Abstract
Serine, glycine and other nonessential amino acids are critical for tumour progression, and strategies to limit their availability are emerging as potential therapies for cancer1,2,3. However, the molecular mechanisms driving this response remain unclear and the effects on lipid metabolism are relatively unexplored. Serine palmitoyltransferase (SPT) catalyses the de novo biosynthesis of sphingolipids but also produces noncanonical 1-deoxysphingolipids when using alanine as a substrate4,5. Deoxysphingolipids accumulate in the context of mutations in SPTLC1 or SPTLC26,7—or in conditions of low serine availability8,9—to drive neuropathy, and deoxysphinganine has previously been investigated as an anti-cancer agent10. Here we exploit amino acid metabolism and the promiscuity of SPT to modulate the endogenous synthesis of toxic deoxysphingolipids and slow tumour progression. Anchorage-independent growth reprogrammes a metabolic network involving serine, alanine and pyruvate that drives the endogenous synthesis and accumulation of deoxysphingolipids. Targeting the mitochondrial pyruvate carrier promotes alanine oxidation to mitigate deoxysphingolipid synthesis and improve spheroid growth, similar to phenotypes observed with the direct inhibition of SPT or ceramide synthesis. Restriction of dietary serine and glycine potently induces the accumulation of deoxysphingolipids while decreasing tumour growth in xenograft models in mice. Pharmacological inhibition of SPT rescues xenograft growth in mice fed diets restricted in serine and glycine, and the reduction of circulating serine by inhibition of phosphoglycerate dehydrogenase (PHGDH) leads to the accumulation of deoxysphingolipids and mitigates tumour growth. The promiscuity of SPT therefore links serine and mitochondrial alanine metabolism to membrane lipid diversity, which further sensitizes tumours to metabolic stress.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Full immunoblots are provided as Supplementary Information. Additional data that support findings are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Knott, S. R. V. et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 554, 378–381 (2018).
LeBoeuf, S. E. et al. Activation of oxidative stress response in cancer generates a druggable dependency on exogenous non-essential amino acids. Cell Metab. 31, 339–350.e4 (2020).
Maddocks, O. D. K. et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 493, 542–546 (2013).
Duan, J. & Merrill, A. H., Jr. 1-Deoxysphingolipids encountered exogenously and made de novo: dangerous mysteries inside an enigma. J. Biol. Chem. 290, 15380–15389 (2015).
Lone, M. A., Santos, T., Alecu, I., Silva, L. C. & Hornemann, T. 1-Deoxysphingolipids. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1864, 512–521 (2019).
Eichler, F. S. et al. Overexpression of the wild-type SPT1 subunit lowers desoxysphingolipid levels and rescues the phenotype of HSAN1. J. Neurosci. 29, 14646–14651 (2009).
Penno, A. et al. Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J. Biol. Chem. 285, 11178–11187 (2010).
Esaki, K. et al. l-Serine deficiency elicits intracellular accumulation of cytotoxic deoxysphingolipids and lipid body formation. J. Biol. Chem. 290, 14595–14609 (2015).
Gantner, M. L. et al. Serine and lipid metabolism in macular disease and peripheral neuropathy. N. Engl. J. Med. 381, 1422–1433 (2019).
Baird, R. D. et al. Phase I safety, pharmacokinetic, and pharmacogenomic trial of ES-285, a novel marine cytotoxic agent, administered to adult patients with advanced solid tumors. Mol. Cancer Ther. 8, 1430–1437 (2009).
Schafer, Z. T. et al. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 461, 109–113 (2009).
Jiang, L. et al. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature 532, 255–258 (2016).
Parker, S. J. et al. LKB1 promotes metabolic flexibility in response to energy stress. Metab. Eng. 43 (Pt B), 208–217 (2017).
Vacanti, N. M. et al. Regulation of substrate utilization by the mitochondrial pyruvate carrier. Mol. Cell 56, 425–435 (2014).
Schell, J. C. et al. A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Mol. Cell 56, 400–413 (2014).
Lewis, C. A. et al. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol. Cell 55, 253–263 (2014).
Jeske, L., Placzek, S., Schomburg, I., Chang, A. & Schomburg, D. BRENDA in 2019: a European ELIXIR core data resource. Nucleic Acids Res. 47, D542–D549 (2019).
Alecu, I. et al. Cytotoxic 1-deoxysphingolipids are metabolized by a cytochrome P450-dependent pathway. J. Lipid Res. 58, 60–71 (2017).
Gao, X. et al. Serine availability influences mitochondrial dynamics and function through lipid metabolism. Cell Rep. 22, 3507–3520 (2018).
Rodriguez, A. E. et al. Serine metabolism supports macrophage IL-1β production. Cell Metab. 29, 1003–1011.e4 (2019).
Guri, Y. et al. mTORC2 promotes tumorigenesis via lipid synthesis. Cancer Cell 32, 807–823 (2017).
Maddocks, O. D. K. et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 544, 372–376 (2017).
Locasale, J. W. et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat. Genet. 43, 869–874 (2011).
Possemato, R. et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476, 346–350 (2011).
Othman, A. et al. Lowering plasma 1-deoxysphingolipids improves neuropathy in diabetic rats. Diabetes 64, 1035–1045 (2015).
Oswald, M. C., West, R. J., Lloyd-Evans, E. & Sweeney, S. T. Identification of dietary alanine toxicity and trafficking dysfunction in a Drosophila model of hereditary sensory and autonomic neuropathy type 1. Hum. Mol. Genet. 24, 6899–6909 (2015).
Alecu, I. et al. Localization of 1-deoxysphingolipids to mitochondria induces mitochondrial dysfunction. J. Lipid Res. 58, 42–59 (2017).
Garofalo, K. et al. Oral l-serine supplementation reduces production of neurotoxic deoxysphingolipids in mice and humans with hereditary sensory autonomic neuropathy type 1. J. Clin. Invest. 121, 4735–4745 (2011).
Han, G. et al. Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl- specificities. Proc. Natl Acad. Sci. USA 106, 8186–8191 (2009).
Wallace, M. et al. Enzyme promiscuity drives branched-chain fatty acid synthesis in adipose tissues. Nat. Chem. Biol. 14, 1021–1031 (2018).
Yu, M. et al. A resource for cell line authentication, annotation and quality control. Nature 520, 307–311 (2015).
Young, J. D. INCA: a computational platform for isotopically non-stationary metabolic flux analysis. Bioinformatics 30, 1333–1335 (2014).
Cordes, T. & Metallo, C. M. Quantifying intermediary metabolism and lipogenesis in cultured mammalian cells using stable isotope tracing and mass spectrometry. Methods Mol. Biol. 1978, 219–241 (2019).
Steiner, R. et al. Elucidating the chemical structure of native 1-deoxysphingosine. J. Lipid Res. 57, 1194–1203 (2016).
Bielawski, J. et al. Comprehensive quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods Mol. Biol. 579, 443–467 (2009).
Wu, J. et al. Quantitative analysis of intracellular nucleoside triphosphates and other polar metabolites using ion pair reversed-phase liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1006, 167–178 (2015).
Acknowledgements
We thank M. Gantner, M. Friedlander and all members of the laboratory of C.M.M. for support and helpful discussions; and N. Mainolfi, V. Suri, A. Friedman and M. Manfredi of Raze Therapeutics for providing PH-755. This work was supported by the NIH (R01CA188652 and R01CA234245 to C.M.M.; U54CA132379), a Camille and Henry Dreyfus Teacher-Scholar Award (to C.M.M.), the National Science Foundation (NSF) Faculty Early Career Development (CAREER) Program (1454425 to C.M.M.), the Helmsley Center for Genomic Medicine (to A.F.M.P) and funding from Ferring Foundation (to A.S.). This work was also supported by NIH grants to the Salk Institute Mass Spectrometry Core (P30CA014195, S10OD021815).
Author information
Authors and Affiliations
Contributions
C.M.M. and T.M. designed the study. T.M., T.C., L.Y., E.W.L. and J.G. performed in vitro cell studies and independently repeated spheroid growth assays. T.M. and M.K.H. performed xenograft experiments. T.M., T.C., M.K.H., L.Y., M.G.B. and A.F.M.P. generated and analysed targeted metabolomics data. A.F.M.P., M.J.K. and M.G.B. generated and analysed untargeted lipidomics data. A.S. and M.W. guided experimental design and analysis. C.M.M. and T.M. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Sarah-Maria Fendt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
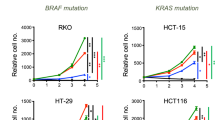
Extended Data Fig. 1 Mitochondrial pyruvate transport and amino acid metabolism influence spheroid growth.
a, HCT116 spheroid growth from 3-, 5- and 8-day cultures. Scale bars, 100 μm. b, Metabolite levels in HCT116 adherent and spheroid cultures (n = 3 culture wells each). c, Alanine levels in adherent and spheroid cultures (n = 3 culture wells each condition and cell line). d, Isotopic labelling (M2 citrate/M3 pyruvate) in HCT116 and MCF7 cells cultured with [U-13C6]glucose for 24 h (n = 3 culture wells each). e, Protein expression of phosphorylated PDH (pPDH), total PDH (tPDH) and β-actin in HCT116 cells. Each lane derived from a single culture well, processed in parallel, and used for quantification. For gel source data, see Supplementary Information. f, Citrate labelling in HCT116 cells cultured with [U-13C5]glutamine in HCT116 (n = 3 culture wells each condition). g, Metabolite levels upon UK5099 treatment in HCT116 spheroid cultures (n = 6 culture wells each). h, Abundances of alanine and serine in A549 spheroid cultures upon treatment with UK5099 (n = 3 culture wells each). i, Spheroid growth in cells upon UK5099 treatment (n = 3 culture wells each). j, k, Adherent growth of A549 (j) and HCT116 (k) cells upon treatment with UK5099 (n = 3 culture wells each). l, m, Adherent growth of A549 (l) and HCT116 (m) cells upon MPC1 or MPC2 knockdown compared to control (shNT) (n = 3 culture wells each). n, o, Isotopologue distributions of serine (n) and citrate (o) in HCT116 spheroid cultures traced with [U-13C6]glucose for 24 h (n = 3 culture wells each). p, Alanine abundances in HCT116 spheroids in the presence of 1 mM alanine and UK5099 (n = 3 culture wells each). q, r, Spheroid growth of HCT116 (q) and MCF7 (r) cells grown in the presence of UK5099 and alanine (n = 3 culture wells each condition). s, Cell number of adherent HCT116 cells in the presence of UK5099 and alanine (n = 3 culture wells each). t–v, Spheroid biomass in HCT116 (t), MCF7 (u) and A549 (v) cells grown in the presence or absence of 0.4 mM serine, 0.4 mM glycine, 1 mM alanine and 1 mM formate (n = 3 culture wells for each cell line and condition). Two-sided Student’s t-test (b–i, n, o), one-way ANOVA (t–v) or two-way ANOVA (j–m, p–s) was performed for each comparison, with no adjustment for multiple comparison. Similar results obtained in two (d, e, h, p, r), three (c, f, g, i, n, o, q), or four (b) independent experiments. Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.0001.
Extended Data Fig. 2 1-Deoxysphingolipid synthesis and degradation influence spheroid growth in vitro.
a, Total (hydrolysed) sphinganine and deoxysphinganine (deoxySA) abundances in adherent and spheroid cultures of HCT116 cells (n = 3 culture wells each). b, Free deoxysphinganine abundances in HCT116 cultures in normal medium or medium without serine and glycine but containing 1 mM alanine (−SG+A) (n = 3 culture wells each). c, Deoxysphinganine/sphinganine (SA) molar ratio in HCT116 cells in cultures from (b) (n = 3 culture wells each). d, HCT116 spheroid growth in the presence or absence of 10 nM deoxysphinganine and treated with vehicle (DMSO) or UK5099 (n = 3 culture wells each). e, Adherent (n = 4 culture wells each) and spheroid (n = 3 culture wells each) biomass of HCT116 cells treated with vehicle or 50 nM sphingoid bases. SO, sphingosine; deoxySO, deoxysphingosine. f, Free deoxysphinganine, summed deoxyDHCER and summed deoxyCER in spheroid cultures from (e) (n = 3 culture wells each). g, Sphinganine and deoxysphinganine abundances in HCT116 spheroid cultures treated with vehicle or 10 nM myriocin (n = 3 culture wells each). h, Deoxysphinganine/ sphinganine molar ratio in HCT116 adherent and spheroid cultures treated with vehicle or 10 nM myriocin (n = 3 culture wells each). i, Free deoxysphinganine abundances in HCT116 spheroid cells in the presence or absence of 1 mM alanine and treated with vehicle or 10 nM myriocin (n = 3 culture wells each). j, Spheroid growth of cell lines cultured in the presence or absence of 1 mM alanine (red), 10 nM myriocin (blue) or both (red outline with checkered blue fill) (n = 3 culture wells each condition). k, A549 spheroid growth under 5 d of culture in myriocin or 10 μM fumonisin B1 (FuB1) (n = 3 culture wells each). l, m, HCT116 spheroid growth (l) and free deoxysphinganine (m) in the presence (+SG) or absence (−SG) of 0.4 mM serine and glycine and treated with DMSO, fenofibrate (FeF), 1 mM alanine or both (n = 3 culture wells for each condition). Two-sided Student’s t-test (a, g), two-way ANOVA (b–d, h, i, l, m) or one-way ANOVA (e, f, j, k) was performed for each comparison with no adjustment for multiple comparison. Similar results obtained in two (b–e) or three (a, g–i) independent experiments. Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.0001, #P < 0.0001.
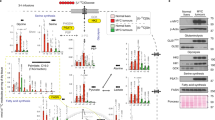
Extended Data Fig. 3 Dietary serine and glycine restriction alters tumour growth and ceramide metabolism.
a, HCT116 xenograft size in mice fed control (n = 16) or −SG (n = 15 day 21) diet. b, Plasma amino acids from mice fed control (n = 8) or −SG (n = 7) diet. c, d, Abundances of deoxyDHCER (c) and deoxyCER (d) in HCT116 xenograft tumours (n = 8 each diet). e, Abundances of total sphingolipid species in the livers from HCT116 tumour-bearing mice fed control or −SG diet (n = 8 each diet). DHCER, dihydroceramide. f–i, Abundances of DHCER (f), CER (g), sphingomyelins (h) and sphingosine-1-phosphate (S1P) (i) in HCT116 xenografts from mice fed control or −SG diet (n = 8 each diet). j, HT29 xenograft size in mice fed control (n = 16), −SG (n = 16) or −SG+A (n = 14) diets. k, Plasma amino acids in mice in j fed control (n = 8), −SG (n = 8) or −SG+A (n = 7) diets. l, Tumour amino acids from mice in j fed control (n = 7), −SG (n = 8) or −SG+A (n = 7). m, Deoxysphinganine and summed ceramide species in HT29 xenograft tumours from mice fed control (n = 16), −SG (n = 16) or −SG+A diets (n = 14). n, Abundances of lactosylceramides in HT29 xenograft tumours from mice fed control (n = 16), −SG (n = 16) or −SG+A diets (n = 14). o, Gpt1 and Gpt2 expression in liver tissue of mice fed control, −SG or −SG+A diets (n = 8 for each). p, Nucleotide phosphate abundances in HCT116 xenograft tumours from mice fed control, −SG or −SG+A diets (n = 8 for each). q, Reduced glutathione (GSH), oxidized glutathione (GSSG) and GSH/GSSG measurements of HCT116 xenograft tumours from mice fed control, −SG or −SG+A diets (n = 8 each). Two-sided Student’s t-test (b–i, p, q) or two-way ANOVA (a, j–o) was performed, with no adjustment for multiple comparison. Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.0001 or #P < 0.0001.
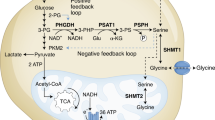
Extended Data Fig. 4 Influence of SPT and PHGDH inhibition on sphingolipid metabolism.
a–d, Abundances of deoxyDHCER (a), deoxyCER (b), DHCER (c) and CER (d) in HCT116 xenograft tumours from mice fed an −SG diet and administered vehicle, 0.03 mg/kg myriocin or 0.3 mg/kg myriocin (n = 16 for each treatment). e–h, Abundances of deoxyDHCER (e), deoxyCER (f), DHCER (g) and CER (h) in livers from HCT116 xenograft-bearing mice fed an −SG diet and administered vehicle (n = 8 for e, f, h and n = 7 for g), 0.03 mg/kg myriocin (n = 8) or 0.3 mg/kg myriocin (n = 8). i, Weight loss in mice after tumour inoculation in mice administered vehicle (n = 7), 0.03 mg/kg myriocin (n = 8) or 0.3 mg/kg myriocin (n = 8). j, Schematic describing in vivo sphingolipid physiology under high- and low-dose myriocin treatments generated using BioRender. k, Fractional labelling of serine (1 − M0) from HCT116 spheroids cultured with [U-13C6]glucose for 24 h and treated with vehicle or 5 μM PH-755 (n = 3 culture wells for each). l, HCT116 spheroid growth in medium containing 0.4 mM or 1.0 mM serine and treated with vehicle or 5 μM PH-755 (n = 3 culture wells for each). m, Total deoxysphinganine abundances in HCT116 spheroids grown for 5 d in medium with 0.4 mM or 1 mM serine, and treated with vehicle or 5 μM PH-755 (n = 3 culture wells for each). n, Total deoxysphinganine abundances in A549, HCT116 and MCF7 spheroids treated with vehicle or 5 μM PH-755 (n = 3 culture wells each). o, Plasma serine, glycine and alanine in mice treated with vehicle or PH-755 (n = 7 each). Two-way ANOVA (a–h, k–n), one-way ANOVA (i), or two-sided Student’s t-test (o) was performed, with no adjustment for multiple comparison. Similar results were obtained in two independent experiments (k–n). Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.0001 or #P < 0.0001.
Supplementary information
Supplementary Information
Supplementary Figure 1. Raw gel data from Extended data Figure 1e depicting β-Actin, total PDH, and phosphorylated PDH in adherent and spheroid cultures of HCT116.
Supplementary Table
Supplementary Table 1. Composition of control and amino acid restricted diets.
Supplementary Table
Supplementary Table 2. Composition of 100X amino acid stock solution used to prepare tracer medium for in vitro studies.
Supplementary Table
Supplementary Table 3. Ion transitions, collision energies, and fragmentor voltages for LC-MS/MS analysis.
Supplementary Table
Supplementary Table 4. RT-PCR primer sequences used in these studies.
Rights and permissions
About this article
Cite this article
Muthusamy, T., Cordes, T., Handzlik, M.K. et al. Serine restriction alters sphingolipid diversity to constrain tumour growth. Nature 586, 790–795 (2020). https://doi.org/10.1038/s41586-020-2609-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2609-x
This article is cited by
-
Comprehensive analysis the prognostic and immune characteristics of mitochondrial transport-related gene SFXN1 in lung adenocarcinoma
BMC Cancer (2024)
-
Serine synthesis and catabolism in starved lung cancer and primary bronchial epithelial cells
Cancer & Metabolism (2024)
-
Selenium reduction of ubiquinone via SQOR suppresses ferroptosis
Nature Metabolism (2024)
-
Amino acid metabolism in tumor biology and therapy
Cell Death & Disease (2024)
-
The effects of metabolism on the immune microenvironment in colorectal cancer
Cell Death Discovery (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.