999, Pei et al. 2000, Zhang et al. 2001), because somehow SA intercepted fluorescence of dichlorofluorescin (Allan and Fluhr 1997). Our findings using CLA, SOD and SHAM suggest that the cell wall enzyme-mediated system participates in the SA-induced ROS production, but the intracellular DPI-sensitive system does not, according to classification by Allan and Fluhr (1997). Irrespective of production mechanism, ROS is involved in stomatal closure induced by treatment of ABA (Pei et al. 2000, Zhang et al. 2001), fungal elicitor (Lee et al. 1999) and SA (Lee 1998, this study). There might be a common signal transduction pathway downstream of ROS in ABA-, fungal elicitor- and SA-induced stomatal closure (Fig. 5).
SHAM inhibits various peroxidases including plant GPXs, by binding to the haem pockets of the enzymes (Ikeda-Saito et al. 1991) and also inhibits the terminal oxidase in the alternative respiratory pathway in mitochondria (Chivasa et al. 1997). Involvement of alternative oxidase in SA-induced O2·– generation could be eliminated, since the experiments with exogenously added SOD clearly showed that O2·– generation is an extracellular event (Fig. 2B).
In contrast to the single transient observed in tobacco suspension culture (Kawano et al. 1998), biphasic O2·– generation was observed in Vicia epidermal peels. We consider the first O2·– generation peak represents the immediate consumption of pre-existing H2O2 by the peroxidase-dependent reaction initiated by the application of SA. The second peak may represent the increase in H2O2 resulted from the inhibition of catalase by SA, and the subsequent peroxidase-mediated generation of O2·–. A sudden increase in SA concentration is not likely to occur in vivo, there must be a gradual increase in SA concentration. Thus the second prolonged O2·– generation may represent the naturally occurring SA response in plants.
SOD and Tiron completely suppressed the SA-induced prolonged O2·– generation, and partially but significantly suppressed the spike of O2·– generation and stomatal closure. The incomplete inhibition of stomatal closure may be due to the remaining portion of the O2·– in the first and rapid phase of O2·– production. Another interpretation is that other signaling pathway(s) may function in parallel with ROS in SA signaling pathways (Fig. 5) and thus we observed the partial effects of the scavengers on the SA-induced stomatal closure.
In summary, ROS generation followed by a possible [Ca2+]cyt elevation is the early event in the SA-induced stomatal closure in V. faba and peroxidase plays an important role in this event.
Materials and Methods
Plant materials and measurement of stomatal aperture
Vicia faba cv. Otafukusanzu were grown as previously reported (Mori and Muto 1997). Abaxial epidermal peels (2×5 mm) were floated on 50 µl of 10 mM MES-KOH (pH 5.7) containing 50 mM KCl and 100 µM CaCl2 on a slide glass, and incubated under a fluorescent lamp at 100 µmol m–2 s–1 for 45 min. A piece of epidermal peel with fully opened stomata was set on the stage of a microscope. SA was applied to the epidermal peel, and stomatal apertures were recorded on a digital camera (Cool pix 950, Nikon, Tokyo, Japan), and the width of the aperture was analyzed with a graphic analysis software (NIH image 1.58, provided by Dr. Wayne Rasband, NIH, Atlanta, GA, U.S.A.).
Chemicals
CLA and Tiron were purchased from Tokyo Kasei Kogyo Co. (Tokyo, Japan). SOD and DPI were from Sigma (St Louis, MO, U.S.A.). SHAM and other chemicals were products of Wako Pure Chemical Industries (Osaka, Japan).
Detection of O2·–
Generation of O2·– was examined by monitoring the CLA-chemiluminescence, essentially as reported by Kawano et al. (1998). Chemiluminescence of CLA was monitored with a luminometer (CHEM-GLOW Photometer, American Instrument Co., MD, U.S.A.) and expressed as relative luminescence unit (rlu). The desired concentration of SA solution was prepared in 20% ethanol and 5 µl of this solution was applied with a syringe to peeled abaxial epidermis (4×30 mm) suspended in 200 µl of a medium consists of 20 µM CLA, 10 mM KCl, 0.1 mM CaCl2 and 5 mM MES-KOH (pH 5.7).
Determination of GPX activity
Abaxial epidermis peeled from 10×30 mm leaf section was washed with 5 ml of distilled water twice and incubated with 3 ml of reaction mixture containing 5 mM guaiacol, 2 mM H2O2 and 10 mM Na-phosphate buffer (pH 7.2) in the presence and absence of 5 mM SHAM at room temperature. Increase in A470 of the reaction mixture was measured every 1 min for 10 min. The activity was expressed as nmol tetraguaiacol (ε = 26.6 µmol–1 cm–1) formed min–1 (cm2 epidermal peel)–1.
Acknowledgements
This work was supported by Grant-in-Aid for Scientific Research (B) (no. 11440235) and for Special Research on Priority Areas (no. 13039008) from Ministry of Education, Science, Sports, Culture and Technology of Japan to S.M. I.C.M. and R.P. were supported by Research Fellowship for Young Scientists (no. 11000465) and Postdoctoral Fellowship for Foreign Researchers (no. 12099005) from Japan Society for the Promotion of Science, respectively.
Present address: Department of Biology, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0116, U.S.A.
Present address: Laboratory of Plant Molecular Genetics, Nara Institute of Science and Technology, 8916-5 Takayama, Ikoma, 630-0101 Japan.
Present address: Graduate School of Sciences, Hiroshima University, 1-3-1 Kagamiyama, Higashihiroshima, Hiroshima, 739-8526 Japan.
Corresponding author: E-mail, h44787a@nucc.cc.nagoya-u.ac.jp; Fax, +81-52-789-5206.
Fig. 1 Salicylic acid-induced stomatal closure in epidermal peels of Vicia faba. Epidermal peels were treated by salicylic acid and stomatal apertures were measured at the indicated times. SA concentrations: 0 mM (open circle), 0.2 mM (open square), 0.5 mM (open triangle), 2.0 mM (open diamond). Error bars indicate standard errors (n = 40).
Fig. 2 Effects of SOD, Tiron and SHAM on SA-induced CLA-chemiluminescence in epidermal peels. (A) Typical traces of CLA-chemiluminescence in the absence and presence of 5 mM SOD, Tiron or SHAM. SA (2 mM) was added at the time indicated by an arrowhead. (B) The height of first chemiluminescence spike (open bars) and the second phase chemiluminescence at the plateau (solid bars) were represented. The plateau of second phase in control is out of the traces in A (at ~10 min). Error bars are standard deviations (n = 3).
Fig. 3 Effect of Tiron, SOD, SHAM and EGTA on SA-induced stomatal closure in epidermal peels. Width of stomata was measured before and 30 min after application of SA and the difference of widths gives closure. Concentrations of SA, Tiron, EGTA, and SHAM, SOD and DMSO were 1, 5, 2 and 5 mM, 330 units ml–1 and 1%, respectively. SHAM was dissolved in DMSO. Student’s t-test reveals significant difference as indicated with *, ** and ***. P = 0.02 against SA, P = 0.039 against DMSO + SA and P <0.001 against SA. Error bars show standard errors (n = 25).
Fig. 4 KO2-induced stomatal closure in epidermal peels. Stomatal width was measured before and 5 min after application of KO2 and the difference of widths gives closure. Error bars show standard errors (n = 40).
Fig. 5 A model for ABA-, elicitor- and SA-signal transduction net work leading stomatal closure.
Abbreviations
- [Ca2+]cyt
cytosolic free calcium ion concentration
- CLA
Cripridina lucigenin-derived chemiluminescent agent (2-methyl-6-phenyl-3,7-dihydroimidazo[1,2-a]pyrasin-3-one)
- DMSO
dimethylsulfoxide
- DPI
diphenylene iodonium
- EGTA
ethylene glycol-bis(β-aminoethyl ether) N,N,N′,N′-tetraacetic acid
- GPX
guaiacol peroxidase
- O2·–
superoxide anion
- rlu
relative luminescence unit
- ROS
reactive oxygen species
- SA
salicylic acid
- SAR
systemic acquired resistance
- SHAM
salicylhydroxamic acid
- SOD
CuZn-superoxide dismutase.
References
Allan, A.C. and Fluhr, R. (
Chen, Z., Silva, H. and Klessig, D.F. (
Chivasa, S., Murphy, A.M., Naylor, M. and Carr, J.P. (
Delaney, T.P., Uknes, S., Vernoij, B., Friedrich, L., Weymann, K., Negrotto, D., Gaffney, T., Gul-Rella, M., Kessmann, H., Ward, E. and Ryals, J. (
Frechilla, S., Zhu, J., Talbott, L.D. and Zeiger, E. (
Gaffney, T., Friedrich, L., Vernooij, B., Negrotto, D., Nye, G., Uknes, S., Ward, E., Kessmann, H. and Ryals, J. (
Ikeda-Saito, M., Shelley, D.A., Lu, L., Booth, K.S., Caughey, W.S. and Kimura, S. (
Kawano, T. and Muto, S. (
Kawano, T., Sahashi, N., Takahashi, K., Uozumi, N. and Muto, S. (
Korthout, H.A. and de Boer, A.H. (
Lee, J.-S. (
Lee, S., Choi, H., Suh, S., Doo, I.-S., Oh, K.-Y., Choi, E.J., Taylor, A.T.S., Low, P.S. and Lee, Y. (
Manthe, B., Schulz, M. and Schnable, H. (
Marra, M., Fullone, M.R., Fogliano, V., Pen, J., Mattei, M., Masi, S. and Aducci, P. (
Mori, I.C. and Muto, S. (
Pei, Z.M., Murata, Y., Benning, G., Thomine, S., Klüsener, B., Allen, G.J., Grill, E. and Schroeder, J.I. (
Ward, E., Uknes, S.J., Williams, S.C., Dincher, S.S., Wiederhol, D.J., Alexander, D.C., Aho-goy, P., Metraux, J.P. and Ryals, J. (