-
PDF
- Split View
-
Views
-
Cite
Cite
Niamh C. O'Sullivan, Thomas R. Jahn, Evan Reid, Cahir J. O'Kane, Reticulon-like-1, the Drosophila orthologue of the Hereditary Spastic Paraplegia gene reticulon 2, is required for organization of endoplasmic reticulum and of distal motor axons, Human Molecular Genetics, Volume 21, Issue 15, 1 August 2012, Pages 3356–3365, https://doi.org/10.1093/hmg/dds167
Close - Share Icon Share
Several causative genes for hereditary spastic paraplegia encode proteins with intramembrane hairpin loops that contribute to the curvature of the endoplasmic reticulum (ER), but the relevance of this function to axonal degeneration is not understood. One of these genes is reticulon2. In contrast to mammals, Drosophila has only one widely expressed reticulon orthologue, Rtnl1, and we therefore used Drosophila to test its importance for ER organization and axonal function. Rtnl1 distribution overlapped with that of the ER, but in contrast to the rough ER, was enriched in axons. The loss of Rtnl1 led to the expansion of the rough or sheet ER in larval epidermis and elevated levels of ER stress. It also caused abnormalities specifically within distal portions of longer motor axons and in their presynaptic terminals, including disruption of the smooth ER (SER), the microtubule cytoskeleton and mitochondria. In contrast, proximal axon portions appeared unaffected. Our results provide direct evidence for reticulon function in the organization of the SER in distal longer axons, and support a model in which spastic paraplegia can be caused by impairment of axonal the SER. Our data provide a route to further understanding of both the role of the SER in axons and the pathological consequences of the impairment of this compartment.
INTRODUCTION
Hereditary spastic paraplegias (HSPs) are a group of neurological disorders characterized by retrograde degeneration of long nerve fibres in the corticospinal tracts and posterior columns, sometimes accompanied by additional, mainly neurological symptoms (1,2). The disease mechanisms are largely unknown, but since distal regions of longer axons appear to be worst affected, the disease may reflect problems in trafficking cell components between the cell body and distal axons that can be up to a metre away. Some clues about disease mechanisms come from the identification of over 20 causative HSP genes (SPGs, spastic paraplegia genes) (3). These encode a heterogeneous group of proteins, but the largest single class are intracellular membrane proteins, principally the endosomal or endoplasmic reticulum (ER). Functions ascribed to these proteins (not mutually exclusive) include the inhibition of BMP signalling (4–6), formation of lipid droplets (7–9) or regulation of ER topology (10–13).
At least four autosomal dominant HSPs are caused by mutations in proteins that have a common feature of ER localization, and an intramembrane hairpin loop that can induce or sense the curvature of ER membranes and form oligomeric complexes among themselves and each other (10–15). These hairpin-loop proteins, SPG3A/atlastin1, SPG4/spastin, SPG12/reticulon2 (RTN2) and SPG31/REEP1, contribute to ER topology in a number of ways. Reticulon and REEP proteins share a partly redundant role in the formation of tubular ER, and in the induction of the curvature at the edges of the sheet ER (10,16). ER tubule elongation is proposed to involve both REEP proteins and the microtubule (MT)-severing activity of spastin, which could potentially nucleate new MT elongation and accompanying tubule extension (13,17–19). The GTPase Atlastin1 is thought to mediate the membrane fusion events that maintain the reticular organization of the ER (12,14,20).
Little protein synthesis occurs in axons (21), and consequently they contain very little rough ER (RER) (22); it is at first sight paradoxical that mutations in ER-modelling proteins could be causative for axonal degeneration. However, axons and presynaptic terminals contain the smooth ER (SER), based on ultrastructural evidence and the presence of calcium homeostasis machinery (22–26). Since smooth and tubular ER are broadly equivalent (16), this could explain why axons are sensitive to mutations in proteins that model tubular ER.
One of the major ER-tubulating protein classes is the reticulon family, one of whose members, RTN2, was recently identified as an SPG gene product (15). Here, we used Drosophila to test the effects on the ER and axons of the loss of reticulon function. Drosophila has a single widely expressed reticulon, reticulon1 (Rtnl1), that is an orthologue of reticulons 1–4 in humans. We show that Rtnl1 is required for ER network organization, and that its loss induces an ER stress response. Furthermore, the loss of Rtnl1 leads to abnormalities of an SER marker, the MT cytoskeleton and mitochondria, in the distal axons or presynaptic termini of longer motor axons. Our findings reveal increased susceptibility of posterior axons to the disruption of ER organization and suggest a mechanism by which the loss of hairpin loop proteins gives rise to axonopathy.
RESULTS
Loss of Rtnl1, the Drosophila orthologue of vertebrate reticulons 1–4, causes age-related locomotor deficits
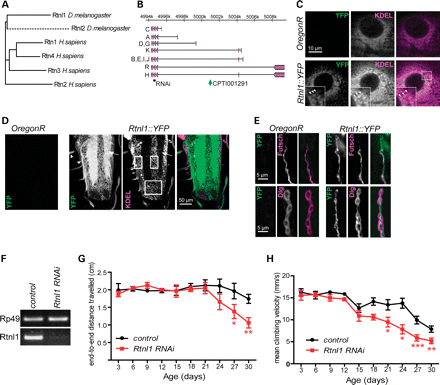
Loss of Drosophila reticulon causes a progressive locomotor deficit. (A) Dendrogram showing similarity of human and Drosophila reticulon proteins, drawn from a Clustal alignment using the neighbour-joining algorithm in MEGA 5.05. Dashed line indicates fast divergence of Drosophila Rtnl2. (B) The Rtnl1 locus showing proteins encoded by transcripts A-R (Flybase Genome Browser R5.41), location of VDRC RNAi fragments (RNAi) and the Rtnl1::YFP insertion (CPTI00291). (C–E) Confocal sections showing localization of Rtnl1::YFP in epidermal cells overlapping with KDEL (arrowheads in inset; C), in the larval ventral nerve cord (D) where it localizes preferentially in neuropil (arrow) and nerve bundles (arrowheads) in contrast to KDEL, which is concentrated within cell bodies (boxes), and in NMJ presynaptic boutons (E), where it follows the path of the axonal MT marker Futsch (upper panel). (F) VDRC RNAi line (construct GD900) successfully knocks down Rtnl1 expression as observed by PCR amplification of Rp49 and Rtnl1 cDNA from progeny of da-GAL4 crossed to either w1118 (control) or Rtnl1 RNAi flies. (G and H) Loss of Rtnl1 causes an age-dependent climbing deficit. Graphs represent end-to-end distance travelled in 15 s (G) and climbing velocity (H) of Rtnl1 RNAi and control flies (mean ± SEM here and in all subsequent graphs; *P < 0.05, **P < 0.01, ***P < 0.005, otherwise P > 0.05; two-way ANOVA and Bonferroni post-tests; n = 3 independent experiments).
An Rtnl1 exon trap insertion tagged with yellow fluorescent protein (YFP) (Fig. 1B) showed widespread expression in third-instar larvae, with the highest levels observed in neural tissues. Its subcellular localization was seen most clearly in epidermal cells, where it had a reticular distribution, overlapping with labelling of the ER retention signal KDEL (Fig. 1C). In contrast, in the CNS, relatively low levels of Rtnl1::YFP were found in cell bodies where most KDEL labelling is found (Fig. 1D); much higher levels were found in the neuropil (containing axons, dendrites and synapses) and in the nerves that connect the CNS to the periphery, which include motor neuron axons. In motor neuron terminals at the neuromuscular junction (NMJ), Rtnl1::YFP followed the distribution of the axonal MT marker Futsch (homologous to mammalian MAP1B), consistent with the established close association of tubular ER and MTs (Fig. 1E) (13,29). Therefore, Rtnl1::YFP overlaps with the ER, but its distinct distribution in neurons suggests that a smooth tubular ER compartment, marked by Rtnl1::YFP, is found preferentially in axons rather than in cell bodies.
To detect whether the loss of Rtnl1 had any overt phenotypic consequences, we tested for any obvious effects of ubiquitous knockdown of Rtnl1, using an RNAi insertion expressed under the control of da-Gal4, which resulted in almost complete depletion of Rtnl1 (Fig. 1F). Rtnl1 knockdown flies were viable, and an automated three-dimensional tracking system that measured the climbing behaviour of these flies in a negative geotaxis assay (30) showed that they had normal locomotor activity as early adults (Fig. 1G and H). However, as adults aged, they showed a progressive loss of locomotor activity relative to controls (Fig. 1G and H), consistent with a degenerative phenotype caused by Rtnl1 loss.
Rtnl1 is required for normal ER organization and function
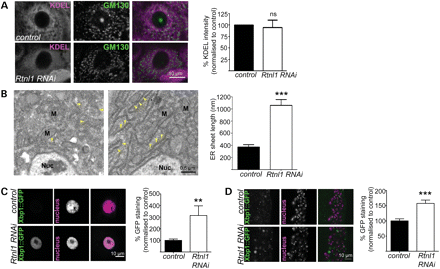
Loss of Rtnl1 causes expansion of ER sheets and increases the ER stress response. (A) KDEL distribution in third-instar larval epidermal cells is reticular in control larvae but more diffuse in Rtnl1 RNAi larvae; distribution of the Golgi marker GM130 is broadly unaffected. Graph shows KDEL staining intensity per cell as a percentage of control levels (n= 4 independent experiments; ns, not significant, P > 0.7). (B) Electron micrographs show increased length of ER sheets in Rtnl1 RNAi epidermal cells compared with controls. Arrowheads, ER sheets; Nuc, nucleus; M, mitochondrion. The graph shows the quantification of ER sheet cross-sectional length (***P < 0.005; n = 3 independent larvae). (C and D) Single-confocal sections of larval epidermal cells (C) and ventral nerve cord (D) show increased ER stress, measured by increased Xbp1::GFP expression in Rtnl1 RNAi larvae compared with controls and quantified in graphs (**P < 0.01, ***P < 0.005; n = 12–16).
The main signalling mechanism controlling ER homeostasis, including the size and the shape, is the ER unfolded protein response (31). This induces expression of a large number of genes that encode components of the ER-resident folding machinery, stimulate lipid biosynthesis and enlarge the ER (32). We therefore tested whether the expansion of ER sheets observed in Rtnl1 knockdown larvae coincided with an ER stress response, using the ER stress reporter Xbp1::enhanced green fluorescent protein (EGFP) in which EGFP is expressed in frame with Xbp1 only after ER-stress activated splicing occurs (33). Rtnl1 knockdown significantly increased the ER stress response compared with controls, both in epidermal cells and neurons (Fig. 2C and D).
Loss of Rtnl1 leads to defects in longer motor axons
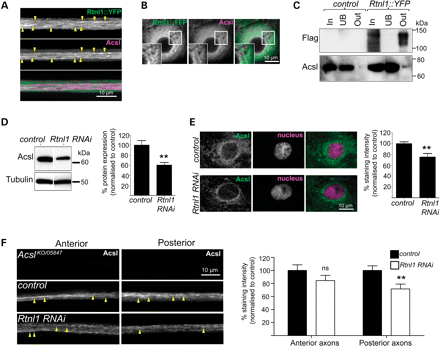
Rtnl1 interacts with the Acyl-CoA synthetase Acsl. Confocal sections showing overlapping localization of Rtnl1::YFP and Acsl in axons (linear staining indicated with arrowheads; A) and epidermal cells (B). (C) Protein lysates from OregonR (control) and Rtnl1::YFP flies were immunoprecipitated with Strep-Tactin Sepharose. Whole lysate (In), unbound (UB) and bound (Out) fractions were analysed by western blot. Loss of Rtnl1 reduces endogenous Acsl protein levels relative to controls in immunoblots of adult lysates (D) and single-confocal sections of larval epidermal cells (E). (F) Anti-Acsl staining of OK6GAL4;UAS-Acsl::myc motor neurons labels structures running along axons (arrowheads). Acsl staining is significantly lowered in posterior but not anterior segments of long motor axons upon knockdown of Rtnl1 (F). Graphs represent Acsl expression levels (ns, not significant, P > 0.2; **P < 0.01; n = 6–8 lysates in D, n = 14–16 larvae in E and F).
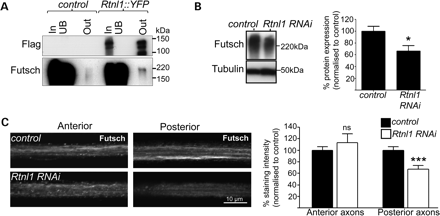
Rtnl1 interacts with the MT-associated protein Futsch. (A) Protein lysates from OregonR (control) and Rtnl1::YFP flies were immunoprecipitated and blotted as in Figure 3A. Loss of Rtnl1 reduces Futsch protein levels relative to controls in immunoblots of adult lysates (B) and in posterior but not anterior segments of long motor axons (C). Graphs represent Futsch expression levels (ns, not significant, P > 0.44; *P < 0.05, ***P < 0.001; n = 6–8 lysates in B, n = 16–19 larvae in C).
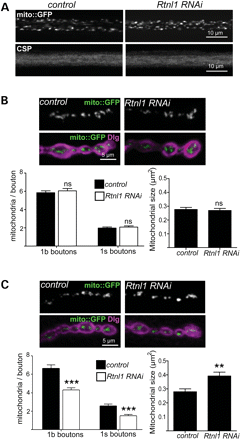
Loss of Rtnl1 causes disrupted mitochondrial organization in posterior NMJs
Effects of Rtnl1 loss on motor neuron mitochondria. (A) Single-confocal sections showing no effect of Rtnl1 RNAi (generated using OK6-GAL4 in this figure) on mito::GFP and CSP in posterior larval motor axons. (B and C) Single-confocal sections showing mito::GFP (green) and Dlg (magenta) at 1b boutons of anterior (B) and posterior (C) abdominal NMJs from control and Rtnl1 RNAi larvae. Graphs show the number (in 1b and 1s boutons) and the size (in 1b boutons) of mitochondria in posterior NMJs (ns, not significant; P > 0.5, **P < 0.01, ***P < 0.0005; n = 18–22 NMJs for 1b boutons, n = 12 NMJs for 1s boutons).
DISCUSSION
The hairpin loop protein reticulon, along with REEP, atlastin and spastin family members, is required for ER network organization. The disruption of the ER network, brought about by mutations affecting any one of these families, results in the degeneration of long axons as observed in HSP. However, how mutations in these proteins can specifically target axonal degeneration is not understood. We describe a novel Drosophila model of HSP caused by loss of the orthologue of SPG12, Rtnl1. This is the first animal model providing evidence of an ER phenotype due to the loss of reticulon and shows that major rearrangements of ER morphology do not noticeably affect organismal survival. These findings are consistent with previous studies investigating reticulon function both in the yeast Saccharomyces cervecisiae and in the plant Arabidopsis thaliana (10,16,42,43). Importantly, our model also allows the investigation of the role of tubular ER in axons; the loss of Rtnl1 selectively effects longer axons, suggesting a mechanistic similarity between the cellular phenotypes of Drosophila Rtnl1 knockdown and of spastic paraplegias that are caused by haplo-insufficient loss-of-function or dominant negative alleles of hairpin-loop proteins (15).
Model of hairpin loop protein dysfunction in motor neuron axonopathy. (A) The schematic diagram represents motor neurons under ‘normal’ or non-diseased conditions. Ribosomal studded RER sheets predominate within the neuronal cell body, whereas tubular SER runs the length of the axon closely associated with the MT cytoskeleton (13,21,29). In addition, the ER has extensive contact points with mitochondria which are required for lipid biosynthesis, calcium homeostasis and mitochondrial division (38,46). (B) Mutation or depletion of any one of the hairpin loop proteins causes disruption of the ER network (either less extensive and/or discontinuous) and degeneration of long motor axons as observed in HSP. Our findings have revealed an increased susceptibility of posterior axons to the disruption of ER organization, resulting in the disruption of tubular SER, MT cytoskeleton and mitochondria. This suggests that the loss of tubular ER from long motor axons may be the mechanism by which the loss of hairpin loop protein function gives rise to motor neuron axonopathy.
Mitochondria and the ER have extensive contact points that are crucial for calcium homoeostasis and lipid biosynthesis (38). These contact points are mediated in part by the mitochondrial fusion protein Mfn2 and are stably maintained during ER sliding along MTs (44,45). The loss of Rtnl1 from motor neurons resulted in fewer, larger mitochondria within boutons of posterior, but not anterior, NMJs (Fig. 5). ER tubules appear to play a role in defining the position of mitochondrial division sites (46); therefore, mitochondrial fission at terminals of the longer motor axons could be more sensitive to ER tubule disruption, and the reduction in mitochondria in posterior axons could thus be a consequence of ER defects caused by loss of Rtnl1 (Fig. 6B). Furthermore, mitochondrial dysfunction is also implicated in some forms of HSP: mutations in a number of mitochondrial proteins can cause HSP (39–41); a number of other HSP proteins, spatacsin, spastizin and spartin (SPG11, SPG15 and SPG20, respectively) are reported to localize at least in part to mitochondria (47–49); and a possible mitochondrial fission defect has been reported in at least one SPG31 patient (50). Our observations indicate the possibility of mechanisms that may link the pathogenesis of HSP proteins involved in ER morphogenesis and mitochondrial dysfunction. It is worth noting however that in addition to mitochondria, the ER contacts other membranous organelles such as endosomes and mutlivesicular bodies as well as the plasma membrane (44,51,52). These interactions may also be affected by disruption of tubular ER.
Our results provide the first direct evidence of SER defects in mature axons in a loss-of-function SPG genotype. They also provide a route to further study the roles of the SER in normal neurons, impairment of which may lead to degeneration. These roles potentially include local effects on processes such as mitochondrial function and calcium homeostasis, but also long-distance communication along SER tubules, which extend throughout neurons, as in the ‘neuron within a neuron’ model (53). The study of HSP model flies in which SER organization is impaired promises to illuminate both the function of this fascinating compartment and the cell physiological processes that may be impaired by its disruption, and that may contribute to progressive axonal degeneration that spreads proximally from distal axonal ends.
MATERIALS AND METHODS
Fly stocks
The Rtnl1 exon trap line CPTI-001291 contains FLAG and StrepII tags in addition to Venus YFP (http://flybase.org/reports/FBal0262134.html). For knockdown experiments, either the UAS-Rtnl1-RNAi line 7866 (construct GD900, which has no predicted off-targets) or the w1118 control stock, 60000 (obtained from the Vienna Drosophila RNAi Center, www.vdrc.at) (54), was crossed with either da-GAL4 (55) or OK6-GAL4 (56); da-GAL4 was used except where indicated otherwise. Other fly stocks used were xbp1::EGFP (33), PBac{RB3.WH3}AcslKO (37), P{PZ}Acsl05847 (34), P{UAS-Acsl.715.MycC}3 (34) and mito::GFP (P{UAS-mito-HA-GFP.AP}3) (57).
Semi-quantitative PCR
Total RNA was purified from five male flies (<3 days post-eclosion) collected in TRIzol reagent (Invitrogen, Paisley, UK). cDNA was produced using the SuperScript III First Strand kit (Invitrogen, UK), 50 µm oligo-dT primer and 2 µg of DNase-treated RNA. Amplification of Rp49 mRNA was used to control for cDNA concentration in the PCR reaction. Primers used were: Rp49-L: CCGACCACGTTACAAGAACTCTC; Rp49-R: CGCTTCAAGGGACAGTATCTGA; Rtnl1-L: ACGTAGGAGCACCGCGAACG; and Rtnl1-R: CTGAAGGCGACGGTGCCGAA. PCR conditions were 95°C for 30 s, 60°C for 30 s and 72°C for 1 min, repeated for 20 cycles for Rp49 and 25 cycles for Rtnl1. At least six different samples for each genotype were analysed. PCR products were visualized with ethidium bromide on a gel, and band intensities quantified using ImageJ.
Immunoprecipitation and immunoblot
Soluble protein lysates were purified from male flies (<3 days post-eclosion) by homogenization in 1% Triton X-100, 25 mm HEPES (pH 7.4), 150 mm NaCl, 2 mm EDTA and Protease Inhibitor Cocktail (Sigma, Poole, UK), followed by centrifugation at 15 000g for 30 min at 4°C. Immunoprecipitation was carried out with Strep-Tactin Sepharose (IBA, Göttingen, Germany). Briefly, protein samples (Input) were incubated overnight at 4°C with Strep-Tactin Sepharose, and unbound and bound proteins were collected by centrifugation. For immunoblot, protein samples were separated on polyacrylamide minigels and electrophoretically transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). The nitrocellulose was blocked in 5% non-fat milk in 10 mm Tris–HCl, 100 mm NaCl and 0.05% Tween-20 and incubated with primary antibodies overnight at 4°C. Primary antibodies used were against: FLAG (F1804; Sigma), Acsl (34), α-tubulin (T9026; Sigma) and Futsch (22C10; Hybridoma) (58). After incubation with HRP (horseradish peroxidase)-coupled secondary antibodies and SuperSignal Chemiluminescent Substrate (Pierce), bands were visualized by autoradiography. Band intensities were determined using ImageJ, and levels from knockdown samples normalized to control samples on the same film.
Histology and immunomicroscopy
Third-instar larvae were dissected in chilled Ca2+-free HL3 solution (59), fixed in 4% formaldehyde in PBS for 30 min and permeabilized in 0.1% Triton X-100 in PBS. Primary antibodies used were as for immunoblotting and against: GFP (ab6556; Abcam, Cambridge, UK), KDEL (MAC256; Abcam), GM130 (Ab32337; Abcam), HRP (P7899; Sigma), Discs-large (4F3) (60) and cysteine string protein (DCSP-2 (6D6) (61). The nuclear stain used was TO-PRO-3 iodide (T3605; Invitrogen). Fixed preparations were mounted in Vectashield (Vector Laboratories) and viewed using a Nikon CSi confocal head mounted on an Eclipse 90i microscope. Motor axons were imaged passing through segment A2 (‘anterior’) and segment A6 (‘posterior’). NMJs were imaged at muscle 6/7 of segment A3 (‘anterior’) and segment A6 (‘posterior’). Images were acquired using a 60×/1.4NA objective and the Nikon EZC1 3.90 software, and imported to ImageJ. Brightness, contrast and colour channels were adjusted using Photoshop (Adobe).
Image analysis
For fluorescence intensity analysis, control and mutant larvae were stained and washed in the same tube, with one genotype tail-clipped for identification. Mean grey intensity for regions of interest was quantified in ImageJ, using at least three independent experiments, each with at least four larvae. Mitochondria were counted manually, blind to genotype. Type 1b and 1s boutons were differentiated by intensity of Dlg staining.
Electron microscopy
Third-instar larvae were dissected in chilled Ca2+-free HL3 solution and fixed in 3% glutaraldehyde, 0.3% hydrogen peroxide and 2 mm sodium EDTA for 4 h at 4°C. After four washes in 0.1 m HEPES buffer, larva were post-fixed in 1% osmium ferricyanide (Oxchem, Edinburgh, UK) in buffer at room temperature for 1 h. After rinsing in de-ionized water, samples were stained in 2% uranyl acetate in maleate buffer, dehydrated in an ethanol series, followed by acetonitrile and then infiltrated with Quetol epoxy resin (Agar Scientific, Stansted, UK) and cured at 60°C for 48 h. Sections were cut on a Leica Ultracut UCT ultra-microtome at 70 nm, using a diamond knife, and contrasted with uranyl acetate and lead citrate. They were viewed using a Tecnai G2 electron microscope operated at 120 kV, and an AMT XR60B camera running the Deben software. EM images were collected from three larvae per genotype, with at least five epidermal cells imaged per larva. Images were blinded for genotype before ER branch length was determined in ImageJ.
Locomotor assay
Locomotor assays were carried out as described (30), using 10 flies per vial, 3 vials per experiment and 3 independent experiments. Flies were kept at 25°C and assayed every 3 days. Climbing ability was determined over 15 s in a vertical glass vial (10 cm length, 2.5 cm diameter). All trajectories were quantified for their end-to-end distance and their velocity.
Statistical analysis
Data were exported to GraphPad Prism 5 (GraphPad Software, Inc.) for statistical analysis. Statistical significance was determined using Student's t-test, except for analysis of climbing, where two-way ANOVA and Bonferroni post hoc analyses were used.
ACKNOWLEDGEMENTS
We thank Z. Wang, H.D. Ryoo, T.E. Rusten, J. Roote, the Developmental Studies Hybridoma Bank and the Bloomington and Vienna Drosophila Stock Centres for antibodies and stocks. We thank J. Skepper and J. Powell for help with EM, K. Lilley for support with mass spectrometry analysis, and D. Crowther and A. Mok for assistance with the fly climbing assay.
Conflict of Interest statement. None declared.
FUNDING
N.C.O'S. was supported by Marie Curie Individual Fellowship 236777. E.R. is a Wellcome Trust Senior Research Fellow in Clinical Science (grant 082381) and is supported by a strategic award from the Wellcome Trust to CIMR (grant 079895). Funding to pay the Open Access publication charges for this article was provided by the Wellcome Trust.