Abstract
Vascular contributions to dementia and Alzheimer’s disease are increasingly recognized1,2,3,4,5,6. Recent studies have suggested that breakdown of the blood–brain barrier (BBB) is an early biomarker of human cognitive dysfunction7, including the early clinical stages of Alzheimer’s disease5,8,9,10. The E4 variant of apolipoprotein E (APOE4), the main susceptibility gene for Alzheimer’s disease11,12,13,14, leads to accelerated breakdown of the BBB and degeneration of brain capillary pericytes15,16,17,18,19, which maintain BBB integrity20,21,22. It is unclear, however, whether the cerebrovascular effects of APOE4 contribute to cognitive impairment. Here we show that individuals bearing APOE4 (with the ε3/ε4 or ε4/ε4 alleles) are distinguished from those without APOE4 (ε3/ε3) by breakdown of the BBB in the hippocampus and medial temporal lobe. This finding is apparent in cognitively unimpaired APOE4 carriers and more severe in those with cognitive impairment, but is not related to amyloid-β or tau pathology measured in cerebrospinal fluid or by positron emission tomography23. High baseline levels of the BBB pericyte injury biomarker soluble PDGFRβ7,8 in the cerebrospinal fluid predicted future cognitive decline in APOE4 carriers but not in non-carriers, even after controlling for amyloid-β and tau status, and were correlated with increased activity of the BBB-degrading cyclophilin A-matrix metalloproteinase-9 pathway19 in cerebrospinal fluid. Our findings suggest that breakdown of the BBB contributes to APOE4-associated cognitive decline independently of Alzheimer’s disease pathology, and might be a therapeutic target in APOE4 carriers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated and/or analysed during this study are either included in this article (and its Supplementary Information) or are available from the corresponding author on reasonable request. Source Data for Figs. 1–4 are provided with the article.
Code availability
All software used in this study are publicly available: Rocketship v1.2 (https://github.com/petmri/ROCKETSHIP/blob/master/dce/compare_gui.m), FreeSurfer (v5.3.0) (http://surfer.nmr.mgh.harvard.edu/), FSL-FLIRT (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FLIRT), SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/), and Quantitative Imaging Toolkit (https://cabeen.io/about/publication/cabeen2018quantitative/).
References
Wardlaw, J. M. et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12, 822–838 (2013).
Kapasi, A., DeCarli, C. & Schneider, J. A. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 134, 171–186 (2017).
Iadecola, C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96, 17–42 (2017).
Iturria-Medina, Y., Sotero, R. C., Toussaint, P. J., Mateos-Pérez, J. M. & Evans, A. C. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat. Commun. 7, 11934 (2016).
Sweeney, M. D., Sagare, A. P. & Zlokovic, B. V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14, 133–150 (2018).
Sweeney, M. D. et al. Vascular dysfunction—the disregarded partner of Alzheimer’s disease. Alzheimers Dement. 15, 158–167 (2019).
Nation, D. A. et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 25, 270–276 (2019).
Montagne, A. et al. Blood–brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302 (2015).
van de Haar, H. J. et al. Neurovascular unit impairment in early Alzheimer’s disease measured with magnetic resonance imaging. Neurobiol. Aging 45, 190–196 (2016).
van de Haar, H. J. et al. Blood–brain barrier leakage in patients with early Alzheimer disease. Radiology 281, 527–535 (2016).
Corder, E. H. et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923 (1993).
Roses, A. D. Apolipoprotein E alleles as risk factors in Alzheimer’s disease. Annu. Rev. Med. 47, 387–400 (1996).
Farrer, L. A. et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. J. Am. Med. Assoc. 278, 1349–1356 (1997).
Genin, E. et al. APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Mol. Psychiatry 16, 903–907 (2011).
Hultman, K., Strickland, S. & Norris, E. H. The APOE ɛ4/ɛ4 genotype potentiates vascular fibrin(ogen) deposition in amyloid-laden vessels in the brains of Alzheimer’s disease patients. J. Cereb. Blood Flow Metab. 33, 1251–1258 (2013).
Halliday, M. R. et al. Accelerated pericyte degeneration and blood–brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J. Cereb. Blood Flow Metab. 36, 216–227 (2016).
Salloway, S. et al. Effect of APOE genotype on microvascular basement membrane in Alzheimer’s disease. J. Neurol. Sci. 203-204, 183–187 (2002).
Zipser, B. D. et al. Microvascular injury and blood–brain barrier leakage in Alzheimer’s disease. Neurobiol. Aging 28, 977–986 (2007).
Bell, R. D. et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485, 512–516 (2012).
Armulik, A. et al. Pericytes regulate the blood–brain barrier. Nature 468, 557–561 (2010).
Bell, R. D. et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 409–427 (2010).
Nikolakopoulou, A. M. et al. Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat. Neurosci. 22, 1089–1098 (2019).
Jack, C. R. Jr et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562 (2018).
Pan, C. et al. Diagnostic values of cerebrospinal fluid T-Tau and Aβ42 using meso scale discovery assays for Alzheimer’s disease. J. Alzheimers Dis. 45, 709–719 (2015).
Roe, C. M. et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology 80, 1784–1791 (2013).
Montagne, A., Zhao, Z. & Zlokovic, B. V. Alzheimer’s disease: a matter of blood-brain barrier dysfunction? J. Exp. Med. 214, 3151–3169 (2017).
Bennett, R. E. et al. Tau induces blood vessel abnormalities and angiogenesis-related gene expression in P301L transgenic mice and human Alzheimer’s disease. Proc. Natl Acad. Sci. USA 115, E1289–E1298 (2018).
Fouquet, M., Besson, F. L., Gonneaud, J., La Joie, R. & Chételat, G. Imaging brain effects of APOE4 in cognitively normal individuals across the lifespan. Neuropsychol. Rev. 24, 290–299 (2014).
Schultz, S. A. et al. Widespread distribution of tauopathy in preclinical Alzheimer’s disease. Neurobiol. Aging 72, 177–185 (2018).
Miners, J. S., Kehoe, P. G., Love, S., Zetterberg, H. & Blennow, K. CSF evidence of pericyte damage in Alzheimer’s disease is associated with markers of blood–brain barrier dysfunction and disease pathology. Alzheimers Res. Ther. 11, 81 (2019).
Stanciu, C., Trifan, A., Muzica, C. & Sfarti, C. Efficacy and safety of alisporivir for the treatment of hepatitis C infection. Expert Opin. Pharmacother. 20, 379–384 (2019).
Morris, J. C. et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis. Assoc. Disord. 20, 210–216 (2006).
Morris, J. C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43, 2412–2414 (1993).
Nation, D. A. et al. Antemortem pulse pressure elevation predicts cerebrovascular disease in autopsy-confirmed Alzheimer’s disease. J. Alzheimers Dis. 30, 595–603 (2012).
Bangen, K. J. et al. Aggregate effects of vascular risk factors on cerebrovascular changes in autopsy-confirmed Alzheimer’s disease. Alzheimers Dement. 11, 394–403.e1 (2015).
Jak, A. J. et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am. J. Geriatr. Psychiatry 17, 368–375 (2009).
Jak, A. J. et al. Neuropsychological criteria for mild cognitive impairment and dementia risk in the Framingham heart study. J. Int. Neuropsychol. Soc. 22, 937–943 (2016).
Weintraub, S. et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis. Assoc. Disord. 23, 91–101 (2009).
Besser, L. et al. Version 3 of the National Alzheimer’s Coordinating Center’s Uniform Data Set. Alzheimer Dis. Assoc. Disord. 32, 351–358 (2018).
Delis, D., Kramer, J., Kaplan, E. & Ober, B. California Verbal Learning Test (PsychCorp, 2000).
Montagne, A. et al. Undetectable gadolinium brain retention in individuals with an age-dependent blood-brain barrier breakdown in the hippocampus and mild cognitive impairment. Alzheimers Dement. 15, 1568–1575 (2019).
Fischl, B. FreeSurfer. Neuroimage 62, 774–781 (2012).
Fischl, B. et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355 (2002).
Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 (2006).
Fischl, B. & Dale, A. M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl Acad. Sci. USA 97, 11050–11055 (2000).
Fischl, B., Sereno, M. I., Tootell, R. B. & Dale, A. M. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 8, 272–284 (1999).
Dinov, I. et al. Neuroimaging study designs, computational analyses and data provenance using the LONI pipeline. PLoS ONE 5, e13070 (2010).
Sepehrband, F. et al. Neuroanatomical morphometric characterization of sex differences in youth using statistical learning. Neuroimage 172, 217–227 (2018).
Cabeen, R. P., Laidlaw, D. H. & Toga, A. W. Quantitative imaging toolkit: software for interactive 3D visualization, data exploration, and computational analysis of neuroimaging datasets. Proc. Intl Soc. Magnetic Resonance in Medicine (ISMRM) vol. 2854 (2018).
Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841 (2002).
Bullich, S. et al. Optimal reference region to measure longitudinal amyloid-β change with 18F-florbetaben PET. J. Nucl. Med. 58, 1300–1306 (2017).
Marquié, M. et al. Lessons learned about [F-18]-AV-1451 off-target binding from an autopsy-confirmed Parkinson’s case. Acta Neuropathol. Commun. 5, 75 (2017).
Mishra, S. et al. AV-1451 PET imaging of tau pathology in preclinical Alzheimer disease: defining a summary measure. Neuroimage 161, 171–178 (2017).
TCW, J. et al. Cholesterol and matrisome pathways dysregulated in human ε4 glia. Preprint at https://www.biorxiv.org/content/10.1101/713362v1 (2019).
Faal, T. et al. Induction of mesoderm and neural crest-derived pericytes from human pluripotent stem cells to study blood-brain barrier interactions. Stem Cell Reports 12, 451–460 (2019).
Aggarwal, C. C. Outlier Analysis (Springer, 2013).
Sagare, A. P., Sweeney, M. D., Makshanoff, J. & Zlokovic, B. V. Shedding of soluble platelet-derived growth factor receptor-β from human brain pericytes. Neurosci. Lett. 607, 97–101 (2015).
Glickman, M. E., Rao, S. R. & Schultz, M. R. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J. Clin. Epidemiol. 67, 850–857 (2014).
Acknowledgements
The work of B.V.Z. is supported by National Institutes of Health (NIH) grant nos. R01AG023084, R01NS034467, R01AG039452, 5P01AG052350 and 5P50AG005142, in addition to an Alzheimer’s Association strategic 509279 grant, Cure Alzheimer’s Fund, the Foundation Leducq Transatlantic Network of Excellence for the Study of Perivascular Spaces in Small Vessel Disease reference no. 16 CVD 05, and Open Philanthropy. D.A.N. is supported by NIH grant nos. R01AG060049, R01AG064228, P01AG052350 and P50AG016573, Alzheimer’s Association grant AARG-17–532905 and Alzheimer’s Association strategic grant 509279. D.P.B and M.G.H. are supported by the L. K. Whittier Foundation, grant nos. P01AG052350, R01AG054434 and R01AG055770. Enrolment of participants into the WashU Knight ADRC is supported by NIH grant nos. P50AG05681, P01AG03991 and P01AG026276 (J.C.M.). E.M.R. is supported by National Institute of Aging (NIA) grant nos. P30AG19610 and R01AG031581, in addition to the state of Arizona. Enrolment of participants into the USC ADRC is supported by NIH grant no. 5P50AG005142 (H.C.C.). Avid Radiopharmaceuticals, a wholly owned subsidiary of Eli Lilly and Company, provided doses of FBP and financial support for FBP scanning at the WashU site. Avid Radiopharmaceuticals also provided the WashU site with AV1451 precursor and technology transfer for producing the tracer on site. Avid Radiopharmaceuticals was not involved in data analysis or interpretation.
Author information
Authors and Affiliations
Contributions
A.M., D.A.N., A.P.S., G.B., M.D.S. and B.V.Z. designed the research study and analysed and interpreted the data. A.M., D.A.N., A.P.S., G.B., M.D.S., A.C., M.P. and Y.C. performed the experiments and analysed the data. A.M. and G.B. performed the MRI analysis. A.M., G.B. and A.C. performed the PET analysis. A.P.S., M.D.S. and M.P. performed the biofluids analysis. D.A.N. performed the neuropsychological analysis. A.P.S., Y.C., B.V.Z. and J.TCW. contributed to human iPSC-pericyte experiments. L.M.D. and A.R.N. prepared and submitted the study to the IRB. M.P., E.J., D.P.B., M.G.H., T.L.S.B., A.M.F., J.M.R., L.S.S., J.C.M., E.M.R., R.J.C., H.C.C., J.TCW., J.P., P.S.C., M.L. and A.W.T. recruited the participants and performed and provided the imaging scans. A.P.S., G.B., M.G.H., T.L.S.B., A.M.F., J.M.R., L.S.S., J.C.M., E.M.R., R.J.C., H.C.C., J.TCW., P.S.C. and A.W.T. provided critical reading of the manuscript. A.M. and D.A.N. contributed to manuscript writing and B.V.Z. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Ronald Thomas, Yi-Fen Yen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Regional BBB Ktrans constant in eight additional brain regions in APOE4 carriers and non-carriers (APOE3) with CDR status 0 and 0.5.
BBB Ktrans constant in the ITG (a), superior frontal gyrus (SFG, b), caudate nucleus (CN, c), thalamus (Thal, d), striatum (Str, e), subcortical watershed normal-appearing white matter (Subcort. WS NAWM, f), corpus callosum (CC, g), and internal capsule (IC, h) in individuals with CDR 0 bearing APOE3 (black, n = 128) and APOE4 (red, n = 68), and with CDR 0.5 bearing APOE3 (black, n = 14) and APOE4 (red, n = 25). Continuous lines, median; dotted lines, IQR. Significance by ANCOVAs for main effects and post hoc comparisons controlling for age, sex, and education.
Extended Data Fig. 2 BBB breakdown in the HC and PHG in APOE4 carriers increases with cognitive domain impairment.
a, b, Ktrans constant in the HC (a) and PHG (b) in individuals with no cognitive domains impaired bearing APOE3 (black, n = 70) or APOE4 (red, n = 40); one cognitive domain impaired bearing APOE3 (n = 18) or APOE4 (n = 21); and two or more cognitive domains impaired bearing APOE3 (n = 7) or APOE4 (n = 12). Continuous lines, median; dotted lines, IQR. c, d, Ktrans (estimated marginal mean ± s.e.m.from ANCOVA models corrected for age, sex, education, CSF Aβ1–42 and pTau status, and HC and PHG volumes) in the HC (c) and PHG (d) in individuals with no cognitive domains impaired bearing APOE3 (n = 70) or APOE4 (n = 40); one cognitive domain impaired bearing APOE3 (n = 18) or APOE4 (n = 21); and two or more cognitive domains impaired bearing APOE3 (n = 7) or APOE4 (n = 12). Significance by ANCOVA for main effects and post hoc comparisons controlling for age, sex, and education. All ANCOVA omnibus tests remained significant at FDR threshold of 0.05.
Extended Data Fig. 3 Regional BBB Ktrans constant in eight additional brain regions in APOE4 carriers and APOE3 carriers with different degrees of cognitive domain impairment.
Ktrans constant in the ITG (a), SFG (b), CN (c), thalamus (d), striatum (e), subcortical WS NAWM (f), CC (g), and IC (h) in individuals with no cognitive domains impaired bearing APOE3 (black, n = 70) or APOE4 (red, n = 40): one cognitive domain impaired bearing APOE3 (n = 18) or APOE4 (n = 21); and two or more cognitive domains impaired bearing APOE3 (n = 7) or APOE4 (n = 12). Continuous lines, median; dotted lines, IQR. Significance tests from ANCOVAs for main effects and post hoc comparisons controlling for age, sex, and education.
Extended Data Fig. 4 Regional BBB Ktrans constant in all studied brain regions in APOE4 carriers and APOE3 carriers in relation to vascular risk factors.
Ktrans constant in the HC (a), PHG (b), ITG (c), SFG (d), CN (e), thalamus (f), striatum (g), subcortical WS NAWM (h), CC (i), and IC (j) in APOE3 (green, n = 80) and APOE4 (brown, n = 42) carriers with 0–1 vascular risk factors (VRFs), and APOE3 (n = 58) and APOE4 (n = 51) carriers with 2+ VRFs. Continuous lines, medians; dotted lines, IQR. Significance by ANCOVAs for main effects and post hoc comparisons controlling for age, sex, and education.
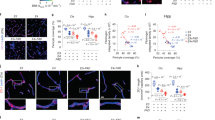
Extended Data Fig. 5 Amyloid and tau PET analysis in APOE4 carriers and correction of 18F-AV1451 off-target binding in the choroid plexus.
All studies were performed in individuals with CDR score 0. Amyloid and tau PET studies were conducted using 18F- FBB or 18F- FBP, and 18F- AV1451, respectively. For amyloid PET data analysis, FBP and FBB data sets were combined. a, Uptake of amyloid tracers by the OFC in APOE4 (n = 29) relative to APOE3 (n = 45) carriers (voxel-wise two-sample one-tailed t-tests). b, Representative amyloid PET SUVR maps from an APOE3 homozygote (top) and an APOE4 carrier (APOE4) (bottom). Slices 1 and 2, regions of interest (ROIs) for amyloid PET and BBB DCE-MRI scans (see e). Arrow, amyloid tracer uptake by OFC. The APOE3 and APOE4 representative images used FBP. c, Uptake of tau tracer shows undetectable tau accumulation in APOE3 (n = 60) or APOE4 (n = 37) carriers (voxel-wise two-sample one-tailed t-tests). d, Representative tau PET SUVR maps from APOE3 (top) and APOE4 (bottom) carriers. Slices 1 and 1′, ROIs for tau PET and BBB DCE-MRI scans, respectively (see e). e, Coronal 3D scans of regions studied in Fig. 2: HC (red), PHG (green), medial OFC (yellow), and ITG (blue). f, Correction of 18F-AV1451 off-target binding in the choroid plexus. Step 1, HC masks were generated from the 3D T1-weighted magnetization prepared–rapid gradient echo (MP-RAGE). Step 2, CP masks were generated from the T1-weighted VIBE post-GBCA image (flip angle, 15°). Step 3, HC and CP masks were overlaid (arrowheads, red). Step 4, areas of CP overlap with HC masks (arrowheads, yellow) were subtracted to obtain CP-corrected HC tau PET signal after adding 6-mm voxel size on top of CP mask generated from DCE data. g, Representative images of HC tau PET signal before (top) and after (bottom) applying the CP correction (arrows and white dotted lines show overlap between HC and CP).
Extended Data Fig. 6 CSF biomarkers of glia and inflammatory response and endothelial and neuronal cell injury in APOE4 and APOE3carriers.
a, CSF astrocytic S100B levels in individuals with CDR 0 bearing APOE3 (black, n = 77) or APOE4 (red, n = 41), and with CDR 0.5 bearing APOE3 (n = 39) or APOE4 (n = 32). b, CSF IL6 levels in individuals with CDR 0 bearing APOE3 (n = 71) or APOE4 (n = 47), and with CDR 0.5 bearing APOE3 (n = 34) or APOE4 (n = 32). c, CSF IFNγ levels in individuals with CDR 0 bearing APOE3 (n = 54) or APOE4 (n = 29), and with CDR 0.5 bearing APOE3 (n = 25) or APOE4 (n = 17). d, CSF IL1β levels in individuals with CDR 0 bearing APOE3 (n = 43) or APOE4 (n = 18), and with CDR 0.5 bearing APOE3 (n = 17) or APOE4 (n = 13). (e) CSF TNFα levels in individuals with CDR 0 bearing APOE3 (n = 70) or APOE4 (n = 46), and with CDR 0.5 bearing APOE3 (n = 34) or APOE4 (n = 32). f, CSF soluble intercellular adhesion molecule 1 (sICAM1) levels in individuals with CDR 0 bearing APOE3 (n = 77) or APOE4 (n = 40), and with CDR 0.5 bearing APOE3 (n = 39) or APOE4 (n = 33). g, CSF NSE levels in individuals with CDR 0 bearing APOE3 (n = 47) or APOE4 (n = 32), and with CDR 0.5 bearing APOE3 (n = 29) or APOE4 (n = 29). Continuous lines, median; dotted lines, IQR. a and b had one outlier each, which were removed before statistical analysis (see Methods). Significance by ANCOVAs for main effects and post hoc comparisons controlling for age, sex, and education.
Extended Data Fig. 7 Decreased CSF Aβ1–42 and increased pTau levels in APOE4 carriers with cognitive impairment.
a, CSF Aβ1–42 levels in individuals with CDR 0 bearing APOE3 (black, n = 141) or APOE4 (red, n = 83) and with CDR 0.5 bearing APOE3 (n = 39) or APOE4 (n = 41). b, CSF Aβ1–42 levels in APOE3 (n = 89) and APOE4 (n = 55) carriers with no cognitive domains impaired, APOE3 (n = 29) and APOE4 (n = 31) carriers with one cognitive domain impaired, and APOE3 (n = 17) and APOE4 (n = 14) carriers with two or more cognitive domains impaired. c, CSF Aβ1-42 levels (estimated marginal means ± s.e.m. from ANCOVA models corrected for age, sex, education, and CSF sPDGFRβ levels) in individuals with CDR 0 bearing APOE3 (n = 141) or APOE4 (n = 83) and with CDR 0.5 bearing APOE3 (n = 39) or APOE4 (n = 41). d, CSF pTau levels in individuals with CDR 0 bearing APOE3 (n = 141) or APOE4 (n = 82) and with CDR 0.5 bearing APOE3 (n = 39) or APOE4 (n = 43). e, CSF pTau levels in APOE3 (n = 89) and APOE4 (n = 56) carriers with no cognitive domains impaired, APOE3 (n = 29) and APOE4 (n = 30) carriers with one cognitive domain impaired, and APOE3 (n = 17) and APOE4 (n = 15) carriers with two or more cognitive domains impaired. f, CSF pTau levels (estimated marginal means ± s.e.m. from ANCOVA models corrected for age, sex, education, and CSF sPDGFRβ levels) in individuals with CDR 0 bearing APOE3 (n = 141) or APOE4 (red, n = 82) and with CDR 0.5 bearing APOE3 (n = 39) or APOE4 (n = 43). Violin plots: continuous lines, median; dotted lines, IQR. CSF Aβ1–42 and pTau values were log10-transformed before statistical analysis because they had a non-normal distribution. Significance tests from ANCOVAs for main effects and post hoc comparisons controlling for age, sex, and education.
Extended Data Fig. 8 Full scans of western blots.
Full scans of western blots for CypA shown in Fig. 4m (top).
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-5, Supplementary Methods, a Supplementary Discussion and Supplementary References.
Rights and permissions
About this article
Cite this article
Montagne, A., Nation, D.A., Sagare, A.P. et al. APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature 581, 71–76 (2020). https://doi.org/10.1038/s41586-020-2247-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2247-3
This article is cited by
-
The role of CD56bright NK cells in neurodegenerative disorders
Journal of Neuroinflammation (2024)
-
Border-associated macrophages in the central nervous system
Journal of Neuroinflammation (2024)
-
Cell type-specific roles of APOE4 in Alzheimer disease
Nature Reviews Neuroscience (2024)
-
Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota–gut–brain axis
Nature Reviews Gastroenterology & Hepatology (2024)
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.