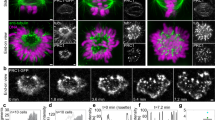
Abstract
A remarkable feature of the microtubule cytoskeleton is the coexistence of subpopulations having different dynamic properties. A prominent example is the anaphase spindle, where stable antiparallel bundles exist alongside dynamic microtubules and provide spatial cues for cytokinesis. How are the dynamics of spatially proximal arrays differentially regulated? We reconstitute a minimal system of three midzone proteins: microtubule-crosslinker PRC1 and its interactors CLASP1 and Kif4A, proteins that promote and suppress microtubule elongation, respectively. We find that their collective activity promotes elongation of single microtubules while simultaneously stalling polymerization of crosslinked bundles. This differentiation arises from (1) strong rescue activity of CLASP1, which overcomes the weaker effects of Kif4A on single microtubules, and (2) lower microtubule- and PRC1-binding affinity of CLASP1, which permits the dominance of Kif4A at overlaps. In addition to canonical mechanisms where antagonistic regulators set microtubule length, our findings illuminate design principles by which collective regulator activity creates microenvironments of arrays with distinct dynamic properties.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The following datasets and their analyses generated during the current study are available from the corresponding author on reasonable request:
raw microscopy images and videos for Figs. 1a,e, 2b–e and 3a–c and Extended Data Figs. 2c,d, 3, 4, 6, 7a,b, 8 and 9a,c;
scanned SDS gel images for Extended Data Figs. 1a and 7d.
Source data for Figs. 1b–f, 4a–f and 5a,b,d and Extended Data Figs. 1b,c, 2a–c,e–i, 4a, 5a,b, 6b,d,f, 7a–c, 8b and 9a–c. Source data are provided with this paper.
References
Bitan, A., Rosenbaum, I. & Abdu, U. Stable and dynamic microtubules coordinately determine and maintain Drosophila bristle shape. Development 139, 1987–1996 (2012).
Pous, C. et al. Functional specialization of stable and dynamic microtubules in protein traffic in WIF-B cells. J. Cell Biol. 142, 153–165 (1998).
Baas, P. W., Rao, A. N., Matamoros, A. J. & Leo, L. Stability properties of neuronal microtubules. Cytoskeleton (Hoboken) 73, 442–460 (2016).
Lindeboom, J. J. et al. A mechanism for reorientation of cortical microtubule arrays driven by microtubule severing. Science 342, 1245533 (2013).
Foe, V. E. & von Dassow, G. Stable and dynamic microtubules coordinately shape the myosin activation zone during cytokinetic furrow formation. J. Cell Biol. 183, 457–470 (2008).
Vukusic, K., Buda, R. & Tolic, I. M. Force-generating mechanisms of anaphase in human cells. J. Cell Sci. 132, jcs231985 (2019).
Rizk, R. S. et al. MCAK and paclitaxel have differential effects on spindle microtubule organization and dynamics. Mol. Biol. Cell 20, 1639–1651 (2009).
Mastronarde, D. N., McDonald, K. L., Ding, R. & McIntosh, J. R. Interpolar spindle microtubules in PTK cells. J. Cell Biol. 123, 1475–1489 (1993).
Saxton, W. M. & McIntosh, J. R. Interzone microtubule behavior in late anaphase and telophase spindles. J. Cell Biol. 105, 875–886 (1987).
Murthy, K. & Wadsworth, P. Dual role for microtubules in regulating cortical contractility during cytokinesis. J. Cell Sci. 121, 2350–2359 (2008).
Uehara, R. & Goshima, G. Functional central spindle assembly requires de novo microtubule generation in the interchromosomal region during anaphase. J. Cell Biol. 191, 259–267 (2010).
Uehara, R. et al. Augmin shapes the anaphase spindle for efficient cytokinetic furrow ingression and abscission. Mol. Biol. Cell 27, 812–827 (2016).
Pamula, M. C. et al. High-resolution imaging reveals how the spindle midzone impacts chromosome movement. J. Cell Biol. 218, 2529–2544 (2019).
Jiang, W. et al. PRC1: a human mitotic spindle-associated CDK substrate protein required for cytokinesis. Mol. Cell 2, 877–885 (1998).
Verni, F. et al. Feo, the Drosophila homolog of PRC1, is required for central-spindle formation and cytokinesis. Curr. Biol. 14, 1569–1575 (2004).
Muller, S. et al. The plant microtubule-associated protein AtMAP65-3/PLE is essential for cytokinetic phragmoplast function. Curr. Biol. 14, 412–417 (2004).
Schuyler, S. C., Liu, J. Y. & Pellman, D. The molecular function of Ase1p: evidence for a MAP-dependent midzone-specific spindle matrix. Microtubule-associated proteins. J. Cell Biol. 160, 517–528 (2003).
Verbrugghe, K. J. & White, J. G. SPD-1 is required for the formation of the spindle midzone but is not essential for the completion of cytokinesis in C. elegans embryos. Curr. Biol. 14, 1755–1760 (2004).
Hu, C. K., Coughlin, M., Field, C. M. & Mitchison, T. J. KIF4 regulates midzone length during cytokinesis. Curr. Biol. 21, 815–824 (2011).
Lee, Y. M. & Kim, W. Kinesin superfamily protein member 4 (KIF4) is localized to midzone and midbody in dividing cells. Exp. Mol. Med. 36, 93–97 (2004).
Rizk, R. S., Discipio, K. A., Proudfoot, K. G. & Gupta, M. L. Jr. The kinesin-8 Kip3 scales anaphase spindle length by suppression of midzone microtubule polymerization. J. Cell Biol. 204, 965–975 (2014).
Bratman, S. V. & Chang, F. Stabilization of overlapping microtubules by fission yeast CLASP. Dev. Cell 13, 812–827 (2007).
Inoue, Y. H. et al. Mutations in orbit/mast reveal that the central spindle is comprised of two microtubule populations, those that initiate cleavage and those that propagate furrow ingression. J. Cell Biol. 166, 49–60 (2004).
Pereira, A. L. et al. Mammalian CLASP1 and CLASP2 cooperate to ensure mitotic fidelity by regulating spindle and kinetochore function. Mol. Biol. Cell 17, 4526–4542 (2006).
Gaillard, J. et al. Two microtubule-associated proteins of Arabidopsis MAP65s promote antiparallel microtubule bundling. Mol. Biol. Cell 19, 4534–4544 (2008).
Loiodice, I. et al. Ase1p organizes antiparallel microtubule arrays during interphase and mitosis in fission yeast. Mol. Biol. Cell 16, 1756–1768 (2005).
Kurasawa, Y., Earnshaw, W. C., Mochizuki, Y., Dohmae, N. & Todokoro, K. Essential roles of KIF4 and its binding partner PRC1 in organized central spindle midzone formation. EMBO J. 23, 3237–3248 (2004).
Liu, J. et al. PRC1 cooperates with CLASP1 to organize central spindle plasticity in mitosis. J. Biol. Chem. 284, 23059–23071 (2009).
Zhu, C. & Jiang, W. Cell cycle-dependent translocation of PRC1 on the spindle by Kif4 is essential for midzone formation and cytokinesis. Proc. Natl Acad. Sci. USA 102, 343–348 (2005).
Rincon, S. A. et al. Kinesin-5-independent mitotic spindle assembly requires the antiparallel microtubule crosslinker Ase1 in fission yeast. Nat. Commun. 8, 15286 (2017).
Maton, G. et al. Kinetochore components are required for central spindle assembly. Nat. Cell Biol. 17, 953 (2015).
Espiritu, E. B., Krueger, L. E., Ye, A. & Rose, L. S. CLASPs function redundantly to regulate astral microtubules in the C. elegans embryo. Dev. Biol. 368, 242–254 (2012).
Subramanian, R., Ti, S. C., Tan, L., Darst, S. A. & Kapoor, T. M. Marking and measuring single microtubules by PRC1 and kinesin-4. Cell 154, 377–390 (2013).
Bieling, P., Telley, I. A. & Surrey, T. A minimal midzone protein module controls formation and length of antiparallel microtubule overlaps. Cell 142, 420–432 (2010).
Aher, A. et al. CLASP suppresses microtubule catastrophes through a single TOG domain. Dev. Cell 46, 40–58 (2018).
Al-Bassam, J. et al. CLASP promotes microtubule rescue by recruiting tubulin dimers to the microtubule. Dev. Cell 19, 245–258 (2010).
Lawrence, E. J., Arpag, G., Norris, S. R. & Zanic, M. Human CLASP2 specifically regulates microtubule catastrophe and rescue. Mol. Biol. Cell 29, 1168–1177 (2018).
Majumdar, S. et al. An isolated CLASP TOG domain suppresses microtubule catastrophe and promotes rescue. Mol. Biol. Cell 29, 1359–1375 (2018).
Patel, K., Nogales, E. & Heald, R. Multiple domains of human CLASP contribute to microtubule dynamics and organization in vitro and in Xenopus egg extracts. Cytoskeleton (Hoboken) 69, 155–165 (2012).
Oda, Y. Cortical microtubule rearrangements and cell wall patterning. Front. Plant Sci. 6, 236 (2015).
Girao, H. et al. CLASP2 binding to curved microtubule tips promotes flux and stabilizes kinetochore attachments. J. Cell Biol. 219, e201905080 (2020).
Wijeratne, S. & Subramanian, R. Geometry of antiparallel microtubule bundles regulates relative sliding and stalling by PRC1 and Kif4A. eLife 7, e32595 (2018).
Hannabuss, J. et al. Self-organization of minimal anaphase spindle midzone bundles. Curr. Biol. 29, 2120–2130 (2019).
Jiang, S. et al. Interplay between the kinesin and tubulin mechanochemical cycles underlies microtubule tip tracking by the non-motile ciliary kinesin Kif7. Dev. Cell 49, 711–730 (2019).
Subramanian, R. et al. Insights into antiparallel microtubule crosslinking by PRC1, a conserved nonmotor microtubule binding protein. Cell 142, 433–443 (2010).
Goshima, G. & Scholey, J. M. Control of mitotic spindle length. Annu. Rev. Cell Dev. Biol. 26, 21–57 (2010).
Bastos, R. N., Cundell, M. J. & Barr, F. A. KIF4A and PP2A-B56 form a spatially restricted feedback loop opposing Aurora B at the anaphase central spindle. J. Cell Biol. 207, 683–693 (2014).
Scholey, J. M., Civelekoglu-Scholey, G. & Brust-Mascher, I. Anaphase B. Biology (Basel) 5, 10.3390/biology5040051 (2016).
Hannak, E. & Heald, R. Xorbit/CLASP links dynamic microtubules to chromosomes in the Xenopus meiotic spindle. J. Cell Biol. 172, 19–25 (2006).
Pinder, C., Matsuo, Y., Maurer, S. P. & Toda, T. Kinesin-8 and Dis1/TOG collaborate to limit spindle elongation from prophase to anaphase A for proper chromosome segregation in fission yeast. J. Cell Sci. 132, jcs232306 (2019).
Hyman, A. et al. Preparation of modified tubulins. Methods Enzymol. 196, 478–485 (1991).
Folta-Stogniew, E. & Williams, K. R. Determination of molecular masses of proteins in solution: implementation of an HPLC size exclusion chromatography and laser light scattering service in a core laboratory. J. Biomol. Tech. 10, 51–63 (1999).
Koppel, D. E. Analysis of macromolecular polydispersity in intensity correlation spectroscopy: the method of cumulants. J. Chem. Phys. 57, 4814–4820 (1972).
Acknowledgements
This work was supported by a grant from the NIH (no. 1DP2GM126894-01), and by funds from the Pew Charitable Trusts and the Smith Family Foundation to R.S. We thank J. Welburn (University of Edinburgh, UK) and M. Zanic (Vanderbilt University, USA) for the generous gift of EB plasmids, and B. K. Dass (ForteBio, Sartorius) for help with troubleshooting BLI assays. The SEC–MALS instrumentation was supported by NIH award no. 1S10RR023748-01 to E. Folta-Stogniew (Yale School of Medicine, USA (The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health).
Author information
Authors and Affiliations
Contributions
R.S. conceptualized the research. N.M. and A.E.N. purified recombinant proteins. S.J., N.M. and R.S. designed all TIRF microscopy experiments. S.J. performed and analyzed data from single-microtubule and dynamic bundle TIRF assays. N.M. performed dynamic bundle TIRF assays with two labeled proteins and EB3 protein. N.M. and S.S.W. analyzed data. S.S.W. designed and performed single-molecule TIRF experiments and analyzed data. N.M. designed BLI experiments. N.M. and A.E.N. performed all biochemical assays. N.M., S.J. and R.S. wrote the original draft. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemical Biology thanks Jawdat Al-Bassam and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Related to Fig. 1.
a, Domain diagram of CLASP1 constructs used in this study (top), along with SDS-PAGE gel of all purified proteins used in this study (bottom). SR-rich: Serine Arginine rich, CLIP-ID: CLIP-Interacting Domain, M: molecular weight marker, with corresponding masses in kDa on the side. b, SEC-MALS profiles showing elution volumes of CLASP1 constructs from a Superose-6 column, and their corresponding molecular weights in solution. Mean and standard deviation of calculated Molecular weight (expected weight of monomer): CLASP1(1-654)-GFP (black) 108.8 ± 0.8 kDa (101.5 kDa); CLASP1(805-1471)-GFP (red) 107.6 ± 10.8 kDa (102.2 kDa); CLASP1(654-1471)-GFP (blue):128.7 ± 0.3 kDa (120 kDa). c, Auto-correlation function for apex of eluting peak of CLASP1(805-1471)-GFP shown in (b), obtained through dynamic light scattering studies. Calculated hydrodynamic radius, Rh = 5.49 ± 0.12 nm.
Extended Data Fig. 2 Related to Fig. 1.
n = number of kymographs analyzed from 3 independent experiments, except where indicated. P values were calculated from an ordinary one-way ANOVA test with Dunnett correction for multiple comparisons. a, Scatter plot with bar graph showing mean of fraction of time microtubules are stalled. Error bars indicate standard deviation. Assay conditions: tubulin control (0.0 ± 0.1, n = 59), 200 nM CLASP1 (0.0 ± 0.0, n = 67), 200 nM CLASP1 + 125 nM Kif4A (0.3 ± 0.3, n = 67), 200 nM CLASP1 + 250 nM Kif4A (0.4 ± 0.3, n = 73), 200 nM CLASP1 + 1000 nM Kif4A (0.6 ± 0.4, n = 73) and 1000 nM Kif4A (0.9 ± 0.1, n = 39). b, Box and whisker plot of average length of microtubule before catastrophe. Plus-sign indicates mean. Horizontal lines within box indicate the 25th, median (center line) and 75th percentile. Error bars indicate minimum and maximum range. Mean and standard deviation for assay conditions: Tubulin control (3.0 ± 2.4 µm, n = 241), 200 nM CLASP1 (6.1 ± 3.2 µm, n = 150), 200 nM CLASP1 + 125 nM Kif4A (4.0 ± 1.9 µm, n = 82), 200 nM CLASP1 + 250 nM Kif4A (3.2 ± 1.8 µm, n = 107), 200 nM CLASP1 + 1000 nM Kif4A (1.7 ± 1.0 µm, n = 152) and 1000 nM Kif4A (0.9 ± 0.6 µm, n = 24). P < < 0.0001 for 1000 nM Kif4A compared to tubulin control. c, Scatter plot of Kif4A-GFP intensity per pixel on taxol-stabilized microtubules in the presence of 150 µM ATP (n = number of microtubules analyzed from 2 independent experiments). Concentrations refer to monomeric Kif4A. Mean and standard deviation of intensity for assay conditions with Kif4A-GFP: 20 nM (504.2 ± 395.8, n = 54), 100 nM (2556 ± 1182, n = 69), 400 nM (7823 ± 1961, n = 68), 1000 nM (8276 ± 3386, n = 69). d, Representative kymographs generated from single molecule TIRF experiments of 1 nM CLASP1-GFP on dynamic microtubules (left, 25 kymographs examined) and 12 nM Kif4A-GFP (right, 34 kymographs analyzed), showing GFP channels. Scale bars: x = 2 μm and y = 15 s. Schematic indicates position and polarity of microtubule at the start of imaging. Red lines on kymographs indicate the position of the microtubule as deduced from the X-rhodamine channel. e, Scatter plot of tip to lattice GFP intensity ratio. Horizontal line indicates the mean. Error bars indicate standard deviation. Tip to lattice intensity ratios for 1 nM CLASP1-GFP (1.01 ± 0.04; n = 25); 10 nM CLASP1-GFP (0.99 ± 0.12; n = 17); 4 nM Kif4A-GFP (1.36 ± 0.36; n = 33); 12 nM Kif4A-GFP (1.71 ± 0.66; n = 34). P = 0.0066 for 1 nM CLASP1 compared to 4 nM Kif4A. f, Scatter plot of the total GFP intensity. Horizontal line indicates the mean. Error bars indicate standard deviation. Total intensity values for 1 nM CLASP1-GFP (0.41 × 106 ± 0.18 × 106; n = 25); 10 nM CLASP1-GFP (1.58 × 106 ± 0.44 × 106; n = 17); 4 nM Kif4A-GFP (0.43 × 106 ± 0.26 × 106; n = 33); 12 nM Kif4A-GFP (0.67 × 106 ± 0.35 × 106; n = 34). P is not significant for 1 nM CLASP1 compared to 4 nM Kif4A. g, Box and whisker plot of average number of catastrophes in 20 minutes. Plus-sign indicates mean. Horizontal lines within box indicate the 25th, median (center line) and 75th percentile. Error bars indicate minimum and maximum range. Mean and standard deviation for assay conditions: tubulin control (5.1 ± 2.2, n = 65), 200 nM CLASP1 (1.9 ± 1.6, n = 76), 200 nM CLASP1 + 125 nM Kif4A (1.5 ± 1.5, n = 69), 200 nM CLASP1 + 250 nM Kif4A (1.8 ± 1.7, n = 73), 200 nM CLASP1 + 1000 nM Kif4A (2.3 ± 2.5, n = 73) and 1000 nM Kif4A (1.6 ± 3.2, n = 38). P < 0.0001 for (i) tubulin control to 200 nM CLASP1. P is not significant (> 0.05) for 200 nM CLASP1 to (i) 200 nM CLASP1 + 125 nM Kif4A, (ii) 200 nM CLASP1 + 250 nM Kif4A, (iii) 200 nM CLASP1 + 1000 nM Kif4A and to (iv) 1000 nM Kif4A. h, Box and whisker plot of average number of rescues in 20 minutes. Plus-sign indicates mean. Horizontal lines within box indicate the 25th, median (center line) and 75th percentile. Error bars indicate minimum and maximum range. Mean and standard deviation for assay conditions: tubulin control (0.1 ± 0.4, n = 60), 200 nM CLASP1 (2.0 ± 1.5, n = 67), 200 nM CLASP1 + 125 nM Kif4A (1.4 ± 1.5, n = 67), 200 nM CLASP1 + 250 nM Kif4A (1.4 ± 1.5, n = 73), 200 nM CLASP1 + 1000 nM Kif4A (0.9 ± 1.3, n = 73) and 1000 nM Kif4A (0.0 ± 0.0, n = 37). P < 0.0001 for tubulin control to 200 nM CLASP1 and P = 0.0025 for 200 nM CLASP1 + 1000 nM Kif4A to 1000 nM Kif4A. i, Scatter plot of CLASP1-GFP fluorescence intensity per pixel on single microtubules in the presence of CLASP1-GFP and Kif4A at indicated concentrations. Red bars indicate mean (center line) and standard error of mean. Mean and standard deviation of intensity per pixel for assay conditions: 200 nM CLASP1 (25.9 ± 4.6, n = 59), 200 nM CLASP1 + 125 nM Kif4A (23.0 ± 6.5, n = 59), 200 nM CLASP1 + 250 nM Kif4A (18.4 ± 6.3, n = 60) and 200 nM CLASP1 + 1000 nM Kif4A (12.8 ± 6.3, n = 59). P values < 0.0001 for 200 nM CLASP1 when compared to (i) 200 nM CLASP1 + 250 nM Kif4A and (ii) 200 nM CLASP1 and 1000 nM Kif4A. n is number of microtubules analyzed from 3 independent experiments. AU, arbitrary units.
Extended Data Fig. 3 Related to Fig. 2.
a, Schematics and montages of representative microtubule bundles (red) grown from microtubule seeds (blue) in the presence of 5 nM Kif4A and 0.5 nM PRC1. A total of 20 events were examined. Schematics indicate the plus end (+) of the microtubules within the bundle. Velocity arrow indicates direction of microtubule sliding. X-Rh MT: X-Rhodamine microtubules. Scale bar represents 2 µm. b,c, Representative image of single microtubule (left) and cross-linked microtubule (right), showing HiLyte 647 (top), X-Rhodamine (middle) and merged (bottom) channels at the start (T = 0) and end (T = 20 min) of dynamic bundle assay. Scale bars represent 2 µm. The schematics below the montages indicate the positions of the seed (blue), microtubules (red) at the start and end of the experiment. Assay conditions are (b) 0.5 nM PRC1 + 200 nM CLASP1-GFP (Events examined: 47 single and 29 overlaps), and (c) 0.5 nM PRC1 + 200 nM CLASP1-GFP + 10 nM Kif4A (Events examined: 42 single and 49 overlaps).
Extended Data Fig. 4 Related to Fig. 3.
a, Summary of dynamics of PRC1 cross-linked microtubules, showing percentage of microtubules exhibiting dynamic instability, no growth or only growth in black. For all kymographs, the total number of events, percentage of events that are complete catastrophes and rescues in the presence of CLASP1-GFP, PRC1 and Kif4A at varying concentrations are shown in blue. b,c, Schematics and representative montages of microtubule bundles (red) grown from microtubule seeds (blue). Schematics indicate the plus end of the microtubules within the bundle. Velocity arrow indicates direction of microtubule sliding. Dotted gray lines indicate regions of overlap. X-Rh MT: X-Rhodamine microtubules. Scale bar represents 2 µm. Assay conditions and number of events (n) examined across 3 independent experiments are (b) 200 nM CLASP1-GFP + 0.5 nM PRC1 + 125 nM Kif4A (n = 30) and (c) 200 nM CLASP1-GFP + 0.5 nM PRC1 + 50 nM Kif4A (n = 39).
Extended Data Fig. 5 Related to Fig. 4.
n = number of kymographs of cross-linked microtubules analyzed. a, Box and whisker plot of average microtubule length before catastrophe of cross-linked microtubules. Plus-sign indicates mean. Horizontal lines within box indicate the 25th, median (center line) and 75th percentile. Error bars indicate minimum and maximum range. Mean and standard deviation for assay conditions: 200 nM CLASP1 + 0.5 nM PRC1 + 125 nM Kif4A (0.0 ± 0.0 μm, n = 30), 200 nM CLASP1 + 0.5 nM PRC1 + 50 nM Kif4A (0.4 ± 1.0 μm , n = 39), 200 nM CLASP1 + 0.5 nM PRC1 + 10 nM Kif4A (0.3 ± 0.7 μm , n = 36), 200 nM CLASP1 + 0.5 nM PRC1 + 5 nM Kif4A (3.9 ± 1.9 μm, n = 28), 200 nM + 0.5 nM PRC1 (9.1 ± 3.6 μm, n = 53), 20 nM CLASP1 + 0.5 nM PRC1 + 5 nM Kif4A (1.5 ± 0.9 μm, n = 55) and 0.5 nM PRC1 + 5 nM Kif4A (0.9 ± 0.4 μm, n = 37). b, Box and whisker plot of average number of rescues in 20 minutes for cross-linked microtubules. Plus-sign indicates mean. Horizontal lines within box indicate the 25th, median (center line) and 75th percentile. Error bars indicate minimum and maximum range. Mean and standard deviation for assay conditions 200 nM CLASP1 + 0.5 nM PRC1 + 125 nM Kif4A (Not Determined, n = 30), 200 nM CLASP1 + 0.5 nM PRC1 + 50 nM Kif4A (Not Determined, n = 39), 200 nM CLASP1 + 0.5 nM PRC1 + 10 nM Kif4A (Not Determined, n = 36), 200 nM CLASP1 + 0.5 nM PRC1 + 5 nM Kif4A (0.7 ± 1.2, n = 37), 200 nM + 0.5 nM PRC1 (1.6 ± 1.5, n = 33), 20 nM CLASP1 + 0.5 nM PRC1 + 5 nM Kif4A (0.9 ± 1.3, n = 23) and 0.5 nM PRC1 + 5 nM Kif4A (Not Determined, n = 20).
Extended Data Fig. 6 Related to Fig. 5.
a, c, e, Schematics and montages of X-rhodamine labeled microtubules (red) which grow from X-rhodamine labeled seeds (red) and form new bundles. Time T = 0 refers to the first image where the two growing ends encounter each other, as judged by the intensity in the tubulin channel. Schematics indicate the plus end of the microtubules within the bundle. Velocity arrow indicates direction of microtubule sliding. Dotted red arrows indicate microtubule growth. Dotted gray lines indicate regions of overlap. X-Rho MT: X-Rhodamine microtubules. Scale bar represents 2 µm. b, d, f, Line scans of intensities of GFP (top) and Alexa-647 (bottom) channels along a line joining the two microtubules and containing the overlap, at times T = 0s, 10 s 30 s and 90 s following bundle formation. Intensities were normalized to a value of 100 for the maximum intensity recorded for each channel, across all time points. Assay conditions are a & b: 200 nM CLASP1-GFP + 0.5 nM Alexa 647-labeled PRC1 + 50 nM Kif4A (8 events analyzed from 2 independent experiments). c & d: 200 nM CLASP1-GFP + 0.5 nM PRC1 + 50 nM Alexa 647-labeled Kif4A (31 events analyzed from 3 independent experiments). e & f: 200 nM CLASP1-GFP + 0.5 nM PRC1 + 5 nM Alexa 647-labeled Kif4A (22 events analyzed from 3 independent experiments).
Extended Data Fig. 7 Related to Fig. 5.
a, Scatter plot of CLASP1-GFP fluorescence intensities on single and cross-linked microtubules. Error bars indicate standard error of mean. Mean intensity and standard error of mean for assay conditions: 0.5 nM PRC1 + 200 nM CLASP1-GFP, single microtubules (24.2 ± 0.9, n = 54), cross-linked microtubules (80.2 ± 8.1, n = 30). n = number of microtubules analyzed from 3 independent experiments in each condition. b, Scatter plot of Kif4A-GFP fluorescence intensities on single and cross-linked microtubules. Error bars indicate standard error of mean. Mean intensity and standard error of mean for assay conditions: 0.5 nM PRC1 + 10 nM Kif4A-GFP, single microtubules (80.1 ± 13, n = 46), cross-linked microtubules (1106 ± 90.1, n = 57). n = number of microtubules analyzed from 3 independent experiments in each condition. c, Bio-Layer interferometry assay to quantify the binding affinity of CLASP1(654-1471)-GFP to PRC1(1-486). Error bars represent standard error of mean. Data from 3 independent experiments were fit to a Hill equation. KD: 1.04 ± 0.44 µM (R2 of fit = 0.90). d, Representative SDS-PAGE gel showing input proteins (lanes 2-4) and proteins appearing in the bound fraction (lanes 5-11) of pull-down assay with immobilized Flag-Kif4A, from one of 3 independent experiments. Reaction conditions corresponding to each lane are described in the table below the gel. Concentration of CLASP1(654-1471)-GFP used in each reaction is indicated in parentheses below the table. M: molecular weight marker, with corresponding masses in kDa on the side. AU, arbitrary units.
Extended Data Fig. 8 Related to Fig. 5.
a, Representative kymographs of dynamic single X-rhodamine-labeled microtubules (X-Rho MTs) with 50 nM Alexa 647-labeled EB3 + 0.5 nM PRC1 (n = 70), 50 nM Alexa 647-labeled EB3 + 0.5 nM PRC1 + 5 nM Kif4A-GFP (n = 93), 50 nM Alexa 647-labeled EB3 + 0.5 nM PRC1 + 50 nM Kif4A-GFP (n = 60). Scale bar represents 2 µm. n = number of kymographs analyzed from 3 independent experiments. b, Scatter plot of intensities of EB3 at microtubule tips with increasing concentrations of Kif4A-GFP. Mean and standard deviation are indicated by black bars. Assay condition (n = number of growth events analyzed): 50 nM Alexa 647-labeled EB3 + 0.5 nM PRC1 (186.40 ± 173.80, n = 281), 50 nM Alexa 647-labeled EB3 + 0.5 nM PRC1 + 5 nM Kif4A-GFP (143.80 ± 155.20, n = 98), 50 nM Alexa 647-labeled EB3 + 0.5 nM PRC1 + 50 nM Kif4A-GFP (28.11 ± 47.85, n = 43). For 50 nM Alexa 647-labeled EB3 + 0.5 nM PRC1 compared to (i) 50 nM Alexa 647-labeled EB3 + 0.5 nM PRC1 + 5 nM Kif4A-GFP, P = 0.048, (ii) 50 nM Alexa 647-labeled EB3 + 0.5 nM PRC1 + 50 nM Kif4A-GFP, P < < 0.0001, in an ordinary one-way ANOVA test with Dunnett correction for multiple comparisons. c, Representative kymographs of a cross-linked X-rhodamine-labeled microtubule bundle in the presence of 50 nM Alexa 647-labeled EB3, 0.5 nM PRC1 and 5 nM Kif4A-GFP. Schematics above and below the merged kymograph indicate positions of the microtubules (red) and PRC1 (gray dashed line) at the start and end of the experiment, respectively. + indicates microtubule polarity. Scale bar represents 2 µm. A total of 45 kymographs from 3 independent experiments were examined. AU, arbitrary units.
Extended Data Fig. 9 Related to Fig. 5.
a, Representative kymographs of cross-linked X-rhodamine-labeled microtubule bundle (X-Rho MTs) in the presence of 0.5 nM PRC1, 200 nM CLASP1-GFP and 50 nM Alexa 647-labeled EB3. Intensity profile below merged kymograph shows the normalized intensities of the GFP (green) and Alexa 647 (magenta) channels at the time-point represented by dashed yellow line on merged kymograph. Gray box indicates the position of microtubule seeds. Intensities were normalized to a value of 100 for the maximum intensity recorded for each channel. Schematics on bottom right represent the positions of microtubules (red), EB3 (magenta) and PRC1 (gray dashed lines) at the same time-point. Scale bar represents 2 µm. A total of 73 kymographs from 3 independent experiments were examined. b, Representative BLI sensorgram of the binding response of 1 µM CLASP1(654-1471)-GFP in solution to immobilized EB3, in the presence of 10 mM (black), 25 mM (blue) and 50 mM (red) KCl. Vertical dotted black line demarcates the binding and dissociation phases. The sensorgrams have been corrected for drift of EB3 from the sensor and non-specific binding of CLASP1(654-1471)-GFP to the sensor. Binding curves from 3 independent experiments were analyzed. c, Representative kymographs of cross-linked X-rhodamine-labeled microtubules (X-Rho MTs) in the presence of 0.5 nM PRC1, 200 nM CLASP1-GFP, 50 nM Kif4A and 50 nM Alexa 647-labeled EB3. Intensity profile below merged kymograph shows the normalized intensities of the GFP (green) and Alexa-647 (magenta) channels at the time-point represented by dashed yellow line on merged kymograph. Gray box indicates the position of microtubule seeds. Intensities were normalized between 0 (0 intensity) and 100 for the maximum intensity recorded for each channel. Schematics on bottom right represent the positions of microtubules (red), EB3 (magenta) and PRC1 (gray dashed lines) at the same time-point. Scale bar represents 2 µm. A total of 67 kymographs from 3 independent experiments were examined.
Supplementary information
Supplementary Video 1
The collective activity of CLASP1 and PRC1 promotes the elongation of single and cross-linked microtubules. Blue, GMPCPP-polymerized, HiLyte 647-labeled microtubule seeds; red, GMPCPP-polymerized, X-rhodamine-labeled microtubule seeds and X-rhodamine-labeled tubulin. Single and crosslinked microtubules are indicated by white and yellow arrows, respectively. The video was taken over 20 min (120 frames) and is displayed at a rate of 20 frames s–1. Assay conditions: 200 nM CLASP1-GFP and 0.5 nM PRC1. Scale bar, 2 µm.
Supplementary Video 2
The collective activity of CLASP1, Kif4A and PRC1 differentially regulates the dynamics of single and crosslinked microtubules. Blue, GMPCPP-polymerized, HiLyte 647-labeled microtubule seeds; red, GMPCPP-polymerized X-rhodamine-labeled microtubule seeds and X-rhodamine-labeled tubulin. Single and crosslinked microtubules are indicated by white and yellow arrows, respectively. The video was taken over 20 min (120 frames) and is displayed at a rate of 20 frames s–1. Assay conditions: 200 nM CLASP1-GFP, 0.5 nM PRC1 and 10 nM Kif4A. Scale bar, 2 µm.
Supplementary Video 3
The levels of enrichment of CLASP1 and Kif4A at overlaps underlie the differential regulation of single and crosslinked microtubules under the same conditions. Red, GMPCPP-polymerized, X-rhodamine-labeled microtubule seeds and X-rhodamine-labeled tubulin; green, CLASP1-GFP; magenta, Alexa 647-labeled Kif4A. MT, microtubules. The video was taken over 10 min (61 frames) and is displayed at a rate of 10 frames s–1. Assay conditions: 200 nM CLASP1-GFP, 0.5 nM PRC1 and 50 nM Alexa 647-labeled Kif4A. Scale bar, 2 µm.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1
Uncropped gels.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Uncropped gels (figure shows only one gel, but quantification of intensity in Fig. 5d was performed on three gels, which are provided as source data).
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
About this article
Cite this article
Mani, N., Jiang, S., Neary, A.E. et al. Differential regulation of single microtubules and bundles by a three-protein module. Nat Chem Biol 17, 964–974 (2021). https://doi.org/10.1038/s41589-021-00800-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-021-00800-y