-
PDF
- Split View
-
Views
-
Cite
Cite
Frederic Lamoth, Shawn R Lockhart, Elizabeth L Berkow, Thierry Calandra, Changes in the epidemiological landscape of invasive candidiasis, Journal of Antimicrobial Chemotherapy, Volume 73, Issue suppl_1, January 2018, Pages i4–i13, https://doi.org/10.1093/jac/dkx444
Close - Share Icon Share
Abstract
The epidemiology of invasive candidiasis has evolved in recent years, warranting a review of the changes and the implications for current and future diagnosis and treatment. The overall burden of invasive candidiasis remains high, particularly in the expanding populations of patients at risk of opportunistic infection, such as the elderly or immunosuppressed. Progressive shifts from Candida albicans to non-albicans Candida spp. have been observed globally. The recent emergence of novel, multiresistant species, such as Candida auris, amplifies the call for vigilance in detection and advances in treatment. Among the current treatment options, fluconazole is still widely used throughout the world. Increased resistance to fluconazole, both acquired and naturally emerging, has been observed. Resistance to echinocandins is presently low but this may change with increased use. Improvement of diagnostic techniques and strategies, development of international surveillance networks and implementation of antifungal stewardship programmes represent major challenges for a better epidemiological control of invasive candidiasis.
Incidence of invasive candidiasis: a research challenge
Long underappreciated as a cause of nosocomial bloodstream infections (BSIs), Candida spp. are one of the primary causes of catheter-associated BSIs in ICUs of US and European hospitals and a significant contributor to morbidity and mortality.1 Other studies have shown it to be among the top four nosocomial bloodstream pathogens, especially in the setting of ICUs.2–4 Invasive candidiasis is not limited to candidaemia, referring instead to a variety of disease states caused by Candida spp., but the majority of the research on invasive candidiasis concentrates on candidaemia. This may be due to the difficulty in diagnosing non-candidaemia candidiasis.5 The majority of invasive candidiasis is diagnosed using blood culture, but in a recent study, only 17% of cases of deep-seated candidiases were detected by blood culture.5 Another study found that blood culture only had a 45% sensitivity for deep-seated candidiasis, suggesting that many cases could be undetected.6
The worldwide incidence of candidaemia is difficult to ascertain, in part because there are no set criteria for an incidence denominator. While a few countries perform population-based surveillance and use census population data as a denominator, smaller studies use patient days, patient discharges, hospital admissions or ICU admissions as a denominator, making comparisons between studies challenging. The rate is also dependent upon a number of other factors, including the age of the patient, especially the number of patients at the extremes of age, the overall health of the population, and the number of patients who have undergone transplants or surgery or who are being treated for malignancies (Figure 1).
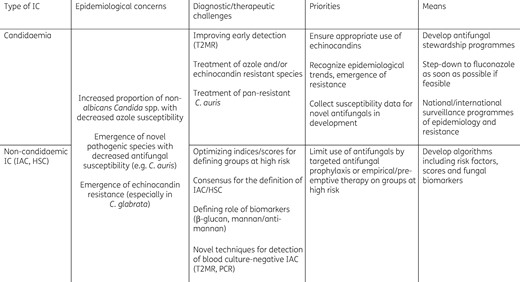
Evolving epidemiology of invasive candidiasis (IC): current challenges and priorities. IAC, intra-abdominal candidiasis; HSC, hepatosplenic candidiasis
Adding to the difficulty in determining the worldwide rate of candidaemia is that the rate can increase or decrease depending upon when the study was conducted. The rates in the USA were seen to rise dramatically in two cities between 1992–2000 and 2011.7 However, in the most recent report the incidence in those same two US cities dropped from 14.1 and 30.9 cases per 100 000 persons to 9.5 and 14.4/100 000, a decline of 33% and 54%, respectively, over the 5 year period between 2008 and 2013.8 This decrease was reflected in a 13.5% decrease in the number of central line-associated bloodstream infections in the USA caused by Candida species between 2004 and 2010.9
The incidence of candidaemia in Australia rose between 2004 and 2015 from 1.8 to 2.4/100 000 but was still moderately low and notably lower than in the USA.10 When examined by Australian state, the rate per 100 000 ranged from a low of 1.6 to a high of 7.2.10 In Norway, the incidence increased between 2003 and 2012 from 2.4 to 3.9 per 100 000.11 There was a marked increase in the candidaemia rate among patients aged 60 years and older, with the rate similar to the overall rates in the USA at >15/100 000 but lower than the 43.3/100 000 seen in those aged >65 years.8 In 2006, Denmark had a rate similar to that seen in the USA at 10/100 000, but by 2009 that decreased to 8.6/100 000.12,13 The rate in Spain in 2011 was 8.1/100 000, much higher than the rate seen in a single Spanish city in 2003, which was only 4.3/100 000.14,15 England and Wales performed population-based surveillance for neonatal and paediatric candidaemia between 2000 and 2009.16 Their overall rate was low at 1.5/100 000 population, but it was notably higher at 11.0/100 000 for patients that were <1 year old. While high, these rates are still lower than the US rate in infants, which was 33.8/100 000 in 2013.8
Although good data exist for North America and Europe, there are no population-based data from Africa, Asia, the Middle East or Latin America from which to establish an overall worldwide rate. There are multicentre and single-institution studies that provide some insight into the rates, although the lack of a consistent denominator precludes comparisons between these studies. In a tertiary care hospital in Turkey the candidaemia rate was 0.3/1000 patient days.17 In South Africa in a single hospital in Soweto the rate was 0.28/1000 admissions in 2002 but jumped to 0.36/1000 admissions in 2007.18 In Taiwan the rate increased from 2003 to 2012 from 0.8 to 1.1 per 1000 discharges in one study and was recorded as 0.37/1000 patient days in another study, making it difficult to compare rates even within a single country.19,20
There are very few rate-based data from Latin America. Two intensive care hospitals in Brazil reported a rate of 1.8/1000 admissions.21 In another Brazilian hospital the rate was 1.9/1000 admissions, which translated to 0.27 cases/1000 patient days.22 Candidaemia surveillance in an additional 11 medical centres in Brazil in 2006 found a rate of 2.5 cases per 1000 admissions, which translated to 0.37 cases per 1000 patient days.23 Another study from 23 hospitals in eight Latin American countries measured the paediatric candidaemia rate as 0.8/1000 admissions.24 Taking these data together, it may be concluded that there is no universal candidaemia rate and there is not even a universal methodology for computing the rate, making the data difficult to compare across regions. Until a unifying denominator is determined a worldwide candidaemia rate will never be determined.
Distribution trends towards non-albicans Candida species
The distribution of Candida species has been changing over the last decade, with a decrease in the proportion of C. albicans and an increase in C. glabrata and C. parapsilosis. Like the candidaemia rate, the overall species distribution is dependent upon geographical location and patient population. In the USA, the proportion of C. albicans has dropped significantly and it now accounts for <50% of Candida infections.25–27 The largest proportional increase in the USA is in C. glabrata, which now accounts for one-third or more of all candidaemia isolates.26,27 This is followed closely by an increase in C. parapsilosis, which accounts for ∼15% of all isolates.8
The trend for increasing C. glabrata is seen in Australia and in some European countries as well. In Australia between 2004 and 2015 C. glabrata increased from 16% to 27% of all isolates.10 In Denmark C. glabrata accounted for 26% of isolates by 2009, similar to the 27% seen in a multicentre study in Belgium.13,28 In Scotland C. glabrata accounts for 21% of isolates, but in Spain C. glabrata only 13%, third behind C. albicans and C. parapsilosis.14,29 In Norway C. glabrata accounts for only 15% of the isolates but is still ranked second behind C. albicans, which made up 68% of all Candida isolates.11
The picture is somewhat different in Latin America and Africa, where the predominant species are C. albicans and C. parapsilosis. Recent surveillance from 16 hospitals in Brazil revealed C. albicans (34%), C. parapsilosis (24%) and C. tropicalis (15%) as the predominant species, numbers that are similar to earlier surveillance data in 11 centres from nine cities: C. albicans (41%), C. parapsilosis (21%) and C. tropicalis (21%).23,30 Similar numbers were seen in a seven-country, 20-centre surveillance study in Latin America, where C. albicans (38%) and C. parapsilosis (27%) were predominant, and a 10-centre study, where again C. albicans (44%) and C. parapsilosis (26%) were predominant.31,32 In South Africa C. albicans and C. parapsilosis are predominant, but data are dependent on whether the hospitals are private or public. In public hospitals it is C. albicans (46%) and C. parapsilosis (35%), while in private sector hospitals it is C. parapsilosis (53%) and then C. albicans (26%).33
The species distribution shifts once more when Asia is considered. In a seven-country, 13-hospital study in the Asian Pacific, C. albicans was most common (36%) but C. tropicalis was second (31%).34 This trend held true in another multicentre study in Asia.20 However, a study from a single centre in Taiwan showed a trend of increasing C. glabrata rates, with C. glabrata going from 1.1% in 2003 to 21.6% in 2012.19 In India and Pakistan C. tropicalis is the most prevalent species, followed by C. albicans.35,36 Interestingly, in Pakistani adults, C. albicans (12%) was fourth most prevalent following C. tropicalis (38%), C.parapsilosis (18%) and C. glabrata (16%).36
Mortality rates of invasive candidiasis
Like incidence and species distribution, mortality due to candidaemia is also dependent upon the specific patient population. Many patients who acquire candidaemia have an underlying medical condition. This makes it difficult to distinguish between mortality due to Candida infection and all-cause mortality, which takes into account underlying medical conditions. In general, mortality from candidaemia is expressed as 30 day all-cause mortality. In recent population-based surveillance from the USA the mortality was 29%.7 In Spain the mortality was similar, at 31%.14 However, mortality can be much higher in other settings, such as a multicentre study in Brazil (54%), in a hospital in South Africa (60%) or a different multicentre study in Brazil (72%).18,23,30
Antifungal resistance in Candida spp.
Both the CLSI and the EUCAST have defined testing methods and established clinical breakpoints for the interpretation of MICs of the most frequent Candida spp. isolated. Despite apparent differences, both approaches have demonstrated their efficiency in discriminating wild-type from non-wild-type isolates and both committees have been recently working for the harmonization of these interpretive criteria.37–39
With a few specific exceptions, the majority of Candida species exhibit high in vitro susceptibility to antifungal agents. For example, in the USA, C. albicans, C. tropicalis and C. parapsilosis have low incidences of fluconazole resistance, at 2%, 5% and 4%, respectively.26 These proportions are similar to those observed in Norway and Switzerland.11,40 The same species exhibit resistance to the echinocandins in <1% of isolates in the USA.26,41 An exception is C. glabrata: population-based surveillance in the USA indicates that ∼10% of C. glabrata are resistant to fluconazole and this rate is also seen in Belgium and Australia.10,26,28 Furthermore, 9% of C. glabrata that are resistant to fluconazole are also resistant to the echinocandins. However, the overall resistance of C. glabrata to the echinocandins in the USA ranges between 0% and 4% but can be higher in single institutions.42,43 In other parts of the world, susceptibility patterns vary. In Taiwan, Australia and Belgium, increasing rates of fluconazole resistance in C. tropicalis are higher than that of the USA; it has been reported at 11%, 17% and 20%, respectively, per country.10,19,28,34 These regions also see far less echinocandin resistance in C. glabrata as compared with the USA. With the increased use of echinocandins it is imperative that we monitor for increasing resistance. Susceptibility testing of echinocandins is generally good for detecting echinocandin resistance, but another powerful tool is the detection of the molecular mechanism of resistance: mutations in the FKS genes.41,42,44 While detection of mechanisms of resistance is available for many bacterial species, it is not yet available outside of a few specialty laboratories for fungi.
Emerging Candida spp.
A discussion on the current epidemiology of candidaemia would be incomplete without mention of Candida auris. First discovered in Japan in 2009, C. auris has since emerged on five continents.45–51 In many ways, this emerging species has altered basic perceptions surrounding candidiasis. It is a colonizer of the skin, unlike most Candida spp., which are found predominantly in the gastrointestinal tract, it can heavily contaminate the hospital environment and it has been responsible for numerous ongoing outbreaks.50–52 In addition, C. auris is frequently resistant to antifungals and some isolates are multidrug resistant.49,53
Increased mortality has been reported with C. auris and may be as much a reflection of the patient population as it is of the severity of the disease or the underlying antifungal resistance. The average number of days spent in the hospital before acquiring a C. auris infection was 19 in one study, an indication of the overall morbidity of the cohort.49 Based on the rapid spread and colonization of this newly emerged species in healthcare environments, C. auris may soon change the landscape of candidaemia.
Epidemiology of invasive candidiasis (IC) in different settings
ICU
According to the Extended Prevalence in Intensive Care (EPIC) II point prevalence study, Candida spp. are the third most frequent cause of infection in ICUs worldwide, accounting for 17% of all ICU infections in culture-positive infected patients.4 Increasing incidence of candidaemia in ICUs has been reported in many parts of the world.35,54,55 Various risk factors associated with the development of IC in ICU patients have been identified, including central venous catheters, treatment with broad-spectrum antibiotics, multifocal Candida colonization, surgery, pancreatitis, parenteral nutrition, haemodialysis, mechanical ventilation and prolonged ICU stay.56,57 Some authors have proposed prediction rules or scores integrating these factors to assess the risk of IC in the ICU.58–61 These prediction and scoring systems have been associated with high negative predictive value (NPV), but low positive predictive value (PPV). A recent study proposed a risk-predictive model categorizing patients into low-risk (PPV 0.24%), intermediate-risk (PPV 1.46%) and high-risk (PPV 11.7%) groups that could help in identifying populations deserving specific testing of fungal markers and/or empirical antifungal therapy.62
While C. albicans remains globally the most frequent species isolated in candidaemia in the ICU, an increased proportion of non-albicans Candida spp., in particular C. glabrata, has been reported.3,54,55,63 When compared with non-ICU cases, candidaemia infections in the ICU are characterized by more frequent pre-exposure to fluconazole with subsequent echinocandin treatment, a lower incidence of C. parapsilosis infections and higher crude mortality rates.54,55,64 Several conditions have been identified as independent risk factors for death associated with candidaemia occurring in the ICU. These include diabetes mellitus, mechanical ventilation, immunosuppression, fever at presentation, high APACHE II score, age, use of an arterial catheter, infection by C. kefyr, pre-exposure to caspofungin and lack of antifungal therapy at the time of blood culture results.54,55,65
IC in the ICU may present as candidaemia, but is often associated with negative blood cultures in patients with intra-abdominal candidiasis (IAC) after complicated abdominal surgery. IAC may occur in the setting of intra-abdominal abscesses (30%–60%), secondary peritonitis (30%–40%), infected pancreatic necrosis (5%–10%), cholecystitis/cholangitis (5%–10%) or primary peritonitis (5%).66–68 It is a mixed bacterial and fungal infection in up to two-thirds of the cases.66,68,69 Candidaemia occurs in only 5%–15% of patients. IAC is a diagnostic challenge for the clinician. Various definitions have been proposed that rely on the detection of Candida spp. by direct examination or culture of an intra-abdominal sample (i.e. peritoneal fluid, intra-abdominal abscess, bile or biopsy of intra-abdominal organ) obtained during surgery or from a drain inserted within the last 24 h in a patient with clinical signs of intra-abdominal infection.66,68–70 Non-culture-based methods may help to guide pre-emptive antifungal therapy in these patients.70 In high-risk patients with recurrent gastrointestinal tract perforations, two consecutive positive 1,3-β-d-glucan tests in serum were shown to have 75% sensitivity and 77% specificity.69 Detection of Candida germ tube antibodies (CAGTA), which may be combined with 1,3-β-d-glucan testing, may improve diagnostic accuracy.71–73 However, in the absence of reliable diagnostic markers, diagnosis remains difficult and the cost associated with empirical antifungal therapy without demonstrated survival benefit is a concern.74,75 The presence of Candida spp. in intra-abdominal specimens is an independent risk factor for mortality.76–79 Indeed, septic shock is present in 20%–40% of cases and the rate of mortality is high, ranging from 25% up to 60%.66–68,70,76–79 Several studies have shown that rapid initiation of appropriate antifungal therapy and early source control (drainage or debridement of infected collections/tissues and removal of foreign material) are key elements for a better outcome.66–68
Haematological malignancies
The incidence of IC in onco-haematological patients has decreased with the systematic use of antifungal prophylaxis and is currently estimated to be <1%.80–83 In the USA and in Europe, IC is the second cause of invasive fungal infections in allogeneic haematopoietic stem cell transplant recipients and patients with haematological malignancies, accounting for 25%–30% of cases.81,82,84,85 Risk factors for IC in patients with haematological malignancies include neutropenia, corticosteroid therapy, mucositis and the presence of central venous catheters.86,87 The proportion of non-albicans Candida spp., in particular C. krusei and C. glabrata, is higher in this population as a possible consequence of prolonged azole exposure.88–92 Other studies have also reported a higher incidence of azole-susceptible non-albicans Candida spp., such as C. tropicalis, C. parapsilosis or C. kefyr.80,83,87,93,94
Hepatosplenic candidiasis (HSC), also referred to as chronic disseminated candidiasis, is typically associated with prolonged neutropenia. Clinical manifestations include persistent fever under broad-spectrum antibiotics, anorexia, nausea, vomiting and abdominal discomfort. HSC is characterized by the presence of nodular lesions in the liver, spleen and other organs (lungs, kidneys and skin) on radiological imaging.95–98 Candidaemia is detected in only 20% of the patients. Exacerbated immune response during the neutrophil recovery phase leading to a type of immune reconstitution inflammatory syndrome may play an important role in the pathogenesis of this clinical entity.97 The estimated incidence of HSC among patients with prolonged neutropenia was around 3%–6% and has possibly decreased below 3% with the widespread use of azole prophylaxis.97,98 Diagnosis remains difficult and relies primarily on the detection of fungal biomarkers (1,3-β-d-glucan, mannan and anti-mannan antibodies) and on typical radiological patterns on CT scan, MRI or ultrasound, such as nodules, microabscesses (typically ‘bull-eye’ lesions), hypoechogenic foci or fibrosis and calcifications, which occur late in the course of the disease.97,99–101
Solid organ transplantation (SOT)
Epidemiological data on IC among SOT recipients are derived from two large prospective North American cohorts, the Transplant-Associated Infection Surveillance Network (TRANSNET) and the Prospective Antifungal Therapy (PATH) Alliance.102–104 IC is the most common invasive fungal infection in SOT patients, accounting for more than half of the cases, with the exception of lung transplant recipients, in whom invasive aspergillosis predominates. Overall, the 1 year post-transplant cumulative incidence of IC was 2%, with the majority of cases occurring during the first 100 days after transplantation. C. albicans was the most frequent species (46%), followed by C. glabrata (24%–37%), while other species accounted each for <10% of cases. Candidaemia was present in 44%–53% of cases and intra-abdominal candidiasis in 14%–37%. IC due to C. parapsilosis or C. tropicalis was associated with the worst prognosis.
Neonates
IC affects mainly low birth weight premature infants, with an incidence of 3%–10% among neonates with a weight <1000 g and <0.3% among those weighing >2500 g.105–109 However, recent reports indicate that the incidence of IC has declined over the last decade.7,110 Candidaemia represents the third cause of bloodstream infections in the general paediatric population. 111,112 In addition to prematurity and low birth weight, maternal vaginal candidiasis and vaginal delivery are risk factors for Candida colonization in neonates and the number of sites of colonization is independently associated with IC.113 Other risk factors include low Apgar score, prolonged use of antibiotics (especially cephalosporins), male gender, parenteral nutrition and lack of enteral nutrition, central venous catheters, H2 blockers, mechanical ventilation, length of hospital stay and disseminated intravascular coagulopathy and shock.105,106,108,114
IC in neonates may present as congenital candidiasis, which is acquired by materno-fetal transmission before or during birth, with a predominance of skin lesions. Postnatal IC may be acquired through the use of central venous catheters and is associated with candidaemia in 70%–95% of cases.105,106,108,109,115 Prolonged candidaemia is frequently observed in neonates, in whom there is higher risk of organ involvement, such as eyes, CNS, kidneys, liver and heart. Ocular lesions may be observed in 5%–10% of cases and endocarditis was documented in up to 15% in one series.116,117 Although extremely rare in adults, Candida meningitis is observed in 1%–10% of neonatal IC and blood cultures may be negative in up to 50% of the cases.105,106,108,109,115
While C. albicans remains the most frequent pathogen in neonatal IC, the proportion of non-albicans Candida spp. usually exceeds 50%, with C. parapsilosis being the most frequent species (20%–40%).105–107,115
Morbidity and mortality of neonatal IC is substantial. Mortality rates of 10%–30% have been reported, which were significantly higher than in patients without IC.105–109,114,115 In extremely low birth weight patients with IC, mortality may be as high as 73%.105 Among survivors, neurodevelopmental impairment and the occurrence of neurological sequelae (cerebral palsy, visual or hearing impairments) were significantly higher than in premature neonates without IC. IC due to C. parapsilosis is usually associated with better prognosis.105,109 Prompt removal of a central venous catheter was also associated with better outcome.105
Conclusions and perspectives
Epidemiological challenges and priorities in IC are summarized in Table 1. In recent years the epidemiology of IC has evolved and the incidence has increased in some US and European centres.7,11,13,118,119 This increasing burden of IC, which is especially observed in the elderly, may be related to changes in the hospital case mix, with an expanding population of immunosuppressed or debilitated patients surviving in the face of severe and formerly fatal diseases. However, the incidence of IC in neonates has decreased.7,110 A progressive shift from C. albicans to non-albicans Candida spp. is also observed in most parts of the world, which is probably related to the increased exposure to azoles.10,13,26,118,120,121 Although fluconazole remains active against the majority of Candida spp., a trend towards increased acquired resistance or the emergence of naturally resistant species has been observed.10,13,26,118,122,123 Despite increasing echinocandin use, the level of echinocandin resistance remains very low.10,13,26 However, the link between echinocandin exposure and development of resistance has been well established.124,125 Emergence of novel pathogenic species with multiresistance patterns, such as C. auris, is a major threat and argues in favour of the development of a worldwide sentinel system to rapidly detect and report the emergence of new species.49
Candidaemia epidemiology from population-based or multicentre studies
| Country . | Years covered . | Number of candidaemia episodes . | Annual incidence rate . | Proportion C. albicans/ non-albicans . | Rate of azole resistance . | 30 day mortality rate . | Reference . |
|---|---|---|---|---|---|---|---|
| USA | 2008–11 | 2675 | 13.3–26.2/100 000 population | 37/63 | 7% | 28%–29% | 7 |
| USA | 2013 | 515 | 9.5–14.4/100 000 population | 35/65 | 5%–7% | NA | 8 |
| Canada | 2003–05 | 453 | 3.0/100 000 population | 62/38 | 4% | NA | 143 |
| Norway | 2004–12 | 1677 | 3.9/100 000 population | 68/32 | 7% | NA | 11 |
| Finland | 2004–07 | 603 | 2.9/100 000 population | 67/33 | NA | 35% | 144 |
| Iceland | 2000–11 | 208 | 5.7/100 000 population | 56/44 | 3% | 30% | 145 |
| Denmark | 2004–09 | 2649 | 8.6/100 000 population | 58/42 | NA | NA | 13 |
| France | 2001–10 | 15 570 | 3.6/100 000 population | NA | NA | NA | 146 |
| Spain | 2010–11 | 773 | 8.1/100 000 population | 45/55 | 21% | 31% | 14 |
| Belgium | 2013–14 | 338 | 0.4/1000 admissions | 50/50 | 8% | NA | 28 |
| Scotland | 2007 | 242 | 4.8/100 000 population | 50/50 | 2% | NA | 29 |
| Australia | 2001–04 | 1095 | 1.8/100 000 population | 47/53 | NA | 28% | 147 |
| Australia | 2014–15 | 527 | 2.4/100 000 population | 44/56 | 6% | NA | 10 |
| Brazil | 2007–10 | 137 | NA | 34/66 | 9% | 72% | 30 |
| Peru | 2013–15 | 157 | 2.0/1000 admissions | 28/72 | 3% | 40% | 148 |
| Latin America | 2008–10 | 672 | 0.3–2.0/1000 admissions | 38/62 | 3% | 41% | 31 |
| South Africa | 2009–10 | 2172 | NA | 46/54 | 18% | NA | 33 |
| Asia-Pacific | 2010–11 | 1601 | 0.3–2.9/1000 discharges | 41/59 | NA | NA | 20 |
| India | 2011–12 | 1400 | 6.5/1000 admissionsa | 21/79 | 12% | 45% | 35 |
| Country . | Years covered . | Number of candidaemia episodes . | Annual incidence rate . | Proportion C. albicans/ non-albicans . | Rate of azole resistance . | 30 day mortality rate . | Reference . |
|---|---|---|---|---|---|---|---|
| USA | 2008–11 | 2675 | 13.3–26.2/100 000 population | 37/63 | 7% | 28%–29% | 7 |
| USA | 2013 | 515 | 9.5–14.4/100 000 population | 35/65 | 5%–7% | NA | 8 |
| Canada | 2003–05 | 453 | 3.0/100 000 population | 62/38 | 4% | NA | 143 |
| Norway | 2004–12 | 1677 | 3.9/100 000 population | 68/32 | 7% | NA | 11 |
| Finland | 2004–07 | 603 | 2.9/100 000 population | 67/33 | NA | 35% | 144 |
| Iceland | 2000–11 | 208 | 5.7/100 000 population | 56/44 | 3% | 30% | 145 |
| Denmark | 2004–09 | 2649 | 8.6/100 000 population | 58/42 | NA | NA | 13 |
| France | 2001–10 | 15 570 | 3.6/100 000 population | NA | NA | NA | 146 |
| Spain | 2010–11 | 773 | 8.1/100 000 population | 45/55 | 21% | 31% | 14 |
| Belgium | 2013–14 | 338 | 0.4/1000 admissions | 50/50 | 8% | NA | 28 |
| Scotland | 2007 | 242 | 4.8/100 000 population | 50/50 | 2% | NA | 29 |
| Australia | 2001–04 | 1095 | 1.8/100 000 population | 47/53 | NA | 28% | 147 |
| Australia | 2014–15 | 527 | 2.4/100 000 population | 44/56 | 6% | NA | 10 |
| Brazil | 2007–10 | 137 | NA | 34/66 | 9% | 72% | 30 |
| Peru | 2013–15 | 157 | 2.0/1000 admissions | 28/72 | 3% | 40% | 148 |
| Latin America | 2008–10 | 672 | 0.3–2.0/1000 admissions | 38/62 | 3% | 41% | 31 |
| South Africa | 2009–10 | 2172 | NA | 46/54 | 18% | NA | 33 |
| Asia-Pacific | 2010–11 | 1601 | 0.3–2.9/1000 discharges | 41/59 | NA | NA | 20 |
| India | 2011–12 | 1400 | 6.5/1000 admissionsa | 21/79 | 12% | 45% | 35 |
Criteria for resistance, 30 day mortality and incidence may vary between the studies and may not directly correlate.
NA, not available.
ICU admissions only.
Candidaemia epidemiology from population-based or multicentre studies
| Country . | Years covered . | Number of candidaemia episodes . | Annual incidence rate . | Proportion C. albicans/ non-albicans . | Rate of azole resistance . | 30 day mortality rate . | Reference . |
|---|---|---|---|---|---|---|---|
| USA | 2008–11 | 2675 | 13.3–26.2/100 000 population | 37/63 | 7% | 28%–29% | 7 |
| USA | 2013 | 515 | 9.5–14.4/100 000 population | 35/65 | 5%–7% | NA | 8 |
| Canada | 2003–05 | 453 | 3.0/100 000 population | 62/38 | 4% | NA | 143 |
| Norway | 2004–12 | 1677 | 3.9/100 000 population | 68/32 | 7% | NA | 11 |
| Finland | 2004–07 | 603 | 2.9/100 000 population | 67/33 | NA | 35% | 144 |
| Iceland | 2000–11 | 208 | 5.7/100 000 population | 56/44 | 3% | 30% | 145 |
| Denmark | 2004–09 | 2649 | 8.6/100 000 population | 58/42 | NA | NA | 13 |
| France | 2001–10 | 15 570 | 3.6/100 000 population | NA | NA | NA | 146 |
| Spain | 2010–11 | 773 | 8.1/100 000 population | 45/55 | 21% | 31% | 14 |
| Belgium | 2013–14 | 338 | 0.4/1000 admissions | 50/50 | 8% | NA | 28 |
| Scotland | 2007 | 242 | 4.8/100 000 population | 50/50 | 2% | NA | 29 |
| Australia | 2001–04 | 1095 | 1.8/100 000 population | 47/53 | NA | 28% | 147 |
| Australia | 2014–15 | 527 | 2.4/100 000 population | 44/56 | 6% | NA | 10 |
| Brazil | 2007–10 | 137 | NA | 34/66 | 9% | 72% | 30 |
| Peru | 2013–15 | 157 | 2.0/1000 admissions | 28/72 | 3% | 40% | 148 |
| Latin America | 2008–10 | 672 | 0.3–2.0/1000 admissions | 38/62 | 3% | 41% | 31 |
| South Africa | 2009–10 | 2172 | NA | 46/54 | 18% | NA | 33 |
| Asia-Pacific | 2010–11 | 1601 | 0.3–2.9/1000 discharges | 41/59 | NA | NA | 20 |
| India | 2011–12 | 1400 | 6.5/1000 admissionsa | 21/79 | 12% | 45% | 35 |
| Country . | Years covered . | Number of candidaemia episodes . | Annual incidence rate . | Proportion C. albicans/ non-albicans . | Rate of azole resistance . | 30 day mortality rate . | Reference . |
|---|---|---|---|---|---|---|---|
| USA | 2008–11 | 2675 | 13.3–26.2/100 000 population | 37/63 | 7% | 28%–29% | 7 |
| USA | 2013 | 515 | 9.5–14.4/100 000 population | 35/65 | 5%–7% | NA | 8 |
| Canada | 2003–05 | 453 | 3.0/100 000 population | 62/38 | 4% | NA | 143 |
| Norway | 2004–12 | 1677 | 3.9/100 000 population | 68/32 | 7% | NA | 11 |
| Finland | 2004–07 | 603 | 2.9/100 000 population | 67/33 | NA | 35% | 144 |
| Iceland | 2000–11 | 208 | 5.7/100 000 population | 56/44 | 3% | 30% | 145 |
| Denmark | 2004–09 | 2649 | 8.6/100 000 population | 58/42 | NA | NA | 13 |
| France | 2001–10 | 15 570 | 3.6/100 000 population | NA | NA | NA | 146 |
| Spain | 2010–11 | 773 | 8.1/100 000 population | 45/55 | 21% | 31% | 14 |
| Belgium | 2013–14 | 338 | 0.4/1000 admissions | 50/50 | 8% | NA | 28 |
| Scotland | 2007 | 242 | 4.8/100 000 population | 50/50 | 2% | NA | 29 |
| Australia | 2001–04 | 1095 | 1.8/100 000 population | 47/53 | NA | 28% | 147 |
| Australia | 2014–15 | 527 | 2.4/100 000 population | 44/56 | 6% | NA | 10 |
| Brazil | 2007–10 | 137 | NA | 34/66 | 9% | 72% | 30 |
| Peru | 2013–15 | 157 | 2.0/1000 admissions | 28/72 | 3% | 40% | 148 |
| Latin America | 2008–10 | 672 | 0.3–2.0/1000 admissions | 38/62 | 3% | 41% | 31 |
| South Africa | 2009–10 | 2172 | NA | 46/54 | 18% | NA | 33 |
| Asia-Pacific | 2010–11 | 1601 | 0.3–2.9/1000 discharges | 41/59 | NA | NA | 20 |
| India | 2011–12 | 1400 | 6.5/1000 admissionsa | 21/79 | 12% | 45% | 35 |
Criteria for resistance, 30 day mortality and incidence may vary between the studies and may not directly correlate.
NA, not available.
ICU admissions only.
Novel diagnostic procedures and therapeutic approaches are expected to shape the future of IC epidemiology. The advent of mass spectrometry (MALDI-TOF) as standard diagnostic procedure for yeast identification may lead to a better recognition of rare Candida spp., such as C. auris, that were previously misdiagnosed or unrecognized.126 The recent FDA approval of T2 magnetic resonance (T2MR, T2 Biosystems, Lexington, MA, USA) for the direct detection of Candida spp. in blood samples may improve the early detection of IC.127 While automated blood cultures systems usually require 1–3 days for the detection of yeasts, T2MR can identify Candida spp. within several hours from the time of sampling. Previous analyses in clinical blood samples and spiked samples have shown a sensitivity and specificity >90% when compared with blood cultures and an increased sensitivity for the detection of C. glabrata.128,129 Advances in molecular techniques with availability of PCR kits for direct detection of microorganisms (including Candida spp.) in blood, such as LightCycler SeptiFast or the Iridica BAC BSI Assay, may also improve the early recognition and microbiological documentation of IC.130–133 While most studies have addressed the performance of these novel methods for the diagnosis of candidaemia, data are lacking for deep-seated and typically blood culture-negative IC, such as intra-abdominal candidiasis or chronic disseminated candidiasis with unmet medical needs.
Increasing consumption of antifungal drugs has been universally reported during the last decade and was associated with shifts in Candida spp. distribution and decreased antifungal susceptibility.118,134 Echinocandins have become the first-line therapy of candidaemia according to North American and European updated guidelines.135–138 Clinical recommendations must be balanced by epidemiological concerns. Increased echinocandin use has been associated with a higher rate of C. parapsilosis infections and higher caspofungin MICs for C. albicans, C. glabrata and C. parapsilosis.134 In the continuously evolving epidemiological landscape of invasive candidiasis, antifungal stewardship programmes are warranted to improve appropriate therapy and limit the emergence of resistance.139–142
Funding
This article is part of a Supplement sponsored by Cidara Therapeutics, Inc. Editorial support was provided by T. Chung (Scribant Medical) with funding from Cidara.
Transparency declarations
F. L. is a member of advisory boards for Basilea and MSD. T. C. is a member of advisory boards for Astellas and Cubist (subsequently acquired by MSD), a consultant to Basilea and Debiopharm, an advisor for Cidara, and a member of a speakers’ bureau and advisory board for MSD. All other authors have none to declare.
The authors received no compensation for their contribution to the supplement. This article was co-developed and published based on all authors’ approval.
Disclaimer
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
Author notes
Frederic Lamoth and Shawn R. Lockhart made an equal contribution to the manuscript.