Abstract
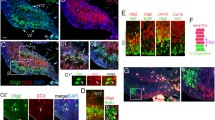
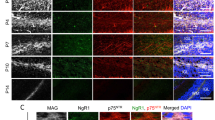
Notch signaling plays an important role in the process of cell-fate assignation during nervous system development. DNER is a neuron-specific transmembrane protein carrying extracellular EGF-like repeats and is expressed in somatodendritic regions.In vitro studies demonstrated that DNER mediates Notch signaling by cell-cell interaction. In the cerebellum, DNER is abundantly expressed in Purkinje cells and moderately in granule cells. DNER-knockout mice showed motor discoordination. The mutant cerebellum showed morphological impairments of Bergmann glia and multiple innervation between climbing fibers and Purkinje cells. Moreover, glutamate clearance at the synapses between parallel fibers and Purkinje cells was significantly weakened, and the expression of GLAST, a glutamate transporter in Bergmann glia, was reduced in the mutant cerebellum. Therefore, DNER contributes to the morphological and functional maturation of Bergmann glia via the Notch signaling pathway, and is essential for precise cerebellar development.
Similar content being viewed by others
References
Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–6.
Mumm JS, Kopan R. Notch signaling: From the outside in. Dev Biol. 2000;228:151–65.
Struhl G, Adachi A. Requirements for presenilin-dependent cleavage of Notch and other transmembrane proteins. Mol Cell. 2000;6:625–36.
Ramain P, Khechumian K, Seugnet L, Arbogast N, Ackermann C, Heitzler P. Novel Notch alleles reveal a Deltex-dependent pathway repressing neural fate. Curr Biol. 2001;11:1729–38.
De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Rayk WJ, Goatek A, Kopan R. A presenillin-1-dependent (-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–22.
Kageyama R, Ohtsuka T, Hatakeyama J, Ohsawa R. Roles of bHLH genes in neural stem cell differentiation. Exp Cell Res. 2005;306:343–8.
Wu L, Aster JC, Blacklow SC, Lake R, Artavanis-Tsakonas S, Griffin JD. MAML1, a human homologue of Drosophila mastermind, is a transcriptional co-activator for Notch receptors. Nat Genet. 2000;26:484–9.
Bailey AM, Posakony JW. Suppressor of hairless directly activates transcription of enhancer of split complex genes in response to Notch receptor activity. Genes Dev. 1995;9: 2609–22.
Matsuno K, Ito M, Hori K, Miyashita F, Suzuki S, Kishi N, Artavanis-Tsakonas S, Okano H. Involvement of a proline-rich motif and RING-H2 finger of Deltex in the regulation of Notch signaling. Development. 2002;129:1049–59.
Liu WH, Lai MZ. Deltex regulates T-cell activation by targeted degradation of active MEKK1. Mol Cell Biol. 2005;25:1367–78.
Anderson DJ. Stem cells and pattern formation in the nervous system: The possible versus the actual. Neuron. 2001;30: 19–35.
Eiraku M, Hirata Y, Takeshima H, Hirano T, Kengaku M. Delta/Notch-like epidermal growth factor (EGF)-related receptor, a novel EGF-like repeat-containing protein targeted to dendrites of developing and adult central nervous system neurons. J Biol Chem. 2002;277:25400–07.
Nishizumi H, Komiyama T, Miyabayashi T, Sakano S, Sakano H. BET, a novel neuronal transmembrane protein with multiple EGF-like motifs. NeuroReport. 2002;13: 909–15.
Handford PA, Mayhew M, Baron M, Winship PR, Campbell ID, Brownlee GG. Key residues involved in calcium-binding motifs in EGF-like domains. Nature. 1991;351:164–7.
Bonifacino JS, Traub LM. Signals for sorting of transmem-brane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447.
Eiraku M, Tohgo A, Ono K, Kaneko M, Fujishima K, Hirano T, Kengaku M. DNER acts as a neuron-specific Notch ligand during Bergmann glial development. Nat Neurosci. 2005;8:873–80.
Shawber C, Nofziger D, Hsieh JJ, Lindsell C, Bogler O, Hayward D, Weinmaster G. Notch signaling inhibits muscle cell differentiation through a CBF1-independent pathway. Development. 1996;122:3765–73.
Patten BA, Peyrin JM, Weinmaster G, Corfas G. Sequential signaling through Notch1 and erbB receptors mediates radial glia differentiation. J Neurosci. 2003;23:6132–40.
Tohgo A, Eiraku M, Miyazaki T, Miura E, Kawaguchi S, Nishi M, Watanabe M, Hirano T, Kengaku M, Takeshima H. Impaired cerebellar functions in mutant mice lacking DNER. Mol Cell Neurosci. 2005 in press.
Watase K, Hashimoto K, Kano M, Yamada K, Watanabe M, Inoue Y, Okuyama S, Sakagawa T, Ogawa S, Kawashima N, Hori S, Takimoto M, Wada K, Tanaka K. Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. Eur J Neurosci. 1998;10:976–88.
Lordkipanidze T, Dunaevsky A. Purkinje cell dendrites grow in alignment with Bergmann glia. Glia. 2005;51: 229–34.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saito, SY., Takeshima, H. DNER as key molecule for cerebellar maturation. Cerebellum 5, 227–231 (2006). https://doi.org/10.1080/14734220600632564
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1080/14734220600632564