-
PDF
- Split View
-
Views
-
Cite
Cite
Takashi Ayaki, Hidefumi Ito, Osamu Komure, Masaki Kamada, Masataka Nakamura, Reika Wate, Hirofumi Kusaka, Yuko Yamaguchi, Fangzhou Li, Hideshi Kawakami, Makoto Urushitani, Ryosuke Takahashi, Multiple Proteinopathies in Familial ALS Cases With Optineurin Mutations, Journal of Neuropathology & Experimental Neurology, Volume 77, Issue 2, February 2018, Pages 128–138, https://doi.org/10.1093/jnen/nlx109
Close - Share Icon Share
Abstract
Optineurin (OPTN) is a causative gene in familial amyotrophic lateral sclerosis (ALS) with transactivation response element DNA-binding protein of 43 kDa (TDP-43) protein pathology. Here, we report multiple proteinopathies in familial ALS cases with OPTN mutations. We examined the TDP-43, tau, and α-synuclein pathology of ALS cases with OPTN mutations including 2 previously reported cases (Cases 1 and 2) and 1 newly autopsied case (Case 3) that was clinically diagnosed as ALS and Parkinson disease with a heterozygous E478G OPTN mutation. Pathologic examination of Case 3 showed motor neuron degeneration and depigmentation of the substantia nigra. Neurofibrillary tangles (NFTs) were seen in the hippocampus, pontine tegmentum, and spinal cord. Accumulation of multiple proteins including phosphorylated TDP-43-positive neuronal cytoplasmic inclusions, phosphorylated tau (AT8)-positive NFTs, and α-synuclein-positive Lewy bodies were observed in the substantia nigra. The other 2 cases had a similar distribution of tau pathology, but lacked synuclein pathology. Consecutive sections of Case 3 revealed pTDP-43, AT8, and α-synuclein-positive inclusions in the same neuron and double immunofluorescence staining showed aggregation of different proteins (tau and α-synuclein, or tau and TDP-43) in the same neuron. Our results support the notion that OPTN mutations may lead to multiple proteins aggregation and neuronal degeneration.
INTRODUCTION
Mutations in the optineurin (OPTN) gene cause familial amyotrophic lateral sclerosis (ALS) (1) and frontotemporal dementia (2). A common neuropathologic feature of patients with OPTN mutations is transactivation response element (TAR) DNA-binding protein of 43 kDa (TDP-43) pathology (2–4). The tau pathology in these patients was reported as argyrophilic grain disease (AGD) and neurofibrillary tangle (NFT) located in the limbic system and temporal lobe (3, 4). However, it remains unknown if this pathology is attributed to coincidental senile change, because the number of autopsied cases with OPTN mutation has been limited.
In this study, we examined the tau and α-synuclein pathology of ALS cases with OPTN mutations including 2 previously reported cases (Cases 1 and 2) and 1 newly autopsied case (Case 3).
MATERIALS AND METHODS
Gene Analysis
The results of OPTN mutation analysis in Cases 1 and 3 are reported in (3), and that of Case 2 is reported in (4). The results are shown in Table 1.
Clinical Information of the 3 Patients With OPTN Mutations
| . | Case 1 . | Case 2 . | Case 3 . |
|---|---|---|---|
| Mutation site | E478G | Q398X | E478G |
| Age of death (y) | 66 | 52 | 81 |
| Disease duration (y) | 10 | 9 | 17 |
| Clinical diagnosis | ALS | ALS | ALS, PD |
| Upper motor sign | + | + | + |
| Lower motor sign | + | + | + |
| Other clinical features | Dystonic finger deformity, personality change | Dystonic finger deformity, glaucoma, forced crying | Dystonic finger deformity, resting tremor, depression |
| Artificial ventilation | – | – | – |
| Cause of death | CO2 narcosis | Respiratory failure | Heart failure |
| . | Case 1 . | Case 2 . | Case 3 . |
|---|---|---|---|
| Mutation site | E478G | Q398X | E478G |
| Age of death (y) | 66 | 52 | 81 |
| Disease duration (y) | 10 | 9 | 17 |
| Clinical diagnosis | ALS | ALS | ALS, PD |
| Upper motor sign | + | + | + |
| Lower motor sign | + | + | + |
| Other clinical features | Dystonic finger deformity, personality change | Dystonic finger deformity, glaucoma, forced crying | Dystonic finger deformity, resting tremor, depression |
| Artificial ventilation | – | – | – |
| Cause of death | CO2 narcosis | Respiratory failure | Heart failure |
Abbreviations: ALS, amyotrophic lateral sclerosis; OPTN, Optineurin, PD, Parkinson disease.
Clinical Information of the 3 Patients With OPTN Mutations
| . | Case 1 . | Case 2 . | Case 3 . |
|---|---|---|---|
| Mutation site | E478G | Q398X | E478G |
| Age of death (y) | 66 | 52 | 81 |
| Disease duration (y) | 10 | 9 | 17 |
| Clinical diagnosis | ALS | ALS | ALS, PD |
| Upper motor sign | + | + | + |
| Lower motor sign | + | + | + |
| Other clinical features | Dystonic finger deformity, personality change | Dystonic finger deformity, glaucoma, forced crying | Dystonic finger deformity, resting tremor, depression |
| Artificial ventilation | – | – | – |
| Cause of death | CO2 narcosis | Respiratory failure | Heart failure |
| . | Case 1 . | Case 2 . | Case 3 . |
|---|---|---|---|
| Mutation site | E478G | Q398X | E478G |
| Age of death (y) | 66 | 52 | 81 |
| Disease duration (y) | 10 | 9 | 17 |
| Clinical diagnosis | ALS | ALS | ALS, PD |
| Upper motor sign | + | + | + |
| Lower motor sign | + | + | + |
| Other clinical features | Dystonic finger deformity, personality change | Dystonic finger deformity, glaucoma, forced crying | Dystonic finger deformity, resting tremor, depression |
| Artificial ventilation | – | – | – |
| Cause of death | CO2 narcosis | Respiratory failure | Heart failure |
Abbreviations: ALS, amyotrophic lateral sclerosis; OPTN, Optineurin, PD, Parkinson disease.
Summary of Clinical Features of the Cases
Case 1 (previously autopsied and reported case) was a 66-year-old woman who developed weakness in her extremities and depression. She died of pneumonia at age 76 (3). Case 2 (previously autopsied and reported case) was a 52-year-old woman who developed dysphagia followed by weakness in her extremities and forced laughing. She died of respiratory failure at age 61 (4). Case 3 (newly autopsied case) was a 64-year-old Japanese woman who noticed weakness in her right arm. The clinical course was previously reported, when she was still alive (3). Her 2 elder sisters had been diagnosed with motor neuron disease. Her muscle weakness gradually progressed and she developed weakness in her extremities. She was diagnosed with ALS. At age 75, the patient developed dysphagia and underwent percutaneous endoscopic gastrostomy. She also had depression and loss of spontaneous movement. Neurologic examination at age 79 showed loss of spontaneous speech, atrophy of extremities, fasciculation in the both arms, and bilateral Babinski signs. Dystonic deformity and resting tremor of the both arms were also noticed, as previously reported (3). Spontaneous eye movement appeared preserved although she could not comply with our instructions. Finger radiograph and blood test did not show evidence of joint inflammation or destruction. The patient lived 17 years after disease onset without respiratory support. She died of respiratory failure at age 81 and an autopsy was performed. All 3 ALS cases with OPTN mutations had mood disorder and dystonic deformity of the finger. The clinical information is summarized in Table 1.
Neuropathologic Examinations
At autopsy, paraffin-embedded blocks were made after fixation in 10% buffered formalin. Formalin-fixed, paraffin-embedded 6-µm sections were deparaffinized followed by hematoxylin and eosin, Klüver–Barrera, or Gallyas–Braak silver staining. For immunohistochemistry, 6-µm sections were deparaffinized, and antigen was retrieved by autoclaving for 20 minutes at 120 °C using the Histofine deparafinizing antigen retrieval buffer, pH 6 (Product Code 415281; Nichirei, Tokyo, Japan), followed by overnight incubation at 4 °C with primary antibodies in phosphate-buffered saline containing 3% bovine serum albumin. Immunohistochemical staining was conducted with the peroxidase polymer-based method using the Histofine Simple Stain MAX-PO Kit (Nichirei) and DAB Substrate Kit (SK 4100; Vector Laboratories, Burlingame, CA). The primary antibodies used were as follows: Rabbit polyclonal TDP-43 (1:2000, No. 10782-2-AP; Proteintech, Rosemont, IL), mouse monoclonal phosphorylated TDP-43 (pTDP-43, 1:2000, No. TIP-PDT-M01; Cosmo Bio Co., Ltd., Tokyo, Japan), mouse monoclonal hyper-phosphorylated tau (1:500, AT8 clone, No. 90206; Innogenetics, Ghent, Belgium), mouse monoclonal phosphorylated α-synuclein pretreated in formic acid for 5 minutes after autoclaving (1:2000, clone #64, No. 015-25191; Wako Chemicals, Richmond, VA), rabbit polyclonal α-synuclein (1:200, No. SC7011R; Santa Cruz Biotechnology, Dallas, TX), rabbit polyclonal amyloid beta pretreated with formic acid for 5 minutes (1:200, No. 18580; IBL America, Minneapolis, MN), mouse monoclonal beta-amyloid pretreated with formic acid for 5 minutes (1:400, clone 6E10, No. 803001; BioLegend, San Diego, CA), and mouse monoclonal CD68 (1:100, clone KP1, No. M0814; Dako, Glostrup, Denmark). Procedures involving the use of human material were performed in accordance with the ethical guidelines set by Kyoto University (Kyoto, Japan). In each region of the tissue section from Case 3, the numbers of pTDP-43-positive neuronal cytoplasmic inclusions (NCIs) and glial cytoplasmic inclusions (GCIs), as well as AT8-positive NFTs, GCIs, and neuropil threads were counted per 400× power field and graded as follows: −, absent; ±, 0 or 1 inclusion; +, 1–2 inclusions; ++, 3–5 inclusions; +++, >5 inclusions. The results are summarized in Table 2.
Summary of pTDP43 and AT8-Immunoreactive Regions in Case 3
| . | . | pTDP43 . | AT8 . | ||||
|---|---|---|---|---|---|---|---|
| Region . | . | NCI . | Neurites . | GCI . | NFT and PreNFT . | Threads . | Glial Inclusion . |
| Frontal | Sup cortical | − | − | − | − | − | − |
| Deep cortical | − | − | − | − | − | − | |
| White matter | − | − | − | − | − | − | |
| Parietal | Sup cortical | − | − | − | − | − | − |
| Deep cortical | − | − | − | − | − | − | |
| White matter | − | − | − | − | − | − | |
| Temporal | Sup cortical | − | − | − | − | − | − |
| Deep cortical | − | − | − | ± | − | − | |
| White matter | − | − | − | − | − | − | |
| Occipital | Sup cortical | − | − | − | − | − | − |
| Deep cortical | − | − | − | − | − | − | |
| White matter | − | − | − | − | − | − | |
| Motor | Sup cortical | − | − | − | − | − | − |
| Deep cortical | ± | − | − | ± | − | − | |
| White matter | − | − | − | − | − | − | |
| Hippocampus | CA4 | ± | − | − | + | +++ | − |
| CA2/3 | − | − | − | +++ | +++ | − | |
| CA1 | ± | − | − | +++ | +++ | − | |
| Entorhinal | − | − | − | +++ | +++ | − | |
| Basal ganglia | Putamen | − | − | ± | − | − | − |
| Capsula interna | − | − | − | − | − | − | |
| Globus pallidus | − | − | + | − | − | GCI ± | |
| Meynert’s nucleus | − | − | ± | ++ | ++ | GCI + | |
| Amygdala | − | − | ± | +++ | Theread +++, grain +++ | GCI +, Astrocytic inclusion ± | |
| Thalamus | − | − | − | + | ± | − | |
| Midbrain | Substantia nigra | + | ± | + | ++ | +++ | Coiled body, GCI +++, Astrocytic inclusion ± |
| Cerebral peduncle | − | − | − | − | − | − | |
| Periaquaductal gray substance | − | − | + | ++ | +++ | Coiled body, GCI +++ | |
| Pons | Locus ceruleus | ± | − | ± | ++ | +++ | Coiled body, GCI +++ |
| Base nucleus | − | − | − | ± | − | − | |
| Base tract | − | − | − | − | − | − | |
| Medulla | Hypoglossal nucleus | − | − | − | ± | ± | − |
| Inferior olive | − | − | − | − | − | GCI ± | |
| Medullary pyramids | − | − | − | − | − | − | |
| Cerebellum | White matter | − | − | − | − | − | − |
| Molecular layer | − | − | − | − | − | − | |
| Granular layer | − | − | − | − | − | − | |
| Dentate nucleus | − | − | − | ± | − | + | |
| Cervical cord | Anterior horn | − | − | + | + | ++ | − |
| Posterior horn | − | − | − | + | + | − | |
| Spinal tract | − | + | − | − | − | − | |
| Lumbar cord | Anterior horn | − | − | ± | + | ++ | − |
| Posterior horn | − | − | − | − | − | − | |
| Spinal tract | − | − | − | − | − | − | |
| . | . | pTDP43 . | AT8 . | ||||
|---|---|---|---|---|---|---|---|
| Region . | . | NCI . | Neurites . | GCI . | NFT and PreNFT . | Threads . | Glial Inclusion . |
| Frontal | Sup cortical | − | − | − | − | − | − |
| Deep cortical | − | − | − | − | − | − | |
| White matter | − | − | − | − | − | − | |
| Parietal | Sup cortical | − | − | − | − | − | − |
| Deep cortical | − | − | − | − | − | − | |
| White matter | − | − | − | − | − | − | |
| Temporal | Sup cortical | − | − | − | − | − | − |
| Deep cortical | − | − | − | ± | − | − | |
| White matter | − | − | − | − | − | − | |
| Occipital | Sup cortical | − | − | − | − | − | − |
| Deep cortical | − | − | − | − | − | − | |
| White matter | − | − | − | − | − | − | |
| Motor | Sup cortical | − | − | − | − | − | − |
| Deep cortical | ± | − | − | ± | − | − | |
| White matter | − | − | − | − | − | − | |
| Hippocampus | CA4 | ± | − | − | + | +++ | − |
| CA2/3 | − | − | − | +++ | +++ | − | |
| CA1 | ± | − | − | +++ | +++ | − | |
| Entorhinal | − | − | − | +++ | +++ | − | |
| Basal ganglia | Putamen | − | − | ± | − | − | − |
| Capsula interna | − | − | − | − | − | − | |
| Globus pallidus | − | − | + | − | − | GCI ± | |
| Meynert’s nucleus | − | − | ± | ++ | ++ | GCI + | |
| Amygdala | − | − | ± | +++ | Theread +++, grain +++ | GCI +, Astrocytic inclusion ± | |
| Thalamus | − | − | − | + | ± | − | |
| Midbrain | Substantia nigra | + | ± | + | ++ | +++ | Coiled body, GCI +++, Astrocytic inclusion ± |
| Cerebral peduncle | − | − | − | − | − | − | |
| Periaquaductal gray substance | − | − | + | ++ | +++ | Coiled body, GCI +++ | |
| Pons | Locus ceruleus | ± | − | ± | ++ | +++ | Coiled body, GCI +++ |
| Base nucleus | − | − | − | ± | − | − | |
| Base tract | − | − | − | − | − | − | |
| Medulla | Hypoglossal nucleus | − | − | − | ± | ± | − |
| Inferior olive | − | − | − | − | − | GCI ± | |
| Medullary pyramids | − | − | − | − | − | − | |
| Cerebellum | White matter | − | − | − | − | − | − |
| Molecular layer | − | − | − | − | − | − | |
| Granular layer | − | − | − | − | − | − | |
| Dentate nucleus | − | − | − | ± | − | + | |
| Cervical cord | Anterior horn | − | − | + | + | ++ | − |
| Posterior horn | − | − | − | + | + | − | |
| Spinal tract | − | + | − | − | − | − | |
| Lumbar cord | Anterior horn | − | − | ± | + | ++ | − |
| Posterior horn | − | − | − | − | − | − | |
| Spinal tract | − | − | − | − | − | − | |
Semiquantitative grading: pTDP-43-positive inclusions, as well as AT8-positive inclusions and neuropil threads were counted per 400× power field and graded as follows: −, absent; ±, 0 or 1 inclusion; +, 1–2 inclusions; ++, 3–5 inclusions; +++, >5 inclusions.
Abbreviations: pTDP43, phosphorylated transactivation response element (TAR) DNA-binding protein of 43 kDa, AT8, hyper-phosphorylated Tau paired helical filament, GCI, glial cytoplasmic inclusion, NCI, neuronal cytoplasmic inclusion, NFT, neurofibrillary tangle.
Summary of pTDP43 and AT8-Immunoreactive Regions in Case 3
| . | . | pTDP43 . | AT8 . | ||||
|---|---|---|---|---|---|---|---|
| Region . | . | NCI . | Neurites . | GCI . | NFT and PreNFT . | Threads . | Glial Inclusion . |
| Frontal | Sup cortical | − | − | − | − | − | − |
| Deep cortical | − | − | − | − | − | − | |
| White matter | − | − | − | − | − | − | |
| Parietal | Sup cortical | − | − | − | − | − | − |
| Deep cortical | − | − | − | − | − | − | |
| White matter | − | − | − | − | − | − | |
| Temporal | Sup cortical | − | − | − | − | − | − |
| Deep cortical | − | − | − | ± | − | − | |
| White matter | − | − | − | − | − | − | |
| Occipital | Sup cortical | − | − | − | − | − | − |
| Deep cortical | − | − | − | − | − | − | |
| White matter | − | − | − | − | − | − | |
| Motor | Sup cortical | − | − | − | − | − | − |
| Deep cortical | ± | − | − | ± | − | − | |
| White matter | − | − | − | − | − | − | |
| Hippocampus | CA4 | ± | − | − | + | +++ | − |
| CA2/3 | − | − | − | +++ | +++ | − | |
| CA1 | ± | − | − | +++ | +++ | − | |
| Entorhinal | − | − | − | +++ | +++ | − | |
| Basal ganglia | Putamen | − | − | ± | − | − | − |
| Capsula interna | − | − | − | − | − | − | |
| Globus pallidus | − | − | + | − | − | GCI ± | |
| Meynert’s nucleus | − | − | ± | ++ | ++ | GCI + | |
| Amygdala | − | − | ± | +++ | Theread +++, grain +++ | GCI +, Astrocytic inclusion ± | |
| Thalamus | − | − | − | + | ± | − | |
| Midbrain | Substantia nigra | + | ± | + | ++ | +++ | Coiled body, GCI +++, Astrocytic inclusion ± |
| Cerebral peduncle | − | − | − | − | − | − | |
| Periaquaductal gray substance | − | − | + | ++ | +++ | Coiled body, GCI +++ | |
| Pons | Locus ceruleus | ± | − | ± | ++ | +++ | Coiled body, GCI +++ |
| Base nucleus | − | − | − | ± | − | − | |
| Base tract | − | − | − | − | − | − | |
| Medulla | Hypoglossal nucleus | − | − | − | ± | ± | − |
| Inferior olive | − | − | − | − | − | GCI ± | |
| Medullary pyramids | − | − | − | − | − | − | |
| Cerebellum | White matter | − | − | − | − | − | − |
| Molecular layer | − | − | − | − | − | − | |
| Granular layer | − | − | − | − | − | − | |
| Dentate nucleus | − | − | − | ± | − | + | |
| Cervical cord | Anterior horn | − | − | + | + | ++ | − |
| Posterior horn | − | − | − | + | + | − | |
| Spinal tract | − | + | − | − | − | − | |
| Lumbar cord | Anterior horn | − | − | ± | + | ++ | − |
| Posterior horn | − | − | − | − | − | − | |
| Spinal tract | − | − | − | − | − | − | |
| . | . | pTDP43 . | AT8 . | ||||
|---|---|---|---|---|---|---|---|
| Region . | . | NCI . | Neurites . | GCI . | NFT and PreNFT . | Threads . | Glial Inclusion . |
| Frontal | Sup cortical | − | − | − | − | − | − |
| Deep cortical | − | − | − | − | − | − | |
| White matter | − | − | − | − | − | − | |
| Parietal | Sup cortical | − | − | − | − | − | − |
| Deep cortical | − | − | − | − | − | − | |
| White matter | − | − | − | − | − | − | |
| Temporal | Sup cortical | − | − | − | − | − | − |
| Deep cortical | − | − | − | ± | − | − | |
| White matter | − | − | − | − | − | − | |
| Occipital | Sup cortical | − | − | − | − | − | − |
| Deep cortical | − | − | − | − | − | − | |
| White matter | − | − | − | − | − | − | |
| Motor | Sup cortical | − | − | − | − | − | − |
| Deep cortical | ± | − | − | ± | − | − | |
| White matter | − | − | − | − | − | − | |
| Hippocampus | CA4 | ± | − | − | + | +++ | − |
| CA2/3 | − | − | − | +++ | +++ | − | |
| CA1 | ± | − | − | +++ | +++ | − | |
| Entorhinal | − | − | − | +++ | +++ | − | |
| Basal ganglia | Putamen | − | − | ± | − | − | − |
| Capsula interna | − | − | − | − | − | − | |
| Globus pallidus | − | − | + | − | − | GCI ± | |
| Meynert’s nucleus | − | − | ± | ++ | ++ | GCI + | |
| Amygdala | − | − | ± | +++ | Theread +++, grain +++ | GCI +, Astrocytic inclusion ± | |
| Thalamus | − | − | − | + | ± | − | |
| Midbrain | Substantia nigra | + | ± | + | ++ | +++ | Coiled body, GCI +++, Astrocytic inclusion ± |
| Cerebral peduncle | − | − | − | − | − | − | |
| Periaquaductal gray substance | − | − | + | ++ | +++ | Coiled body, GCI +++ | |
| Pons | Locus ceruleus | ± | − | ± | ++ | +++ | Coiled body, GCI +++ |
| Base nucleus | − | − | − | ± | − | − | |
| Base tract | − | − | − | − | − | − | |
| Medulla | Hypoglossal nucleus | − | − | − | ± | ± | − |
| Inferior olive | − | − | − | − | − | GCI ± | |
| Medullary pyramids | − | − | − | − | − | − | |
| Cerebellum | White matter | − | − | − | − | − | − |
| Molecular layer | − | − | − | − | − | − | |
| Granular layer | − | − | − | − | − | − | |
| Dentate nucleus | − | − | − | ± | − | + | |
| Cervical cord | Anterior horn | − | − | + | + | ++ | − |
| Posterior horn | − | − | − | + | + | − | |
| Spinal tract | − | + | − | − | − | − | |
| Lumbar cord | Anterior horn | − | − | ± | + | ++ | − |
| Posterior horn | − | − | − | − | − | − | |
| Spinal tract | − | − | − | − | − | − | |
Semiquantitative grading: pTDP-43-positive inclusions, as well as AT8-positive inclusions and neuropil threads were counted per 400× power field and graded as follows: −, absent; ±, 0 or 1 inclusion; +, 1–2 inclusions; ++, 3–5 inclusions; +++, >5 inclusions.
Abbreviations: pTDP43, phosphorylated transactivation response element (TAR) DNA-binding protein of 43 kDa, AT8, hyper-phosphorylated Tau paired helical filament, GCI, glial cytoplasmic inclusion, NCI, neuronal cytoplasmic inclusion, NFT, neurofibrillary tangle.
Immunostaining for 3-Repeat Tau and 4-Repeat Tau
Regions with abundant AT8 immunoreactivity in NFTs among the 3 cases (hippocampus, brainstem, basal ganglia, and spinal cord) were immunostained with primary antibodies against 3-repeat (3R) tau (1:3000, isoform RD3, No. 05-803; Millipore, Billerica, MA) and 4-repeat (4R) tau (1:1000, isoform RD4, No. 05-804; Millipore). For 3R and 4R immunohistochemistry, pretreatment in potassium permanganate (0.25% for 15 minutes), oxalic acid (2% for 3 minutes), and formic acid (99% for 30 minutes) was performed as previously reported (5). In each region of the 3 cases, the numbers of RD3- or RD4-positive inclusions and neuropil threads were counted per 400× power field and graded as follows: −, absent; ±, 0 or 1 inclusion; +, 1–2 inclusions; ++, 3–5 inclusions; +++, >5 inclusions. The results are summarized in Tables 3 and 4.
Density of RD3-immunoreactive Areas in the 3 Cases
| . | . | Case 1 (E478G) . | Case 2 (Q398X) . | Case 3 (E478G) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region . | . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . |
| Hippocampus | CA4 | − | − | − | − | − | − | ± | ++ | − |
| CA2/3 | − | − | − | − | − | − | + | ++ | − | |
| CA1 | ± | + | − | ± | + | − | ++ | ++ | − | |
| Subiculum | ++ | ++ | − | ++ | ++ | − | +++ | +++ | − | |
| Entorhinal | +++ | +++ | − | +++ | +++ | − | +++ | +++ | − | |
| Basal ganglia | Putamen | − | − | − | ± | − | − | − | − | − |
| Capsula interna | − | − | − | − | − | − | − | − | − | |
| Globus pallidus | − | − | − | − | ± | − | − | − | − | |
| Amygdala | ++ | ++ | − | + | + | − | − | ± | − | |
| Midbrain | Substantia nigra | ++ | + | Coiled body + | − | − | − | + | + | Coiled body ± |
| Cerebral peduncle | − | − | − | − | − | − | − | − | − | |
| Periaquaductal gray substance | ± | − | − | − | − | − | ++ | ++ | Coiled body, GCI + | |
| Pons | Tegmentum | +++ | +++ | Coiled body + | ++ | − | Coiled body, GCI + | ± | − | Coiled body, GCI + |
| Pontine nuclei | − | − | − | − | − | − | − | − | − | |
| Transverse tract | − | − | − | − | − | − | − | − | − | |
| Cervical cord | Anterior horn | − | − | − | ++ | ± | GCI + | ++ | + | − |
| Posterior horn | − | − | − | − | − | − | − | − | − | |
| Spinal tract | − | − | − | − | − | − | − | − | − | |
| Lumbar cord | Anterior horn | − | − | − | − | − | GCI + | ++ | + | − |
| Posterior horn | − | − | − | − | − | − | − | ± | − | |
| Spinal tract | − | − | − | − | − | − | − | − | − | |
| . | . | Case 1 (E478G) . | Case 2 (Q398X) . | Case 3 (E478G) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region . | . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . |
| Hippocampus | CA4 | − | − | − | − | − | − | ± | ++ | − |
| CA2/3 | − | − | − | − | − | − | + | ++ | − | |
| CA1 | ± | + | − | ± | + | − | ++ | ++ | − | |
| Subiculum | ++ | ++ | − | ++ | ++ | − | +++ | +++ | − | |
| Entorhinal | +++ | +++ | − | +++ | +++ | − | +++ | +++ | − | |
| Basal ganglia | Putamen | − | − | − | ± | − | − | − | − | − |
| Capsula interna | − | − | − | − | − | − | − | − | − | |
| Globus pallidus | − | − | − | − | ± | − | − | − | − | |
| Amygdala | ++ | ++ | − | + | + | − | − | ± | − | |
| Midbrain | Substantia nigra | ++ | + | Coiled body + | − | − | − | + | + | Coiled body ± |
| Cerebral peduncle | − | − | − | − | − | − | − | − | − | |
| Periaquaductal gray substance | ± | − | − | − | − | − | ++ | ++ | Coiled body, GCI + | |
| Pons | Tegmentum | +++ | +++ | Coiled body + | ++ | − | Coiled body, GCI + | ± | − | Coiled body, GCI + |
| Pontine nuclei | − | − | − | − | − | − | − | − | − | |
| Transverse tract | − | − | − | − | − | − | − | − | − | |
| Cervical cord | Anterior horn | − | − | − | ++ | ± | GCI + | ++ | + | − |
| Posterior horn | − | − | − | − | − | − | − | − | − | |
| Spinal tract | − | − | − | − | − | − | − | − | − | |
| Lumbar cord | Anterior horn | − | − | − | − | − | GCI + | ++ | + | − |
| Posterior horn | − | − | − | − | − | − | − | ± | − | |
| Spinal tract | − | − | − | − | − | − | − | − | − | |
Semiquantitative grading: Inclusions and neuropil threads were counted per 400× power field and graded as follows: −, absent; ±, 0 or 1 inclusion; +, 1–2 inclusions; ++, 3–5 inclusions; +++, >5 inclusions.
Abbreviations: RD3, antiTau 3-repeat isoform antibody; NFT, neurofibrillary tangle.
Density of RD3-immunoreactive Areas in the 3 Cases
| . | . | Case 1 (E478G) . | Case 2 (Q398X) . | Case 3 (E478G) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region . | . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . |
| Hippocampus | CA4 | − | − | − | − | − | − | ± | ++ | − |
| CA2/3 | − | − | − | − | − | − | + | ++ | − | |
| CA1 | ± | + | − | ± | + | − | ++ | ++ | − | |
| Subiculum | ++ | ++ | − | ++ | ++ | − | +++ | +++ | − | |
| Entorhinal | +++ | +++ | − | +++ | +++ | − | +++ | +++ | − | |
| Basal ganglia | Putamen | − | − | − | ± | − | − | − | − | − |
| Capsula interna | − | − | − | − | − | − | − | − | − | |
| Globus pallidus | − | − | − | − | ± | − | − | − | − | |
| Amygdala | ++ | ++ | − | + | + | − | − | ± | − | |
| Midbrain | Substantia nigra | ++ | + | Coiled body + | − | − | − | + | + | Coiled body ± |
| Cerebral peduncle | − | − | − | − | − | − | − | − | − | |
| Periaquaductal gray substance | ± | − | − | − | − | − | ++ | ++ | Coiled body, GCI + | |
| Pons | Tegmentum | +++ | +++ | Coiled body + | ++ | − | Coiled body, GCI + | ± | − | Coiled body, GCI + |
| Pontine nuclei | − | − | − | − | − | − | − | − | − | |
| Transverse tract | − | − | − | − | − | − | − | − | − | |
| Cervical cord | Anterior horn | − | − | − | ++ | ± | GCI + | ++ | + | − |
| Posterior horn | − | − | − | − | − | − | − | − | − | |
| Spinal tract | − | − | − | − | − | − | − | − | − | |
| Lumbar cord | Anterior horn | − | − | − | − | − | GCI + | ++ | + | − |
| Posterior horn | − | − | − | − | − | − | − | ± | − | |
| Spinal tract | − | − | − | − | − | − | − | − | − | |
| . | . | Case 1 (E478G) . | Case 2 (Q398X) . | Case 3 (E478G) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region . | . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . |
| Hippocampus | CA4 | − | − | − | − | − | − | ± | ++ | − |
| CA2/3 | − | − | − | − | − | − | + | ++ | − | |
| CA1 | ± | + | − | ± | + | − | ++ | ++ | − | |
| Subiculum | ++ | ++ | − | ++ | ++ | − | +++ | +++ | − | |
| Entorhinal | +++ | +++ | − | +++ | +++ | − | +++ | +++ | − | |
| Basal ganglia | Putamen | − | − | − | ± | − | − | − | − | − |
| Capsula interna | − | − | − | − | − | − | − | − | − | |
| Globus pallidus | − | − | − | − | ± | − | − | − | − | |
| Amygdala | ++ | ++ | − | + | + | − | − | ± | − | |
| Midbrain | Substantia nigra | ++ | + | Coiled body + | − | − | − | + | + | Coiled body ± |
| Cerebral peduncle | − | − | − | − | − | − | − | − | − | |
| Periaquaductal gray substance | ± | − | − | − | − | − | ++ | ++ | Coiled body, GCI + | |
| Pons | Tegmentum | +++ | +++ | Coiled body + | ++ | − | Coiled body, GCI + | ± | − | Coiled body, GCI + |
| Pontine nuclei | − | − | − | − | − | − | − | − | − | |
| Transverse tract | − | − | − | − | − | − | − | − | − | |
| Cervical cord | Anterior horn | − | − | − | ++ | ± | GCI + | ++ | + | − |
| Posterior horn | − | − | − | − | − | − | − | − | − | |
| Spinal tract | − | − | − | − | − | − | − | − | − | |
| Lumbar cord | Anterior horn | − | − | − | − | − | GCI + | ++ | + | − |
| Posterior horn | − | − | − | − | − | − | − | ± | − | |
| Spinal tract | − | − | − | − | − | − | − | − | − | |
Semiquantitative grading: Inclusions and neuropil threads were counted per 400× power field and graded as follows: −, absent; ±, 0 or 1 inclusion; +, 1–2 inclusions; ++, 3–5 inclusions; +++, >5 inclusions.
Abbreviations: RD3, antiTau 3-repeat isoform antibody; NFT, neurofibrillary tangle.
Density of RD4-immunoreactive Areas in the 3 Cases
| . | . | Case 1 (E478G) . | Case 2 (Q398X) . | Case 3 (E478G) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region . | . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . |
| Hippocampus | CA4 | – | – | – | – | – | – | ++ | ++ | – |
| CA2/3 | – | – | – | – | – | – | ++ | ++ | – | |
| CA1 | – | – | – | – | – | – | ++ | ++ | – | |
| Subiculum | + | + | – | + | + | – | +++ | +++ | – | |
| Entorhinal | +++ | + | – | + | + | – | ++ | ++ | – | |
| Basal ganglia | Putamen | + | + | – | – | – | – | ± | ± | – |
| Capsula interna | – | – | – | – | – | – | ± | ± | – | |
| Globus pallidus | + | + | – | – | – | – | ± | ± | – | |
| Amygdala | + | Grains +++, Threads +++ | – | +++ | Grains +++, Theads +++ | Astrocytic inclusion ± | + | Grains +++, threads +++ | Astrocytic inclusion ± | |
| Midbrain | Substantia nigra | + | – | – | – | – | Coiled body + | ++ | +++ | Coiled body + |
| Cerebral peduncle | – | – | – | – | – | – | – | – | – | |
| Periaquaductal gray substance | – | – | – | ± | – | – | ++ | ++ | Coiled body + | |
| Pons | Tegmentum | + | – | – | + | + | – | ++ | ++ | Coiled body + |
| Pontine nuclei | – | – | – | – | – | – | ± | ± | Coiled body + | |
| Transverse tract | – | – | – | – | – | – | – | – | – | |
| Cervical cord | Anterior | – | – | – | – | – | – | – | – | – |
| Posterior | – | – | – | – | – | – | – | – | – | |
| Spinal tract | – | – | – | – | – | – | – | – | – | |
| Lumbar cord | Anterior | – | – | – | – | – | – | – | – | – |
| Posterior | – | – | – | – | – | – | – | – | – | |
| Spinal tract | – | – | – | – | – | – | – | – | – | |
| . | . | Case 1 (E478G) . | Case 2 (Q398X) . | Case 3 (E478G) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region . | . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . |
| Hippocampus | CA4 | – | – | – | – | – | – | ++ | ++ | – |
| CA2/3 | – | – | – | – | – | – | ++ | ++ | – | |
| CA1 | – | – | – | – | – | – | ++ | ++ | – | |
| Subiculum | + | + | – | + | + | – | +++ | +++ | – | |
| Entorhinal | +++ | + | – | + | + | – | ++ | ++ | – | |
| Basal ganglia | Putamen | + | + | – | – | – | – | ± | ± | – |
| Capsula interna | – | – | – | – | – | – | ± | ± | – | |
| Globus pallidus | + | + | – | – | – | – | ± | ± | – | |
| Amygdala | + | Grains +++, Threads +++ | – | +++ | Grains +++, Theads +++ | Astrocytic inclusion ± | + | Grains +++, threads +++ | Astrocytic inclusion ± | |
| Midbrain | Substantia nigra | + | – | – | – | – | Coiled body + | ++ | +++ | Coiled body + |
| Cerebral peduncle | – | – | – | – | – | – | – | – | – | |
| Periaquaductal gray substance | – | – | – | ± | – | – | ++ | ++ | Coiled body + | |
| Pons | Tegmentum | + | – | – | + | + | – | ++ | ++ | Coiled body + |
| Pontine nuclei | – | – | – | – | – | – | ± | ± | Coiled body + | |
| Transverse tract | – | – | – | – | – | – | – | – | – | |
| Cervical cord | Anterior | – | – | – | – | – | – | – | – | – |
| Posterior | – | – | – | – | – | – | – | – | – | |
| Spinal tract | – | – | – | – | – | – | – | – | – | |
| Lumbar cord | Anterior | – | – | – | – | – | – | – | – | – |
| Posterior | – | – | – | – | – | – | – | – | – | |
| Spinal tract | – | – | – | – | – | – | – | – | – | |
Semiquantitative grading: Inclusions and neuropil threads were counted per 400× power field and graded as follows: −, absent; ±, 0 or 1 inclusion; +, 1–2 inclusions; ++, 3–5 inclusions; +++, >5 inclusions.
Abbreviations: RD4, antiTau 4-repeat isoform antibody; NFT, neurofibrillary tangle.
Density of RD4-immunoreactive Areas in the 3 Cases
| . | . | Case 1 (E478G) . | Case 2 (Q398X) . | Case 3 (E478G) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region . | . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . |
| Hippocampus | CA4 | – | – | – | – | – | – | ++ | ++ | – |
| CA2/3 | – | – | – | – | – | – | ++ | ++ | – | |
| CA1 | – | – | – | – | – | – | ++ | ++ | – | |
| Subiculum | + | + | – | + | + | – | +++ | +++ | – | |
| Entorhinal | +++ | + | – | + | + | – | ++ | ++ | – | |
| Basal ganglia | Putamen | + | + | – | – | – | – | ± | ± | – |
| Capsula interna | – | – | – | – | – | – | ± | ± | – | |
| Globus pallidus | + | + | – | – | – | – | ± | ± | – | |
| Amygdala | + | Grains +++, Threads +++ | – | +++ | Grains +++, Theads +++ | Astrocytic inclusion ± | + | Grains +++, threads +++ | Astrocytic inclusion ± | |
| Midbrain | Substantia nigra | + | – | – | – | – | Coiled body + | ++ | +++ | Coiled body + |
| Cerebral peduncle | – | – | – | – | – | – | – | – | – | |
| Periaquaductal gray substance | – | – | – | ± | – | – | ++ | ++ | Coiled body + | |
| Pons | Tegmentum | + | – | – | + | + | – | ++ | ++ | Coiled body + |
| Pontine nuclei | – | – | – | – | – | – | ± | ± | Coiled body + | |
| Transverse tract | – | – | – | – | – | – | – | – | – | |
| Cervical cord | Anterior | – | – | – | – | – | – | – | – | – |
| Posterior | – | – | – | – | – | – | – | – | – | |
| Spinal tract | – | – | – | – | – | – | – | – | – | |
| Lumbar cord | Anterior | – | – | – | – | – | – | – | – | – |
| Posterior | – | – | – | – | – | – | – | – | – | |
| Spinal tract | – | – | – | – | – | – | – | – | – | |
| . | . | Case 1 (E478G) . | Case 2 (Q398X) . | Case 3 (E478G) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region . | . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . | NFT and PreNFT . | Neuropil Threads . | Glial Inclusion . |
| Hippocampus | CA4 | – | – | – | – | – | – | ++ | ++ | – |
| CA2/3 | – | – | – | – | – | – | ++ | ++ | – | |
| CA1 | – | – | – | – | – | – | ++ | ++ | – | |
| Subiculum | + | + | – | + | + | – | +++ | +++ | – | |
| Entorhinal | +++ | + | – | + | + | – | ++ | ++ | – | |
| Basal ganglia | Putamen | + | + | – | – | – | – | ± | ± | – |
| Capsula interna | – | – | – | – | – | – | ± | ± | – | |
| Globus pallidus | + | + | – | – | – | – | ± | ± | – | |
| Amygdala | + | Grains +++, Threads +++ | – | +++ | Grains +++, Theads +++ | Astrocytic inclusion ± | + | Grains +++, threads +++ | Astrocytic inclusion ± | |
| Midbrain | Substantia nigra | + | – | – | – | – | Coiled body + | ++ | +++ | Coiled body + |
| Cerebral peduncle | – | – | – | – | – | – | – | – | – | |
| Periaquaductal gray substance | – | – | – | ± | – | – | ++ | ++ | Coiled body + | |
| Pons | Tegmentum | + | – | – | + | + | – | ++ | ++ | Coiled body + |
| Pontine nuclei | – | – | – | – | – | – | ± | ± | Coiled body + | |
| Transverse tract | – | – | – | – | – | – | – | – | – | |
| Cervical cord | Anterior | – | – | – | – | – | – | – | – | – |
| Posterior | – | – | – | – | – | – | – | – | – | |
| Spinal tract | – | – | – | – | – | – | – | – | – | |
| Lumbar cord | Anterior | – | – | – | – | – | – | – | – | – |
| Posterior | – | – | – | – | – | – | – | – | – | |
| Spinal tract | – | – | – | – | – | – | – | – | – | |
Semiquantitative grading: Inclusions and neuropil threads were counted per 400× power field and graded as follows: −, absent; ±, 0 or 1 inclusion; +, 1–2 inclusions; ++, 3–5 inclusions; +++, >5 inclusions.
Abbreviations: RD4, antiTau 4-repeat isoform antibody; NFT, neurofibrillary tangle.
Double Immunofluorescence Staining
For double immunofluorescence staining, primary antibodies were detected with Alexa Fluor 488-labeled goat antimouse IgG and Alexa Fluor 546-labeled goat antirabbit IgG (1:200; Molecular Probes, Eugene, OR). Vectashield (Vector Laboratories) was used to mount the slides, and staining was observed with an FLUOVIEW FV-1000 confocal laser scanning microscope (Olympus, Tokyo, Japan).
RESULTS
Neuropathologic Findings of Case 3
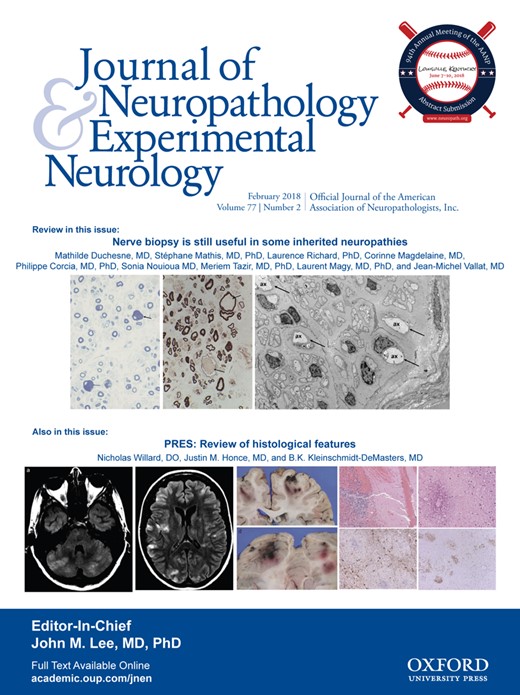
The brain tissue mass from Case 3 was 1032 g. Macroscopic examination showed atrophy of the hippocampus and amygdala, depigmentation of the right substantia nigra, and slight depigmentation of the locus ceruleus. In the spinal cord, Klüver–Barrera staining showed mild degeneration of the corticospinal tract. The anterior horn of the spine showed mild gliosis and neuronophagia. CD68-positive macrophages were observed in the corticospinal tract and anterior horn of the spinal cord. pTDP-43-positive GCIs were observed in the anterior horn (Fig. 1A), whereas pTDP-43-positive NCIs were not found. Nucleus of spinal neurons was normally immunostained with TDP-43. Some remaining motor neurons in the anterior horn had globose-type NFTs (Fig. 1B), which were immunostained with the AT8 antibody (Fig. 1C). This antibody also immunostained GCIs (Fig. 1D). Bunina bodies were not detected. In the motor cortex, slight neuronal loss and neuronophagia were seen. Immunohistochemistry with pTDP-43 and AT8 antibodies did not detect abnormal accumulation in the motor cortex. In the dentate gyrus of the hippocampus, pTDP-43-positive NCIs were scattered (Fig. 1E), and they were more frequently immunostained with the AT8 antibody (Fig. 1F). AT8-positive NFTs were abundant in the hippocampus and entorhinal cortex. NFTs were frequently seen in the temporal lobe and only occasionally seen in other cortices including the frontal, parietal or occipital cortex. In the substantia nigra of the midbrain, there was neuronal loss and free melanin. Some remaining neurons in the locus ceruleus and substantia nigra had Lewy bodies and pale bodies (Fig. 1G). Lewy bodies and Lewy neurites were immunostained with the α-synuclein antibody (Fig. 1H). Cortical or limbic α-synuclein pathology was not observed. The pathology of Case 3 corresponded to Braak stage III of Parkinson disease (6).
Neuropathologic findings of Case 3. pTDP-43-positive glial cytoplasmic inclusions (GCIs) are seen (A, arrow) in the cervical cord. Hematoxylin and eosin (H&E) staining revealed neurofibrillary tangles (NFTs) (B), which were AT8-positive (C) in the lumbar cord. Granular neuronal cytoplasmic inclusion (NCI) was also observed in the anterior horn of the cervical cord (D). pTDP-43-positive NCIs (E, arrow) and AT8-positive NCIs in the dentate gyrus (F, arrow). H&E staining showed Lewy bodies in the pons (G) and α-synuclein (α-syn)-positive Lewy bodies were observed in the substantia nigra of the midbrain (H, arrow). pTDP-43-positive skein-like NCIs in the substantia nigra of the midbrain (I, arrow). Immunostaining with the AT8 antibody revealed NFTs (J), astrocytic inclusions of tau (K, arrow), and coiled bodies (L, arrow) in the substantia nigra of the midbrain. Scale bars: A, G, H, L = 10 μm; B, D, F, J = 25 μm; C, E, I, K = 20 μm.
In the substantia nigra of the midbrain, pTDP-43-positive skein-like NCIs were seen (Fig. 1I). AT8-positive NFTs (Fig. 1J), tau-positive astrocytic inclusions (Fig. 1K), and coiled bodies were also identified (Fig. 1L). In the hypoglossal nucleus, neurons were depleted in number and AT8-positive NFTs were observed. In the amygdala and ambient gyrus, argyrophilic grains and ballooned neurons were immunostained with the AT8 antibody. The pathology of this case corresponded with AGD stage III (7). Amyloid beta-positive senile plaques were not seen in the cortex, and this case was graded 0 according to the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) (8). The distribution of pTDP-43 and AT8-positive inclusions are summarized in Table 2.
Tauopathy and α-Synucleinopathy Examination of Cases 1 and 2
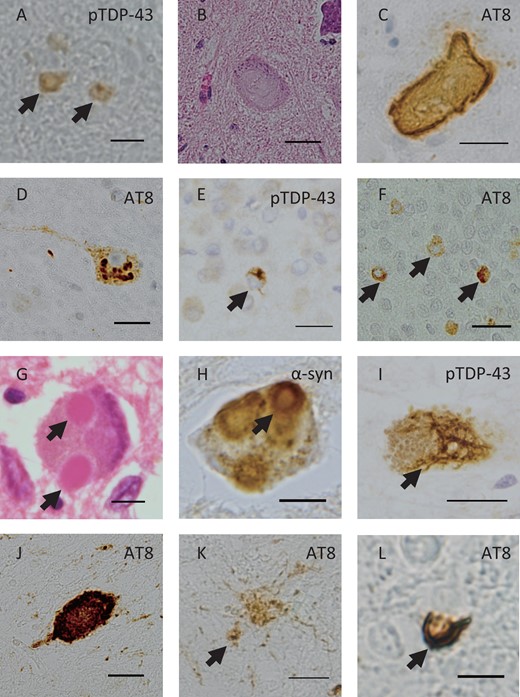
In Case 1 (heterozygous E478G mutation), NFTs were observed in the hippocampus, as previously reported (3). AT8-positive globose-type NFTs were seen in the pontine tegmentum (Fig. 2A), and bush-like tau-positive astrocytic inclusions (Fig. 2B) and argyrophilic grains were observed in the amygdala. In Case 2 (homozygous Q398X mutation), NFTs were observed in the hippocampus, as previously reported (4). AT8-positive NFTs were observed in the pontine tegmentum, and AT8-positive NCIs were observed in the anterior horn of the lumbar cord (Fig. 2C). Astrocytic inclusions of tau were observed in the amygdala (Fig. 2D). TDP-43 pathology was seen as previously reported (3, 4). α-Synuclein accumulation was not found in either case (data not shown).
Tau pathology of Cases 1 and 2. Case 1: AT8-positive NFTs (A, arrow) in the locus ceruleus of the pons and astrocytic inclusions of tau were observed in the amygdala (B, arrow). Case 2: Immunostaining with the AT8 antibody revealed NFTs (C, arrow) and granular NCIs (C, arrowhead) in the anterior horn of the cervical cord, and astrocytic inclusions of tau in the amygdala (D, arrow). Scale bars: A, C = 100 μm; B, D = 25 μm.
Density and Distribution of 3R and 4R Tau in All 3 Cases
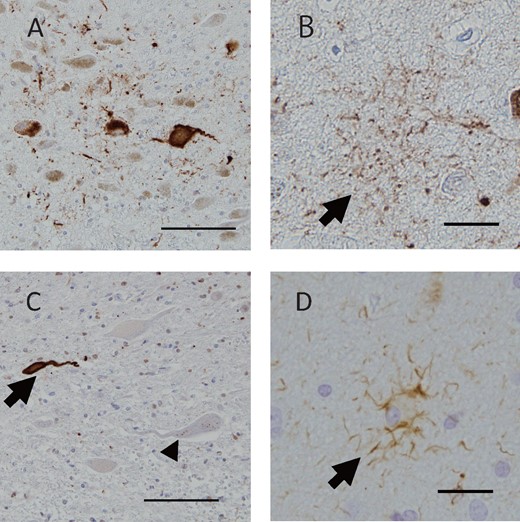
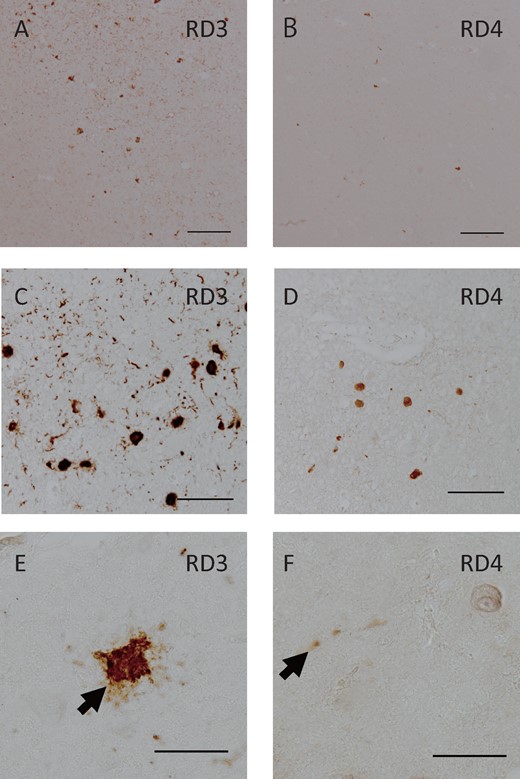
A number of NFTs and neuropil threads in the entorhinal cortex were immunopositive for 3R and 4R tau. The 3R tau antibody more frequently labeled NFTs in all cases (Fig. 3A;Tables 3 and 4). In Case 1, NFTs were frequently immunopositive for 3R tau (Fig. 3C) and were occasionally immunopositive for 4R tau in the pontine tegmentum (Fig. 3D). While Case 1 also exhibited 3R tau dominancy in this area, 4R tau was relatively dominant in Case 3 (Tables 3 and 4). In the anterior horn of the cervical spine, most NFTs and neuropil threads were immunopositive for 3R tau (Fig. 3E). The antibody for 4R tau occasionally labeled neuropil threads in Cases 1 and 3 (Fig. 3F). The amygdala and temporal lobe showed 4R tau-positive argyrophilic grain pathology and ballooned neurons, which were consistent with AGD. The distribution of 3R and 4R tau inclusions is summarized in Tables 3 and 4.
RD3 and RD4 pathology of 3 cases. In the hippocampus entorhinal cortex of Case 2, RD3 (A) immunoreactivity of NFTs and neuropil threads predominated compared with RD4 (B). In the pontine tegmentum of Case 1, RD3-positive NFTs (C) were more frequently seen than RD4-positive NFTs (D). In the anterior horn of the cervical spine of Case 3, the RD3 antibody labeled NFTs (E) whereas the RD4 antibody occasionally labeled neurites (f). Scale bars: A, B = 200 μm; C, D = 100 μm; E, F = 50 μm.
Tau, TDP-43, and α-Synuclein Pathology in the Same Neuron
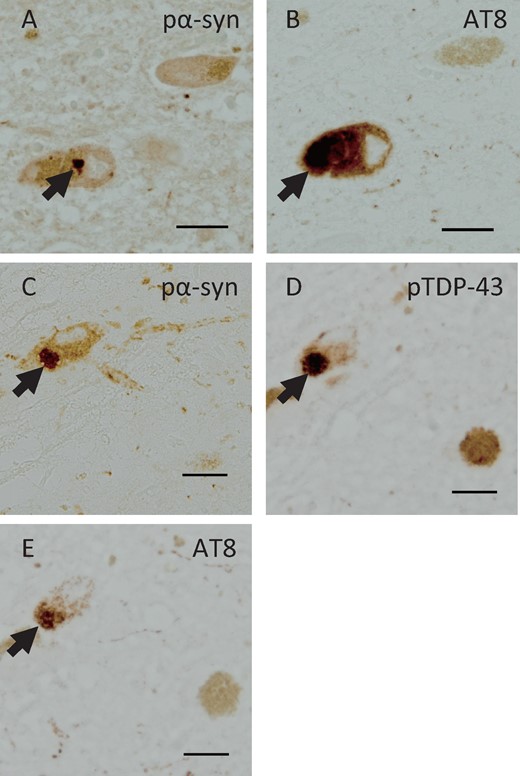
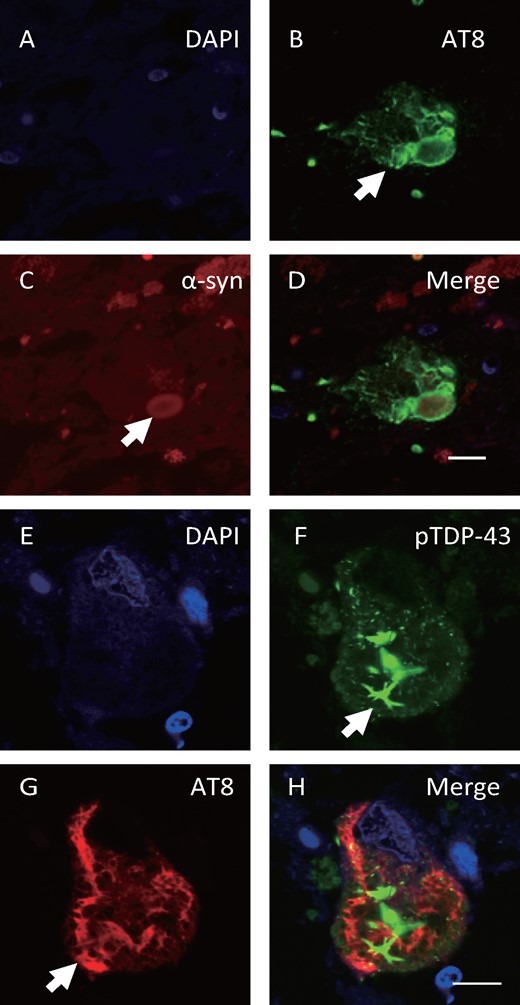
Consecutive sections from Case 3 had phosphorylated α-synuclein-positive inclusions (Fig. 4A), and AT8-positive NFTs (Fig. 4B) were observed in the same neuron in the pons. In serial sections of the midbrain, phosphorylated α-synuclein-positive inclusions (Fig. 4C), pTDP-43-positive inclusions (Fig. 4D), and AT8-positive NFTs (Fig. 4E) were observed in the same neuron. Double immunofluorescence staining revealed that NFTs were immunostained with the AT8 antibody (Fig. 5B) and Lewy bodies were immunostained with the α-synuclein antibody (Fig. 5C) in the same neuron in the pons. In the substantia nigra of the midbrain, pTDP-43-positive cytoplasmic inclusions (Fig. 5F) and AT8-positive NFTs (Fig. 5G) were also observed in the same neuron. In double immunofluorescence images, AT8 and α-synuclein (Fig. 5D) or AT8 and pTDP-43 (Fig. 5H) antibodies labeled different aggregates in the neurons, and partial merging of the proteins was observed.
Immunohistochemistry using serial sections in Case 3. Consecutive sections revealed phosphorylated α-synuclein (pα-syn)-positive inclusions (A) and AT8-positive NFTs (B) in the same neuron in the pons, and pα-syn-positive inclusions (C), pTDP-43-positive inclusions (D) and AT8-positive NFTs (E) in the same neuron in the midbrain. Scale bars: A–E = 25 μm.
Double-labeling immunofluorescence for AT8, α-synuclein and pTDP-43 in Case 3. Double immunofluorescence staining demonstrated AT8-positive NFTs (B, arrow, green) and α-synuclein-positive (C, arrow, red) Lewy bodies in the same neuron in the pons, and pTDP-43-positive cytoplasmic inclusions (F, arrow, green) and AT8-positive NFTs (G, arrow, red) in the same neuron of the midbrain. Scale bars: A–H = 10 μm.
DISCUSSION
Our 3 ALS cases with OPTN mutations showed RD3- and RD4-positive NFTs in the hippocampus, brainstem, and spinal cord and TDP-43 pathology. In most of these areas, 3R tau NFTs were more frequently seen than 4R tau NFTs. Another common feature was RD4-positive AGD pathology. Interestingly, the newly autopsied case (Case 3) showed α-synuclein pathology and aggregation of different proteins (tau and α-synuclein, or tau and TDP-43) in the same neuron in the serial sections. Clinically, the 3 cases were characterized with ALS, focal dystonia of the fingers due to the affected basal ganglia, and mood disorder due to AGD pathology.
The most common cause of 3R and 4R tauopathy is Alzheimer disease (AD). While typical tau pathology in AD progresses from the limbic system to the neocortex (9) and extends to the brainstem and spinal cord in the advanced stage (10, 11), our cases had tau pathology in the brainstem and spinal cord without evident neocortex tau pathology. Amyloid beta-positive senile plaques, as neurologic hallmarks of AD, were not dominant in our cases. In addition, the tau distribution and lack of amyloid beta pathology in these cases with OPTN were not consistent with AD. Although globose-type NFTs in the basal ganglia and brain stem were similar to the pathologic feature of supranuclear palsy (PSP), our cases showed dominant 3R tau tauopathy, which is not consistent with PSP composed of 4R tau. Pick disease has predominant 3R tauopathy, but Pick bodies were not observed in our cases. Given the observed NFT distribution and tau isoform, the tauopathy resulting from OPTN mutations may be unique, and not one that can be explained by a common tauopathy. The limitation of our study includes the quality of the antibodies for 3R and 4R tau, as they are less sensitive than the antibodies for AT8. Studies with more cases are needed to determine the predominance of 3R and 4R tau in cases with OPTN mutations.
Although the 3 cases had similar neuropathologic features, there were also some differences. For example, whereas Case 3 exhibited α-synuclein pathology, the elder sister with the same OPTN mutation (Case 1) lacked this pathology. There was also a difference in severity of the tau and TDP-43 pathology among cases. One reason for these differences may have been disease duration. Because the disease duration of Case 1 was 10 years and that of Case 3 was 17 years, Case 1 might have died before developing synucleinopathy. Another possibility is that mutation of OPTN results in different degrees of penetrance to various diseases. For example, mutations in valosin-containing protein (VCP) causes autosomal dominant syndrome with inclusion body myopathy, which is associated with Paget disease of the bone and frontotemporal dementia and ALS. Because the penetrance of each disease is different, family members with the same VCP gene mutation can have different phenotypes (12).
All 3 cases exhibited various proteinopathies including tau, TDP-43, and α-synuclein. Importantly, in Case 3, AT8, pTDP-43, and α-synuclein antibodies labeled different inclusions in the same neuron, and each aggregate had its own structure. These findings are consistent with the notion that OPTN mutations can cause abnormal accumulation of various degenerative proteins through dysfunctions of OPTN mediated mechanisms, rather than the possibility that normal proteins are entrapped and secondarily aggregate with inclusions of other abnormal proteins.
Cellular functions of OPTN include regulation of selective autophagy (13–5), mitophagy (16, 17), NF-κB (18–20), and necroptosis (21). Several reports have suggested the possibility that autophagy plays an important role in various neurodegenerative diseases including ALS (22–4) and tauopathy (25–7). OPTN works as an autophagy receptor that mediates the interaction between ubiquitinated misfolded proteins and the LC3-interacting region (LIR) in autophagosomes (13–5). TANK-binding kinase 1 (TBK1), which is one of the familial ALS causative genes (28), phosphorylates OPTN in its LIR motif (Ser177), and facilitates autophagy (13). Mutant OPTN reportedly impairs autophagy by disrupting the interaction between OPTN and associated proteins including TBK1 (15) and ubiquitin (13). It is plausible that disruption of this OPTN-mediated process leads to aggregation of degenerative proteins including TDP-43 and tau, which was observed in our cases. As an autophagy receptor, OPTN also recognizes damaged mitochondria in PTEN-induced kinase 1 (PINK1)-Parkin-mediated mitophagy. Because mitophagy is mediated by PINK1-Parkin, a causative gene in familial Parkinson disease, dysfunction of mitophagy could be the pathogenesis of Parkinson disease. Interestingly, Case 3 was the only one that showed clinical and neuropathologic features of Parkinson disease. For determination of the correlation between OPTN mutations and Parkinson disease, further clinicopathologic studies with additional cases with OPTN mutations are needed. OPTN mediates neuronal survival through the regulation of apoptosis (20) and necroptosis (21). Thus, mutant OPTN may lead to neurodegeneration by disruption of neuronal death process.
Together, our results support the notion that mutant OPTN causes neurodegeneration and abnormal accumulation of TDP-43, tau, and α-synuclein through various pathways. Thus, OPTN may be a therapeutic target of neurodegenerative diseases with various proteinopathies.
REFERENCES
Author notes
Takashi Ayaki and Hidefumi Ito contributed equally to this work.
This study was supported by the MEXT KAKENHI grant numbers JP15H04270 and JP16K18383.
The authors have no duality or conflicts of interest to declare.