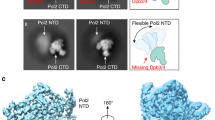
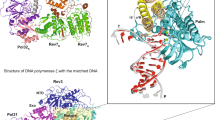
Abstract
DNA polymerase δ (Polδ) plays pivotal roles in eukaryotic DNA replication and repair. Polδ is conserved from yeast to humans, and mutations in human Polδ have been implicated in various cancers. Saccharomyces cerevisiae Polδ consists of catalytic Pol3 and the regulatory Pol31 and Pol32 subunits. Here, we present the near atomic resolution (3.2 Å) cryo-EM structure of yeast Polδ holoenzyme in the act of DNA synthesis. The structure reveals an unexpected arrangement in which the regulatory subunits (Pol31 and Pol32) lie next to the exonuclease domain of Pol3 but do not engage the DNA. The Pol3 C-terminal domain contains a 4Fe−4S cluster and emerges as the keystone of Polδ assembly. We also show that the catalytic and regulatory subunits rotate relative to each other and that this is an intrinsic feature of the Polδ architecture. Collectively, the structure provides a framework for understanding DNA transactions at the replication fork.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The cryo-EM density map has been deposited in the Electron Microscopy Data Bank under accession code EMD-20235. Atomic coordinates have been deposited in the Protein Data Bank (https://www.rcsb.org) with accession code 6P1H. All reagents and relevant data are available from the authors upon reasonable request.
References
Johnson, R. E., Klassen, R., Prakash, L. & Prakash, S. A major role of DNA polymerase delta in replication of both the leading and lagging DNA strands. Mol. Cell 59, 163–175 (2015).
Hartwell, L. H. Sequential function of gene products relative to DNA synthesis in the yeast cell cycle. J. Mol. Biol. 104, 803–817 (1976).
Stillman, B. Reconsidering DNA polymerases at the replication fork in eukaryotes. Mol. Cell 59, 139–141 (2015).
Jain, R., Aggarwal, A. K. & Rechkoblit, O. Eukaryotic DNA polymerases. Curr. Opin. Struct. Biol. 53, 77–87 (2018).
Burgers, P. M. J. & Kunkel, T. A. Eukaryotic DNA replication fork. Annu. Rev. Biochem. 86, 417–438 (2017).
Boulet, A., Simon, M., Faye, G., Bauer, G. A. & Burgers, P. M. Structure and function of the Saccharomyces cerevisiae CDC2 gene encoding the large subunit of DNA polymerase III. EMBO J. 8, 1849–1854 (1989).
Simon, M., Giot, L. & Faye, G. The 3′ to 5′ exonuclease activity located in the DNA polymerase delta subunit of Saccharomyces cerevisiae is required for accurate replication. EMBO J. 10, 2165–2170 (1991).
Gerik, K. J., Li, X., Pautz, A. & Burgers, P. M. Characterization of the two small subunits of Saccharomyces cerevisiae DNA polymerase delta. J. Biol. Chem. 273, 19747–19755 (1998).
Johansson, E., Garg, P. & Burgers, P. M. The Pol32 subunit of DNA polymerase delta contains separable domains for processive replication and proliferating cell nuclear antigen (PCNA) binding. J. Biol. Chem. 279, 1907–1915 (2004).
Johansson, E., Majka, J. & Burgers, P. M. Structure of DNA polymerase delta from Saccharomyces cerevisiae. J. Biol. Chem. 276, 43824–43828 (2001).
Sanchez Garcia, J., Ciufo, L. F., Yang, X., Kearsey, S. E. & MacNeill, S. A. The C-terminal zinc finger of the catalytic subunit of DNA polymerase delta is responsible for direct interaction with the B-subunit. Nucleic Acids Res. 32, 3005–3016 (2004).
Mondol, T., Stodola, J. L., Galletto, R. & Burgers, P. M. PCNA accelerates the nucleotide incorporation rate by DNA polymerase delta. Nucleic Acids Res. 47, 1977–1986 (2019).
Acharya, N., Klassen, R., Johnson, R. E., Prakash, L. & Prakash, S. PCNA binding domains in all three subunits of yeast DNA polymerase delta modulate its function in DNA replication. Proc. Natl Acad. Sci. USA 108, 17927–17932 (2011).
Klinge, S., Nunez-Ramirez, R., Llorca, O. & Pellegrini, L. 3D architecture of DNA Pol alpha reveals the functional core of multi-subunit replicative polymerases. EMBO J. 28, 1978–1987 (2009).
Nakane, T., Kimanius, D., Lindahl, E. & Scheres, S. H. Characterisation of molecular motions in cryo-EM single-particle data by multi-body refinement in RELION. Elife 7, e36861 (2018).
Swan, M. K., Johnson, R. E., Prakash, L., Prakash, S. & Aggarwal, A. K. Structural basis of high-fidelity DNA synthesis by yeast DNA polymerase delta. Nat. Struct. Mol. Biol. 16, 979–986 (2009).
Baranovskiy, A. G. et al. X-ray structure of the complex of regulatory subunits of human DNA polymerase delta. Cell Cycle 7, 3026–3036 (2008).
Netz, D. J. et al. Eukaryotic DNA polymerases require an iron-sulfur cluster for the formation of active complexes. Nat. Chem. Biol. 8, 125–132 (2011).
Chen, Z. et al. Programmable design of orthogonal protein heterodimers. Nature 565, 106–111 (2019).
Suwa, Y. et al. Crystal structure of the human Pol alpha B subunit in complex with the C-terminal domain of the catalytic subunit. J. Biol. Chem. 290, 14328–14337 (2015).
Baranovskiy, A. G. et al. Crystal structure of the human Pol B-subunit in complex with the C-terminal domain of the catalytic subunit. J. Biol. Chem. 292, 15717–15730 (2017).
Adman, E., Watenpaugh, K. D. & Jensen, L. H. NH···S hydrogen bonds in Peptococcus aerogenes ferredoxin, Clostridium pasteurianum rubredoxin and Chromatium high potential iron protein. Proc. Natl Acad. Sci. USA 72, 4854–4858 (1975).
Walters, M. A., Roche, C. L., Rheingold, A. L. & Kassel, S. W. N–H···S hydrogen bonds in a ferredoxin model. Inorg. Chem. 44, 3777–3779 (2005).
Carter, C. W. Jr, Kraut, J., Freer, S. T. & Alden, R. A. Comparison of oxidation–reduction site geometries in oxidized and reduced Chromatium high potential iron protein and oxidized Peptococcus aerogenes ferredoxin. J. Biol. Chem. 249, 6339–6346 (1974).
Chen, K. et al. Crystal structures of ferredoxin variants exhibiting large changes in [Fe-S] reduction potential. Nat. Struct. Biol. 9, 188–192 (2002).
Bartels, P. L., Stodola, J. L., Burgers, P. M. J. & Barton, J. K. A redox role for the [4Fe4S] cluster of yeast DNA polymerase delta. J. Am. Chem. Soc. 139, 18339–18348 (2017).
Tse, E. C. M., Zwang, T. J. & Barton, J. K. The oxidation state of [4Fe4S] clusters modulates the DNA-binding affinity of DNA repair proteins. J. Am. Chem. Soc. 139, 12784–12792 (2017).
Sanchez Garcia, J. et al. Functional mapping of the fission yeast DNA polymerase delta B-subunit Cdc1 by site-directed and random pentapeptide insertion mutagenesis. BMC Mol. Biol. 10, 82 (2009).
Johnson, R. E., Prakash, L. & Prakash, S. Pol31 and Pol32 subunits of yeast DNA polymerase delta are also essential subunits of DNA polymerase zeta. Proc. Natl Acad. Sci. USA 109, 12455–12460 (2012).
Jin, Y. H. et al. The multiple biological roles of the 3′→5′ exonuclease of Saccharomyces cerevisiae DNA polymerase delta require switching between the polymerase and exonuclease domains. Mol. Cell Biol. 25, 461–471 (2005).
Marquez, L. A. & Reha-Krantz, L. J. Using 2-aminopurine fluorescence and mutational analysis to demonstrate an active role of bacteriophage T4 DNA polymerase in strand separation required for 3′→5′-exonuclease activity. J. Biol. Chem. 271, 28903–28911 (1996).
Shamoo, Y. & Steitz, T. A. Building a replisome from interacting pieces: sliding clamp complexed to a peptide from DNA polymerase and a polymerase editing complex. Cell 99, 155–166 (1999).
Giot, L., Chanet, R., Simon, M., Facca, C. & Faye, G. Involvement of the yeast DNA polymerase delta in DNA repair in vivo. Genetics 146, 1239–1251 (1997).
Jozwiakowski, S. K., Kummer, S. & Gari, K. Human DNA polymerase delta requires an iron-sulfur cluster for high-fidelity DNA synthesis. Life Sci. Alliance 2, e201900321 (2019).
Jain, R. et al. Structural insights into yeast DNA polymerase delta by small angle X-ray scattering. J. Mol. Biol. 394, 377–382 (2009).
Rodrigues, C. H., Pires, D. E. & Ascher, D. B. DynaMut: predicting the impact of mutations on protein conformation, flexibility and stability. Nucleic Acids Res. 46, W350–W355 (2018).
Jin, Y. H., Ayyagari, R., Resnick, M. A., Gordenin, D. A. & Burgers, P. M. Okazaki fragment maturation in yeast. II. Cooperation between the polymerase and 3′-5′-exonuclease activities of Pol delta in the creation of a ligatable nick. J. Biol. Chem. 278, 1626–1633 (2003).
Campbell, B. B. et al. Comprehensive analysis of hypermutation in human cancer. Cell 171, 1042–1056 e1010 (2017).
Rayner, E. et al. A panoply of errors: polymerase proofreading domain mutations in cancer. Nat. Rev. Cancer 16, 71–81 (2016).
Flohr, T. et al. Detection of mutations in the DNA polymerase delta gene of human sporadic colorectal cancers and colon cancer cell lines. Int. J. Cancer 80, 919–929 (1999).
da Costa, L. T. et al. Polymerase delta variants in RER colorectal tumours. Nat. Genet. 9, 10–11 (1995).
Elouej, S. et al. Exome sequencing reveals a de novo POLD1 mutation causing phenotypic variability in mandibular hypoplasia, deafness, progeroid features and lipodystrophy syndrome (MDPL). Metabolism 71, 213–225 (2017).
Zhuang, Z. et al. Regulation of polymerase exchange between Poleta and Poldelta by monoubiquitination of PCNA and the movement of DNA polymerase holoenzyme. Proc. Natl Acad. Sci. USA 105, 5361–5366 (2008).
Suloway, C. et al. Automated molecular microscopy: the new Leginon system. J. Struct. Biol. 151, 41–60 (2005).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Lander, G. C. et al. Appion: an integrated, database-driven pipeline to facilitate EM image processing. J. Struct. Biol. 166, 95–102 (2009).
Voss, N. R., Yoshioka, C. K., Radermacher, M., Potter, C. S. & Carragher, B. DoG Picker and TiltPicker: software tools to facilitate particle selection in single particle electron microscopy. J. Struct. Biol. 166, 205–213 (2009).
Scheres, S. H. et al. Maximum-likelihood multi-reference refinement for electron microscopy images. J. Mol. Biol. 348, 139–149 (2005).
Sorzano, C. O. et al. XMIPP: a new generation of an open-source image processing package for electron microscopy. J. Struct. Biol. 148, 194–204 (2004).
Sorzano, C. O. et al. A clustering approach to multireference alignment of single-particle projections in electron microscopy. J. Struct. Biol. 171, 197–206 (2010).
Roseman, A. M. FindEM—a fast, efficient program for automatic selection of particles from electron micrographs. J. Struct. Biol. 145, 91–99 (2004).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-. Elife 7, e42166 (2018).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Scheres, S. H. & Chen, S. Prevention of overfitting in cryo-EM structure determination. Nat. Methods 9, 853–854 (2012).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
The PyMOL Molecular Graphics System, Version 2.0 (Schrödinger).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Barad, B. A. et al. EMRinger: side chain-directed model and map validation for 3D cryo-electron microscopy. Nat. Methods 12, 943–946 (2015).
Acknowledgements
We thank B. Carragher, C. Potter and E. Eng for helpful advice and discussions throughout the project. Some of the work was supported by grant GM129689 from the NIH (S.P.). Initial EM screening was performed at the Icahn School of Medicine microscope facility supported by a shared instrumentation grant from the NIH (1S10RR026473). Some of this work was performed at the Simons Electron Microscopy Center and National Resource for Automated Molecular Microscopy located at the New York Structural Biology Center, supported by grants from the Simons Foundation (SF349247), NYSTAR and the NIH National Institute of General Medical Sciences (GM103310), with additional support from Agouron Institute (F00316), NIH (OD019994) and NIH (RR029300). Computing resources needed for this work were provided in part by the High Performance Computing facility of the Icahn School of Medicine at Mount Sinai. Molecular graphics and analyses were performed with UCSF Chimera, developed by the Resource for Biocomputing, Visualization and Informatics at the University of California, San Francisco, with support from NIH P41-GM103311.
Author information
Authors and Affiliations
Contributions
A.K.A. and R.J. conceived the project and designed the experiments. R.E.J. expressed the complex in yeast. R.J. purified the complex for cryo-EM. R.M. prepared the grids. R.J. and W.J.R. collected the data and reconstructed the 3D structures. R.J. built and refined the atomic models. A.K.A. guided the overall project, I.U.-B. guided some of the cryo-EM experiments, and S.P. and L.P. guided the protein expression studies. R.J. and A.K.A. prepared the manuscript, with input from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Beth Moorefield was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Cryo-EM image processing.
Particles from three different data sets were merged and 3D refinement was performed with both cryoSPARC and RELION 3.0 beta to yield consensus maps at nominal resolution of 3.2 and 3.4 Å respectively. Consensus map from RELION was subjected to multi-body refinement, resulting in major improvement in density for Pol32N (circled in red) and regions of Pol31 that are furthest from the catalytic module.
Supplementary Figure 2 Comparison of Polδ regulatory module and CysBD with those in homologs.
a, The CysBD interacting interface of the regulatory module is substantially different between yeast Polδ, human Polδ, human Polα and human Polε. b, CysBD of yeast Polδ is much smaller in size than the counterparts from pols α and ε. CysAD is disordered in yeast Polδ.
Supplementary Figure 3 Model of Polδ–PCNA complex.
Model of yeast Polδ (this work) and PCNA (PDB 2OD8) derived by threading the Polδ bound DNA through the central hole of the PCNA.
Supplementary Figure 4 CysBD mediated changes in the Pol3 catalytic module.
a, CysBD draws the exo and thumb domains of the catalytic module closer than their positions in the structure of the isolated catalytic module (3IAY) and results in an overall compaction of the catalytic module by >2.5 Å. b, A primer modeled at the exo active side is within van der Waals distance from Tyr496, which is part of the exo domain loop encompassing amino acids 490–497 that interacts with CysBD. This loop is disordered in the structure of the isolated catalytic module (3IAY).
Supplementary Figure 5 Multi-body refinement and principal component analysis of the relative orientations of the catalytic and regulatory modules.
a, Consensus and multi-body maps colored by local resolution. Multi-body analysis results in a substantial improvement in resolution for regions of Pol31 and Pol32 that are farthest from the catalytic module, for example, Pol32N and the OB fold of Pol31. b, Contribution of all eigenvectors to the variance. c, Histograms of amplitudes along the first two eigenvectors. Both histograms are unimodal, indicating continuous motion.
Supplementary Figure 6 Trajectories from normal mode analysis compared to motion from multi-body analysis.
End of range conformations for the regulatory module are shown in green and red. The first eigenvector from principal component analysis of multi-body refinement represents rocking motion of the regulatory module parallel to the catalytic module (middle), while the second eigenvector represents rocking motion towards the catalytic module (bottom). Trajectories for the first non-trivial mode of molecular motion from normal mode analysis (top) correspond to the motion represented by the first eigenvector (middle).
Supplementary Figure 7 Disease mutations in human Polδ.
Cancer driver mutations in human Polδ mapped on the structure of yeast Polδ (top). These mutations are distributed on the NTD, exo, palm, fingers and CysAD domains. The oncogenic R506H (exo) mutation and the MDPL associated R507C (exo) and I1070N (CysBD) mutations map to the interface between the catalytic and regulatory modules.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7.
Supplementary Video 1
Motion represented by the first eigenvector from multi-body refinement. Motion represented by the first eigenvector corresponds to a rocking motion of the regulatory module parallel to the catalytic module. The DNA bound catalytic module is shown in cyan and CysBD and the regulatory module are in green.
Supplementary Video 2
Motion represented by the first eigenvector from multi-body refinement. Motion represented by the first eigenvector corresponds to a rocking motion of the regulatory module parallel to the catalytic module. DNA bound catalytic module is shown in cyan and CysBD and the regulatory module are in green.
Rights and permissions
About this article
Cite this article
Jain, R., Rice, W.J., Malik, R. et al. Cryo-EM structure and dynamics of eukaryotic DNA polymerase δ holoenzyme. Nat Struct Mol Biol 26, 955–962 (2019). https://doi.org/10.1038/s41594-019-0305-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-019-0305-z
This article is cited by
-
Cryo-EM structures of human monkeypox viral replication complexes with and without DNA duplex
Cell Research (2023)
-
Structure of the processive human Pol δ holoenzyme
Nature Communications (2020)
-
Structure and mechanism of B-family DNA polymerase ζ specialized for translesion DNA synthesis
Nature Structural & Molecular Biology (2020)
-
Structure of DNA polymerase ζ: capturing the getaway driver
Nature Structural & Molecular Biology (2020)