Abstract
CD8 T cell-mediated autoimmune diseases result from the breakdown of self-tolerance mechanisms in autoreactive CD8 T cells1. How autoimmune T cell populations arise and are sustained, and the molecular programmes defining the autoimmune T cell state, are unknown. In type 1 diabetes, β-cell-specific CD8 T cells destroy insulin-producing β-cells. Here we followed the fate of β-cell-specific CD8 T cells in non-obese diabetic mice throughout the course of type 1 diabetes. We identified a stem-like autoimmune progenitor population in the pancreatic draining lymph node (pLN), which self-renews and gives rise to pLN autoimmune mediators. pLN autoimmune mediators migrate to the pancreas, where they differentiate further and destroy β-cells. Whereas transplantation of as few as 20 autoimmune progenitors induced type 1 diabetes, as many as 100,000 pancreatic autoimmune mediators did not. Pancreatic autoimmune mediators are short-lived, and stem-like autoimmune progenitors must continuously seed the pancreas to sustain β-cell destruction. Single-cell RNA sequencing and clonal analysis revealed that autoimmune CD8 T cells represent unique T cell differentiation states and identified features driving the transition from autoimmune progenitor to autoimmune mediator. Strategies aimed at targeting the stem-like autoimmune progenitor pool could emerge as novel and powerful immunotherapeutic interventions for type 1 diabetes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The RNA-seq data have been deposited in the Gene Expression Omnibus under accession GSE151652. Comparisons to publicly available published data used the following datasets: GSE11994324, GSE13013025 and GSE7081356. Additional information and materials will be made available upon request. Source data are provided with this paper.
Code availability
All custom scripts used in this study are deposited at https://github.com/abcwcm/GeartySchietinger and https://github.com/soccin/ScreenSEQ/tree/feature/simpleScripts.
References
Bluestone, J. A., Bour-Jordan, H., Cheng, M. & Anderson, M. T cells in the control of organ-specific autoimmunity. J. Clin. Invest. 125, 2250–2260 (2015).
Kaech, S. M. & Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12, 749–761 (2012).
Philip, M. & Schietinger, A. CD8+ T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-021-00574-3 (2021).
Bluestone, J. A., Herold, K. & Eisenbarth, G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 464, 1293–1300 (2010).
Herold, K. C., Vignali, D. A., Cooke, A. & Bluestone, J. A. Type 1 diabetes: translating mechanistic observations into effective clinical outcomes. Nat. Rev. Immunol. 13, 243–256 (2013).
Anderson, M. S. & Bluestone, J. A. The NOD mouse: a model of immune dysregulation. Annu. Rev. Immunol. 23, 447–485 (2005).
Unanue, E. R. Antigen presentation in the autoimmune diabetes of the NOD mouse. Annu. Rev. Immunol. 32, 579–608 (2014).
Tsai, S., Shameli, A. & Santamaria, P. CD8+ T cells in type 1 diabetes. Adv. Immunol. 100, 79–124 (2008).
Zakharov, P. N., Hu, H., Wan, X. & Unanue, E. R. Single-cell RNA sequencing of murine islets shows high cellular complexity at all stages of autoimmune diabetes. J. Exp. Med. 217, e20192362 (2020).
Coppieters, K. T. et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J. Exp. Med. 209, 51–60 (2012).
Ferris, S. T. et al. A minor subset of Batf3-dependent antigen-presenting cells in islets of Langerhans is essential for the development of autoimmune diabetes. Immunity 41, 657–669 (2014).
Lieberman, S. M. et al. Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc. Natl Acad. Sci. USA 100, 8384–8388 (2003).
Escobar, G., Mangani, D. & Anderson, A. C. T cell factor 1: a master regulator of the T cell response in disease. Sci Immunol 5, eabb9726 (2020).
van Loosdregt, J. & Coffer, P. J. The role of WNT signaling in mature T cells: T cell factor is coming home. J. Immunol. 201, 2193–2200 (2018).
Reya, T. et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423, 409–414 (2003).
Weissman, I. L., Anderson, D. J. & Gage, F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu. Rev. Cell Dev. Biol. 17, 387–403 (2001).
Christianson, S. W., Shultz, L. D. & Leiter, E. H. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Relative contributions of CD4+ and CD8+ T-cells from diabetic versus prediabetic NOD.NON-Thy-1a donors. Diabetes 42, 44–55 (1993).
Verdaguer, J. et al. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J. Exp. Med. 186, 1663–1676 (1997).
Graef, P. et al. Serial transfer of single-cell-derived immunocompetence reveals stemness of CD8+ central memory T cells. Immunity 41, 116–126 (2014).
Lapidot, T. et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367, 645–648 (1994).
Maki, T., Gottschalk, R., Ogawa, N. & Monaco, A. P. Prevention and cure of autoimmune diabetes in nonobese diabetic mice by continuous administration of FTY720. Transplantation 79, 1051–1055 (2005).
Brinkmann, V. et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 9, 883–897 (2010).
Ehlers, M. R. & Rigby, M. R. Targeting memory T cells in type 1 diabetes. Curr. Diab. Rep. 15, 84 (2015).
Yao, C. et al. Single-cell RNA-seq reveals TOX as a key regulator of CD8+ T cell persistence in chronic infection. Nat. Immunol. 20, 890–901 (2019).
Schauder, D. M. et al. E2A-regulated epigenetic landscape promotes memory CD8 T cell differentiation. Proc. Natl Acad. Sci. USA 118, e2013452118 (2021).
Abdelsamed, H. A. et al. Beta cell-specific CD8+ T cells maintain stem cell memory-associated epigenetic programs during type 1 diabetes. Nat. Immunol.21, 578–587 (2020).
Culina, S. et al. Islet-reactive CD8+ T cell frequencies in the pancreas, but not in blood, distinguish type 1 diabetic patients from healthy donors. Sci Immunol 3, eaao4013 (2018).
Pelletier, D. & Hafler, D. A. Fingolimod for multiple sclerosis. N. Engl. J. Med. 366, 339–347 (2012).
Herold, K. C. et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N. Engl. J. Med. 381, 603–613 (2019).
Sims, E. K. et al. Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals. Sci. Transl. Med. 13, eabc8980 (2021).
Magnuson, A. M. et al. Population dynamics of islet-infiltrating cells in autoimmune diabetes. Proc. Natl Acad. Sci. USA 112, 1511–1516 (2015).
Thivolet, C., Bendelac, A., Bedossa, P., Bach, J. F. & Carnaud, C. CD8+ T cell homing to the pancreas in the nonobese diabetic mouse is CD4+ T cell-dependent. J. Immunol. 146, 85–88 (1991).
Sinnathamby, G. et al. Priming and activation of human ovarian and breast cancer-specific CD8+ T cells by polyvalent Listeria monocytogenes-based vaccines. J. Immunother. 32, 856–869 (2009).
Chee, J. et al. Effector-memory T cells develop in islets and report islet pathology in type 1 diabetes. J. Immunol. 192, 572–580 (2014).
Li, D. S., Yuan, Y. H., Tu, H. J., Liang, Q. L. & Dai, L. J. A protocol for islet isolation from mouse pancreas. Nat. Protoc. 4, 1649–1652 (2009).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Harrow, J. et al. GENCODE: the reference human genome annotation for the ENCODE project. Genome Res. 22, 1760–1774 (2012).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
DEGreport: Report of DEG analysis (2020).
Sergushichev, A. An algorithm for fast preranked gene set enrichment analysis using cumulative statistic calculation. Preprint at https://doi.org/10.1101/060012 (2016).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Amezquita, R. A. et al. Orchestrating single-cell analysis with Bioconductor. Nat. Methods 17, 137–145 (2020).
McCarthy, D. J., Campbell, K. R., Lun, A. T. & Wills, Q. F. Scater: pre-processing, quality control, normalization and visualization of single-cell RNA-seq data in R. Bioinformatics 33, 1179–1186 (2017).
Kim, H. J., Lin, Y., Geddes, T. A., Yang, J. Y. H. & Yang, P. CiteFuse enables multi-modal analysis of CITE-seq data. Bioinformatics 36, 4137–4143 (2020).
Lun, A. T., Bach, K. & Marioni, J. C. Pooling across cells to normalize single-cell RNA sequencing data with many zero counts. Genome Biol. 17, 75 (2016).
Haghverdi, L., Lun, A. T. L., Morgan, M. D. & Marioni, J. C. Batch effects in single-cell RNA-sequencing data are corrected by matching mutual nearest neighbors. Nat. Biotechnol. 36, 421–427 (2018).
Lun, A. T., McCarthy, D. J. & Marioni, J. C. A step-by-step workflow for low-level analysis of single-cell RNA-seq data with Bioconductor. F1000Res 5, 2122 (2016).
Angerer, P. et al. destiny: diffusion maps for large-scale single-cell data in R. Bioinformatics 32, 1241–1243 (2016).
Ji, Z. & Ji, H. TSCAN: Pseudo-time reconstruction and evaluation in single-cell RNA-seq analysis. Nucleic Acids Res. 44, e117 (2016).
Robinson, M. D. & Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11, R25 (2010).
Marini, F. & Binder, H. pcaExplorer: an R/Bioconductor package for interacting with RNA-seq principal components. BMC Bioinf. 20, 331 (2019).
Galili, T. dendextend: an R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics 31, 3718–3720 (2015).
Doench, J. G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR–Cas9. Nat. Biotechnol. 34, 184–191 (2016).
Mackay, L. K. et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 352, 459–463 (2016).
Acknowledgements
We thank the members of the Schietinger laboratory, M. Li (MSKCC) and S. Reiner (Columbia University) for helpful discussions; K. Herold (Yale University) for reviewing data and discussion; H. Appleby and E. Bruno for technical support; S. Miakicheva for help with experiments; the MSKCC Flow Cytometry Core, especially R. Gardner; the Integrated Genomics Operation Core, especially A. Farina; M. Philip (Vanderbilt University) for critical feedback on the manuscript; and S. Monette (Tri-Institutional Laboratory of Comparative Pathology, MSKCC) for pathological evaluation of insulitis. This work was supported by the following funding sources: Medical Scientist Training Programme grant NIH T32GM007739 to the Weill Cornell/Rockefeller/Sloan Kettering Tri-Institutional MD-PhD Program (S.V.G.), NIH NIDDK F30DK122691 (S.V.G.), NIH NCI DP2CA225212 (A.S.), Lloyd Old STAR Award of the Cancer Research Institute (A.S.), Josie Robertson Foundation (A.S), the MSKCC Core Grant P30CA008748, Weill Cornell Medicine Core Laboratories Center (P.Z., F.D. and D.B.), NIH NIAID R01AI123730 (T.P.D.). F.J.S.-R. was partially supported by the MSKCC TROT programme (5T32CA160001), a GMTEC Postdoctoral Researcher Innovation Grant and is a HHMI Hanna Gray Fellow. The MSKCC Integrated Genomics Operation Core was supported by Cycle for Survival and the Marie-Josée and Henry R. Kravis Center for Molecular Oncology.
Author information
Authors and Affiliations
Contributions
S.V.G. and A.S. conceived and designed the study and analysed and interpreted data. F.D., P.Z. and D.B. performed RNA-seq, scRNA-seq, scTCR-seq and CITE-seq analyses. N.D.S. performed genetic fate-mapping analyses. M.S. and G.E.-C. carried out experiments. F.J.S.-R. and S.W.L. designed barcodes for genetic fate mapping. P.L. provided the Listeria strain. N.M. and A.V. provided advice on scRNA-seq, scTCR-seq and CITE-seq. P.T. provided advice on study design. T.P.D. provided critical advice on study design and T cell epitopes in NOD mice. S.V.G. and A.S. wrote the manuscript, with all authors contributing to the writing and providing feedback.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review information
Nature thanks Stephen Turner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Phenotypic characterization of IGRP-specific CD8 T cells in NOD mice.
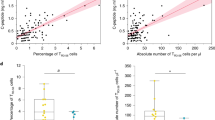
a, Depletion of CD8 T cells prevents T1D. 12-week-old female WT NOD mice were treated with anti-CD8α depleting antibody (clone 53-6.7, 250ug/mouse intraperitoneally (i.p.) twice weekly) for 4 months. Control mice received PBS i.p. n = 4 per group. Statistical testing was conducted via log-rank (Mantel-Cox) test. b, Absolute numbers (top) or frequency (bottom) of IGRP-specific (NRP-V7 tetramer+) CD8+ T cells in pancreatic lymph node (pLN; blue) and pancreas (red) at 5-9, 10-14, 15-20, and 21-30 week-old NOD mice, respectively. For absolute numbers: pLN: n = 6 (5-9wk), n = 4 (10-14wk), n = 4 (15-20wk), n = 3 (21-30wk). pancreas: n = 3 (5-9wk), n = 4 (10-14wk), n = 4 (15-20wk), n = 3 (21-30wk). For frequencies: pLN: n = 10 (5-9wk), n = 8 (10-14wk), n = 17 (15-20wk), n = 16 (21-30wk). Pancreas: n = 5 (5-9wk), n = 7 (10-14wk), n = 15 (15-20wk), n = 15 (21-30wk). Each symbol represents an individual mouse. Data are mean ± SEM. Statistical testing via unpaired two-tailed Student’s t-test. ns, not significant. c, Expression of CD44, PD1, CD38, CD39, LAG3, and TIGIT of IGRP-specific CD8+ T cells from pLN (blue) and pancreas (PA; red). Naïve T cells (NA; NRP-V7-CD44-CD8+ NOD splenocytes from the same mouse) are shown as controls (grey); for CD38: NRP-V7 CD8+ NOD splenocytes. Representative of 2-3 independent experiments, n=4-5. d, Expression of transcription factors EOMES, TBET, BLIMP1 and TOX in IGRP-specific CD8+ (NRP-V7 tet+) T cells isolated from pLN (blue) and pancreas (PA; red); Naïve T cells (NA; NRP-V7 tet- CD44- CD8+ splenocytes) are shown as controls (grey). Data are normalized to NA mean fluorescence intensity (MFI). EOMES and TOX, pooled data from 2 independent experiments, n = 9 (EOMES) and n = 7 (TOX). TBET and BLIMP1, data representative of 2 independent experiments, n=3. Statistical testing via paired two-tailed Student’s t-test. Data are mean ± SEM.
Extended Data Fig. 2 Phenotypic characterization of IGRP-specific and Insulin B (InsB)-specific CD8+ T cells in various tissues of NOD mice.
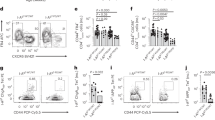
a, Expression of TCF1 versus TOX in IGRP-specific CD8+ T cells from pancreatic lymph node (pLN) and pancreas; Naïve (NA) T cells are shown as controls. Inset numbers show percentage of cells per gate. Data is representative of 2-3 independent experiments, n = 3-6. b, Bifurcation of IGRP-specific CD8 T cells into TCF1hi and TCF1lo T cell populations is not found iLN or spleen. Expression profiles of TCF1, CD44, CD39, PD1, and TOX of IGRP-specific CD8 T cells isolated from inguinal LN (iLN; purple) and spleen (green) of NOD mice; naïve T cells are shown as controls (NA). Inset numbers show percentage for each gate. Right panel, percentage of TCF1loCD39+ cells of NRP-V7 tet+ CD44+ CD8+ T cells in iLN and spleen (iLN, n=5; spleen, n = 3). Statistical testing via unpaired two-tailed Student’s t-test. Data are mean ± SEM. Representative of 2 (iLN) and 5 (spleen) independent experiments. c, IGRP-specific and Insulin B (InsB)-specific CD8 T cells bifurcate into phenotypically similar populations in the pancreatic lymph node. Left, NRP-V7+ and InsB+ CD8 T cell populations in pancreas; FACS plot is gated on live CD8+ T cells. Right, expression of TCF1, CD39, PD1, and CD44 in pLN TCF1hi and TCF1lo NRP-V7+ (grey) and InsB+ (blue) CD8 T cells in pancreatic lymph node (top), and NRP-V7+ (grey) and InsB+ (red) CD8 T cells in pancreas (bottom). pLN cells pooled from multiple mice. Representative of 2-3 independent experiments with n = 3-14 (pLN) and n = 6-9 (pancreas). d, Expression levels of CD44, TCF1, CD39, PD1, and CD62L of TCF1hi and TCF1lo IGRP-specific (NRP-V7 tet+) and Insulin B (InsB tet+) CD8 T cells from pLN and pancreas. For pLN, each symbol represents 3 pooled mice; for pancreas, each symbol represents one individual mouse. Data are mean ± SEM. Statistical testing conducted via unpaired two-tailed Student’s t-test. e, TCF1 and PD1 expression profiles of NRP-V7+ and polyclonal CD44+CD39+CD8+ T cells from pLN and pancreas. pLN cells pooled from multiple mice.
Extended Data Fig. 3 Transcriptome analysis of IGRP-specific CD8 T cells from pancreatic lymph node and pancreas.
a, MA plot of RNA-seq of pLN TCF1hi and pLN TCF1lo IGRP-specific CD8 T cells. Significantly differentially expressed genes are colored in light or dark blue and labeled with *. Selected genes are highlighted. b, Gene ontology (GO) term analysis of differentially expressed genes in pLN RNA-seq data set. GeneRatio>20/X. P-adj<0.01. Statistical testing conducted via hypergeometric test with Benjamini-Hochberg correction. c, GSEA of pLN TCF1hi and pLN TCF1lo cells including Hallmark and KEGG gene sets. d, Over-representation of KEGG and Hallmark pathways and GO terms in groups 1 and 4. P-adj<0.05.
Extended Data Fig. 4 IGRP-specific autoimmune CD8 T cell populations are phenotypically and transcriptionally distinct from IGRP-specific effector and memory CD8 T cells generated during acute infections.
a, Experimental scheme showing generation of NRP-V7 tet+ effector and memory CD8 T cells during an acute Listeria infection in comparison to NRP-V7 tet+ autoimmune CD8 T cells in NOD mice. A Listeria monocytogenes strain was generated expressing the NRP-V7 mimotope (L.m. NRP-V7); host mice (Balb/c or NOD) were infected with L.m. NRP-V7 and effector and memory T cells from indicated tissues were analyzed at indicated time points using NRP-V7 tetramer. Effector and memory T cells were compared with NRP-V7+ autoimmune T cell populations from NOD mice and tissues as indicated. b, Top, identification of NRP-V7+ CD8 T cells in pancreas (effectors (Eff); black) at day 6-7 post infection of Balb/c mice or autoimmune NRP-V7+ CD8 T cells in pancreas of uninfected NOD mice (AI; red). Right, frequency of NRP-V7+ of CD8 T cells. Expression levels of KLRG1, CD127, TBET, and TCF1 in Eff and AI NRP-V7+ CD8 T cells from pancreas of Balb/c and NOD, respectively, are shown and quantified. Eff, n=4 (%NRP-V7+, %KLRG1+, %CD127+, TCF1 MFI) or n = 6 (TBET MFI); AI, n=3 (%NRP-V7+, %KLRG1+, %CD127+, TBET MFI) or n = 4 (TCF1 MFI). c, Expression of KLRG1 and CD127 of NRP-V7+ effectors from spleens of L.m.-infected Balb/c mice. b, c, Representative of 3 independent experiments. d, Frequency of KLRG1+ of NRP-V7+ CD8 T cells from pancreas (left) and spleen (right) of L.m.-infected Balb/c and NOD mice at day 6 post L.m. NRP-V7 infection compared to autoimmune (AI) NRP-V7+ CD8 T cells from NOD mice. Eff, n=3; NOD Eff, n = 4; NOD, n = 3 (pancreas), n=4 (spleen). Representative of 1 experiment. e, Top, identification of NRP-V7+ CD8 T cells in pancreatic lymph node (pLN) (memory (Mem); black) at day 21-28 post infection and autoimmune (AI) NRP-V7+ CD8 T cells in pLN of uninfected NOD mice (blue). Frequency of NRP-V7+ of CD8 T cells quantified on right. Expression profiles and frequencies of KLRG1, CD127, TCF1, CD62L, CD122, and CXCR3 of Mem and AI NRP-V7+ CD8 T cells from pLN of Balb/c and NOD, respectively. Mem, n = 7 (%NRP-V7+), n=6 (%TCF1+CD44+), n = 9 (%CD62L+), n = 3 (%CD127+, CD122 MFI, CXCR3 MFI); AI, n = 3 (NRP-V7+), n=7 (%TCF1+CD44+), n = 11 (%CD62L+), n=4 (%CD127+, CD122 MFI, CXCR3 MFI), n = 3 (%NRP-V7+). Representative of 3 independent experiments; %CD62L+ shows 3 combined experiments. Statistical testing conducted by Student’s two-tailed unpaired t-test. Data are mean ± SEM. f, Gene set enrichment analysis of RNA-seq data from IGRP-specific pLN TCF1hi CD8 T cells (see Fig. 2) compared to central memory CD8 T cells generated during acute LCMV infection; dataset from Mackay et al, 2016, GSE70813. NES, normalized enrichment score.
Extended Data Fig. 5 Longitudinal analysis of IGRP-specific 8.3 CD8 T cells post transfer into NOD Scid mice.
a, NRP-V7+ tetramer staining of CD8+ splenocytes from 8.3 TCR transgenic mouse. Representative of at least 3 independent experiments, n = 2. b, Expression of TCF1, CD44, CD39, PD1, and TOX of naïve 8.3 CD8 T cells pre-transfer (Day 0). c, Expression of indicated molecules in pLN and pancreas of NOD Scid hosts at day 2, 5, 12, and 20 post transfer (note: transferred donor T cells were not detected in pancreas before day 12). b, c, Representative of 2 (day 2, day 5, and 12) or 3 (day 20) independent experiments with n=2 (day 2, day 5, and 12) or n = 6 (day 20) replicates. d, Expression of indicated molecules in inguinal lymph nodes (iLN) and spleens of NOD Scid hosts at day 20 post transfer. Representative of 2 independent experiments with n=2 replicates.
Extended Data Fig. 6 Analysis of transferred TCF1hi or TCF1lo CD8 T cells in 2nd NOD Scid hosts.
a–c, 5,000 pLN TCF1hi or 5,000 pancreatic TCF1lo 8.3 CD45.2+ CD8 T cells (isolated from 1st NOD Scid hosts) were transferred to 2nd NOD Scid hosts. a, Top, blood glucose curve of 2nd NOD Scid hosts receiving 5,000 pLN TCF1hi (blue, n = 5) or pancreatic TCF1lo (red, n = 4) 8.3 CD8 T cells. Each line represents an individual mouse. Bottom, percentage of non-diabetic mice over time in indicated cohorts. Statistical testing conducted via two-sided log-rank (Mantel-Cox) test. Representative of at least three independent experiments with n = 3-6 mice per group; mice were monitored for 50 days post transfer. b, FACS plots show donor TCF1hi or TCF1lo 8.3 found in pLN and pancreas 7 days (TCF1hi, n = 2; TCF1lo, n = 3) and 19-21 days (TCF1hi, n = 5; TCF1lo, n = 6) post transfer into NOD Scid 2nd hosts. c, Absolute numbers of donor T cells recovered in indicated tissues of 2nd NOD Scid hosts 19-21 days post transfer. Top, recovery of 5,000 8.3 TCF1hi (blue) or TCF1lo (red) donor CD8 T cells. Bottom, recovery of 1x106 polyclonal CD4 T cells co-transferred with 5,000 TCF1hi or TCF1lo CD8 T cells. Total donor T cells per spleen, pair of pLN or inguinal LN (iLN), or per 100mg pancreas. Data are mean ± SEM. Statistical testing conducted via unpaired two-tailed Student’s t-test. d, Flow cytometric analysis of active caspases 3 and 7 in IGRP-specific CD8+ T cells isolated from pLN and pancreas. Shown is mean fluorescence intensity (MFI) normalized to MFI of naïve splenocytes. Each symbol represents an individual mouse, lines indicate populations from the same mouse. Pooled data from 2 independent experiments, n = 7 total. Statistical testing conducted by paired Student’s two-tailed t-test. e–g (related to Fig. 3f–h), analysis at day 31 post FTY720 treatment. e, number of 8.3 cells per 100mg pancreas relative to the average number of cells per 100mg pancreas at day 15 (left); flow cytometric analysis of apoptosis using apotracker detection reagent (see Methods) (PBS, n=4; FTY720, n = 3) (right); f, Absolute cell counts per pLN. g, Flow cytometric analysis of apoptosis of 8.3 donor T cells in spleens using apotracker detection reagent. f and g, n = 4 per group. Representative of 1 (e, g) or 2 (f) independent experiments. Each symbol represents an individual mouse. Statistical testing was conducted by unpaired two-tailed Student’s t-tests. Data are mean ± SEM.
Extended Data Fig. 7 Stem-like functionality of pLN TCF1hi CD8 T cells and generation of differentiated TCF1lo CD8 T cells are independent of lymphopenic conditions.
a, Top, experimental scheme of 8.3 adoptive T cell transfer into NOD Scid (1st host) and wild-type (WT) NOD hosts (2nd host). Bottom, TCF1 and CD39 expression profiles of endogenous NRP-V7 tet+ CD8+ T cells (top) or transferred donor pLN TCF1hi 8.3 CD8+ T cells (bottom) isolated from pLN and pancreas from same WT NOD 2nd hosts 35 days post transfer. Flow plots are representative of n=6 mice. b, Top, experimental scheme of 8.3 adoptive T cell transfer into WT NOD (1st host) and NOD Scid hosts (2nd host). Bottom, Ly108 and CD39 expression profiles of donor 8.3 CD8 T cells in WT NOD 1st hosts isolated from pLN and pancreas 29 days post transfer. Representative of 3 independent experiments with n = 10-15 NOD 1st hosts, n = 5-10 pLN pooled for sort. Right, TCF1 and CD39 expression profiles of donor pLN TCF1hi cells isolated from pLN and pancreas of NOD Scid 2nd hosts 41 days post transfer. Representative of 2 independent experiments with n = 3 mice per group. c, Blood glucose curves of NOD Scid 2nd hosts receiving 200-500 pLN TCF1hi (blue) or 1,000 pancreatic TCF1lo (red) 8.3 CD8 T cells. Each line represents an individual mouse. Representative of 2 independent experiments with n = 3 mice per group.
Extended Data Fig. 8 Single cell (sc) RNA-seq of pLN IGRP-specific (NRP-V7+) CD8 T cells reveals intermediate cell states during differentiation of pLN TCF1hi to pLN TCF1lo cells.
a, Numbers of T cells in each cluster classified as TCF1hiCD62Lhi, TCF1lo, and intermediate cells in pLN based on scRNA-seq data in individual mice (n=29). b, Smoothed conditional means of expression of selected genes across individual cells ordered by pseudotime; gray shading indicates 95% confidence interval and the bar on top represents the cluster color code. c, Diffusion maps colored by normalized expression values of individual genes. d, Heatmap of pLN scRNA-seq expression (row-based z-score of normalized log counts) for cluster marker genes (false discovery rate (FDR) < 5%). Cells were ordered based on pseudotime. Red bars on top indicate normalized protein expression values as determined by CITE-seq. e, Left, Expression profiles of TCF1, CD62L, and CD39 in pLN IGRP-specific CD8 T cells by flow cytometric analysis; Right, expression of CD62L and CD39 via CITE-seq antibody-derived tags. f, Overrepresented Gene Ontology Biological Process terms based on the marker genes of each cluster.
Extended Data Fig. 9 scTCR-Seq and genetic barcoding experiments reveal derivation of IGRP-specific pancreatic T cells from pLN TCF1hi population.
a, Alluvial plots tracking the size of non shared (yellow) and shared clonotypes in pLN TCF1hi, pLN TCF1lo, and pancreas (PA) populations from n = 21 mice. Each non-yellow stratum represents a clonotype that is found across multiple populations. b, Number of pancreatic T cells from individual mice that contain clonotypes that were either found in pLN TCF1hi (red) or not found in pLN TCF1hi (orange) population. Each row represents one individual mouse. c, Number of clonotypes across all mice that are shared with at least one other population (blue) or non-shared (yellow) in pLN TCF1hi and pLN TCF1lo populations. d, Fraction of clonotypes in pLN TCF1hi and pLN TCF1lo populations from individual mice that are shared with at least one other population (blue) or non-shared (yellow). Each row represents one mouse. e, Bar plot (top) and Venn diagram (bottom) displaying the different populations from which a given clonotype was recovered. The numbers are based on all clonotypes from all mice for which paired information from pLN and pancreas was available. f, Top, pie charts represent number of cells in each population containing clonotypes from pLN TCF1hi that are shared with at least one other population (n = 127 clonotypes, identified in Extended Data Fig. 9c). Bottom, median number of cells per clonotype in each population split by shared and non shared clonotypes. Each dot represents the cell count from a single mouse. For box plots, center indicates median, lower and upper hinges indicate 25th and 75th percentiles, respectively, and whiskers extend up to 1.5xIQR (inter-quartile range) from the smallest/highest values; points shown individually fall outside that range. Notches extend 1.58xIQR/sqrt(n), roughly indicating 95% confidence interval for comparing medians. g, Cells from one example shared clonotype are shown within the coordinates of the scRNA-seq based diffusion map. Individual cells are colored according to their cluster membership. h, Left, Alluvial plots from genetic barcoding experiment show barcodes detected in pLN TCF1hi 8.3 and pancreatic 8.3 for n = 2 mice; Right, Venn diagrams show numbers of barcodes detected in pLN TCF1hi 8.3 (to achieve 99% barcode coverage) and expanded in pancreas (>1% of barcodes detected).
Extended Data Fig. 10 Autoimmune NOD pLN IGRP-specific CD8 T cells in T1D are distinct from CD8 T cells generated during acute and chronic infections.
scRNA-seq datasets from pLN NRP-V7+ CD8 T cells (Fig. 4) were compared to scRNA-seq datasets of memory precursor effector cells (MPEC) and central memory CD8 T cells (TCM) generated during acute LCMV infection (day 7 and day 129 respectively), as well as to progenitor exhausted CD8 T cells (TPEX) from day 7 during chronic (clone 13) LCMV infection. a, Principal component analysis (PCA) of pseudo-bulk RNA-seq samples representing TCF1hiCD62Lhi, TCF1hiCD62Llo (intermediate), and TCF1loCD62Llo NOD pLN NRP-V7+ CD8 T cells from 3 technical replicates from Gearty et al (Fig. 4; T1D), TCM cells from Schauder et al (PNAS 2021; GSE130130; acute LCMV, day 129) and MPEC as well as TPEX as defined by Yao et al (Nat. Immunology, 2019; GSE119943; acute and chronic LCMV, day 7). The bar charts to the left and on the bottom of the PCA plot represent the genes that are most strongly (anti)correlated with a given principal component (PC). b, Dendrogram representing the results of hierarchical clustering based on the pseudo-bulk RNA-seq samples. c, Heatmap displaying row-normalized expression values of individual cells for genes identified as marker genes when comparing the single cells of the different populations (TCF1hiCD62Lhi, intermediate, and TCF1lo, MPEC, TPEX, TCM) to each other. d, Top, GO terms (biological processes) that are enriched based on genes that are specifically overexpressed in the different populations compared to all others. Bottom, the network-like representations display the genes (small circles) that belong to the respective GO terms (large circles), highlighting genes shared among individual GO terms and upregulated in indicated cell populations.
Supplementary information
Supplementary Figure 1
Representative flow cytometry gating strategies.
Rights and permissions
About this article
Cite this article
Gearty, S.V., Dündar, F., Zumbo, P. et al. An autoimmune stem-like CD8 T cell population drives type 1 diabetes. Nature 602, 156–161 (2022). https://doi.org/10.1038/s41586-021-04248-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-04248-x
This article is cited by
-
CD4+ T cell immunity is dependent on an intrinsic stem-like program
Nature Immunology (2024)
-
The immunology of type 1 diabetes
Nature Reviews Immunology (2024)
-
TYK2 signaling promotes the development of autoreactive CD8+ cytotoxic T lymphocytes and type 1 diabetes
Nature Communications (2024)
-
Lymphatic vessels in the age of cancer immunotherapy
Nature Reviews Cancer (2024)
-
TCF1–LEF1 co-expression identifies a multipotent progenitor cell (TH2-MPP) across human allergic diseases
Nature Immunology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.