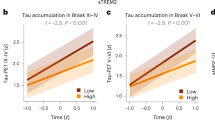
Abstract
Alzheimer disease (AD) is the most common form of neurodegenerative disease, estimated to contribute 60–70% of all cases of dementia worldwide. According to the prevailing amyloid cascade hypothesis, amyloid-β (Aβ) deposition in the brain is the initiating event in AD, although evidence is accumulating that this hypothesis is insufficient to explain many aspects of AD pathogenesis. The discovery of increased levels of inflammatory markers in patients with AD and the identification of AD risk genes associated with innate immune functions suggest that neuroinflammation has a prominent role in the pathogenesis of AD. In this Review, we discuss the interrelationships between neuroinflammation and amyloid and tau pathologies as well as the effect of neuroinflammation on the disease trajectory in AD. We specifically focus on microglia as major players in neuroinflammation and discuss the spatial and temporal variations in microglial phenotypes that are observed under different conditions. We also consider how these cells could be modulated as a therapeutic strategy for AD.
Key points
-
Neuroinflammation has demonstrated a key role in the pathogenesis of Alzheimer disease (AD), the most prevalent form of dementia.
-
Neuroinflammation encompasses a variety of inflammatory events in the CNS under pathological conditions.
-
Among the innate immune cells, microglia are the primary players in neuroinflammation.
-
Activated microglia exhibit diverse phenotypes and have multifaceted interactions with amyloid-β and tau species as well as with neuronal circuits.
-
Activated microglia might have diverse influences on the progression of AD, depending on the stage of disease, individual susceptibility and state of microglial priming.
-
Microglia could potentially be modulated at various points in the AD trajectory to either prevent or modify disease progression.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 14, 367–429 (2018).
Kawas, C., Gray, S., Brookmeyer, R., Fozard, J. & Zonderman, A. Age-specific incidence rates of Alzheimer’s disease - the Baltimore longitudinal study of aging. Neurology 54, 2072–2077 (2000).
McKhann, G. M. et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 263–269 (2011).
Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991).
Hardy, J. & Selkoe, D. J. Medicine — the amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002).
Herrup, K. The case for rejecting the amyloid cascade hypothesis. Nat. Neurosci. 18, 794–799 (2015).
Calsolaro, V. & Edison, P. Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement. 12, 719–732 (2016).
Heneka, M. T. et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 14, 388–405 (2015).
Obulesu, M. & Jhansilakshmi, M. Neuroinflammation in Alzheimer’s disease: an understanding of physiology and pathology. Int. J. Neurosci. 124, 227–235 (2014).
Jonsson, T. et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 368, 107–116 (2013).
Rohn, T. T. The triggering receptor expressed on myeloid cells 2: “TREM-ming” the inflammatory component associated with Alzheimer’s disease. Oxid. Med. Cell. Longev. 2013, 860959 (2013).
Thinakaran, G. & Koo, E. H. Amyloid precursor protein trafficking, processing, and function. J. Biol. Chem. 283, 29615–29619 (2008).
Selkoe, D. J. Soluble oligomers of the amyloid β-protein impair synaptic plasticity and behavior. Behav. Brain Res. 192, 106–113 (2008).
Hardy, J. & Allsop, D. Amyloid deposition as the central event in the etiology of Alzheimers-disease. Trends Pharmacol. Sci. 12, 383–388 (1991).
Hardy, J. A. & Higgins, G. A. Alzheimer’s disease: the amyloid cascade hypothesis. Science 256, 184–185 (1992).
Lannfelt, L., Relkin, N. R. & Siemers, E. R. Amyloid-ss-directed immunotherapy for Alzheimer’s disease. J. Intern. Med. 275, 284–295 (2014).
Small, S. A. & Duff, K. Linking Aβ and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron 60, 534–542 (2008).
Wang, Y. & Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 17, 5–21 (2016).
Jack, C. R. et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 9, 119–128 (2010).
Bischof, G. N., Endepols, H., van Eimeren, T. & Drzezga, A. Tau-imaging in neurodegeneration. Methods 130, 114–123 (2017).
Johnson, K. A. et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann. Neurol. 79, 110–119 (2016).
DiSabato, D. J., Quan, N. & Godbout, J. P. Neuroinflammation: the devil is in the details. J. Neurochem. 139 (Suppl. 2), 136–153 (2016).
Heneka, M. T., Kummer, M. P. & Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 14, 463–477 (2014).
Lyman, M., Lloyd, D. G., Ji, X., Vizcaychipi, M. P. & Ma, D. Neuroinflammation: the role and consequences. Neurosci. Res. 79, 1–12 (2014).
Mishra, A., Kim, H. J., Shin, A. H. & Thayer, S. A. Synapse loss induced by interleukin-1β requires pre- and post-synaptic mechanisms. J. Neuroimmune Pharmacol. 7, 571–578 (2012).
Micheau, O. & Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190 (2003).
Hong, S. et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716 (2016).
Sofroniew, M. V. & Vinters, H. V. Astrocytes: biology and pathology. Acta Neuropathol. 119, 7–35 (2010).
Attwell, D. et al. Glial and neuronal control of brain blood flow. Nature 468, 232–243 (2010).
Simard, M. & Nedergaard, M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience 129, 877–896 (2004).
Pekny, M. et al. Astrocytes: a central element in neurological diseases. Acta Neuropathol. 131, 323–345 (2016).
Rouach, N., Koulakoff, A., Abudara, V., Willecke, K. & Giaume, C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science 322, 1551–1555 (2008).
Eroglu, C. & Barres, B. A. Regulation of synaptic connectivity by glia. Nature 468, 223–231 (2010).
Jessen, N. A., Munk, A. S., Lundgaard, I. & Nedergaard, M. The glymphatic system: a beginner’s guide. Neurochem. Res. 40, 2583–2599 (2015).
Tarasoff-Conway, J. M. et al. Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 11, 457–470 (2015).
Pekny, M., Wilhelmsson, U. & Pekna, M. The dual role of astrocyte activation and reactive gliosis. Neurosci. Lett. 565, 30–38 (2014).
Liddelow, S. A. & Barres, B. A. Reactive astrocytes: production, function, and therapeutic potential. Immunity 46, 957–967 (2017).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
Martinez, F. O. & Gordon, S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6, 13 (2014).
Khakh, B. S. & Sofroniew, M. V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 18, 942–952 (2015).
Thal, D. R. et al. Amyloid β-protein (Aβ)-containing astrocytes are located preferentially near N-terminal-truncated Aβ deposits in the human entorhinal cortex. Acta Neuropathol. 100, 608–617 (2000).
Funato, H. et al. Astrocytes containing amyloid β-protein (Aβ)-positive granules are associated with Aβ40-positive diffuse plaques in the aged human brain. Am. J. Pathol. 152, 983–992 (1998).
Wyss-Coray, T. et al. Adult mouse astrocytes degrade amyloid-β in vitro and in situ. Nat. Med. 9, 453–457 (2003).
Jo, S. et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat. Med. 20, 886–896 (2014).
Chang, J. et al. NF-κB inhibits osteogenic differentiation of mesenchymal stem cells by promoting β-catenin degradation. Proc. Natl Acad. Sci. USA 110, 9469–9474 (2013).
Winkler, E. A. et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat. Neurosci. 18, 521–530 (2015).
Kisler, K., Nelson, A. R., Montagne, A. & Zlokovic, B. V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 18, 419–434 (2017).
Heneka, M. T. et al. Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J. Neuroinflammation 2, 22 (2005).
Harry, G. J. Microglia during development and aging. Pharmacol. Ther. 139, 313–326 (2013).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010).
Kierdorf, K. et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 16, 273–280 (2013).
Bruttger, J. et al. Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity 43, 92–106 (2015).
Priller, J. et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat. Med. 7, 1356–1361 (2001).
Mildner, A. et al. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain 132, 2487–2500 (2009).
Sweeney, M. D., Sagare, A. P. & Zlokovic, B. V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14, 133–150 (2018).
Salter, M. W. & Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 23, 1018–1027 (2017).
Ousman, S. S. & Kubes, P. Immune surveillance in the central nervous system. Nat. Neurosci. 15, 1096–1101 (2012).
Paolicelli, R. C. et al. Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458 (2011).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005).
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758 (2005).
Sole-Domenech, S., Cruz, D. L., Capetillo-Zarate, E. & Maxfield, F. R. The endocytic pathway in microglia during health, aging and Alzheimer’s disease. Ageing Res. Rev. 32, 89–103 (2016).
Bajetto, A., Bonavia, R., Barbero, S. & Schettini, G. Characterization of chemokines and their receptors in the central nervous system: physiopathological implications. J. Neurochem. 82, 1311–1329 (2002).
Owens, T., Khorooshi, R., Wlodarczyk, A. & Asgari, N. Interferons in the central nervous system: a few instruments play many tunes. Glia 62, 339–355 (2014).
Norden, D. M. & Godbout, J. P. Review: Microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol. Appl. Neurobiol. 39, 19–34 (2013).
Spittau, B. Aging microglia-phenotypes, functions and implications for age-related neurodegenerative diseases. Front. Aging Neurosci. 9 (2017).
Stence, N., Waite, M. & Dailey, M. E. Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia 33, 256–266 (2001).
Davies, D. S., Ma, J., Jegathees, T. & Goldsbury, C. Microglia show altered morphology and reduced arborization in human brain during aging and Alzheimer’s disease. Brain Pathol. 27, 795–808 (2017).
Rawji, K. S. et al. Immunosenescence of microglia and macrophages: impact on the ageing central nervous system. Brain 139, 653–661 (2016).
Bisht, K. et al. Dark microglia: a new phenotype predominantly associated with pathological states. Glia 64, 826–839 (2016).
Plescher, M. et al. Plaque-dependent morphological and electrophysiological heterogeneity of microglia in an Alzheimer’s disease mouse model. Glia 66, 1464–1480 (2018).
Sanchez-Mejias, E. et al. Soluble phospho-tau from Alzheimer’s disease hippocampus drives microglial degeneration. Acta Neuropathol. 132, 897–916 (2016).
Navarro, V. et al. Microglia in Alzheimer’s disease: activated, dysfunctional or degenerative. Front. Aging Neurosci. 10, 140 (2018).
Doorn, K. J. et al. Increased amoeboid microglial density in the olfactory bulb of Parkinson’s and Alzheimer’s patients. Brain Pathol. 24, 152–165 (2014).
Tischer, J. et al. Inhomogeneous distribution of Iba-1 characterizes microglial pathology in Alzheimer’s disease. Glia 64, 1562–1572 (2016).
Streit, W. J., Braak, H., Xue, Q. S. & Bechmann, I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathol. 118, 475–485 (2009).
Yin, Z. R. et al. Immune hyperreactivity of Aβ plaque-associated microglia in Alzheimer’s disease. Neurobiol. Aging 55, 115–122 (2017).
Nguyen, H. M. et al. Differential Kv1.3, KCa3.1, and Kir2.1 expression in “classically” and “alternatively” activated microglia. Glia 65, 106–121 (2017).
Minett, T. et al. Microglial immunophenotype in dementia with Alzheimer’s pathology. J. Neuroinflammation 13, 135 (2016).
Hopperton, K. E., Mohammad, D., Trepanier, M. O., Giuliano, V. & Bazinet, R. P. Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: a systematic review. Mol. Psychiatry 23, 177–198 (2018).
Varnum, M. M. & Ikezu, T. The classification of microglial activation phenotypes on neurodegeneration and regeneration in Alzheimer’s disease brain. Arch. Immunol. Ther. Ex. 60, 251–266 (2012).
Ransohoff, R. M. A polarizing question: do M1 and M2 microglia exist? Nat. Neurosci. 19, 987–991 (2016).
Walker, D. G. & Lue, L. F. Immune phenotypes of microglia in human neurodegenerative disease: challenges to detecting microglial polarization in human brains. Alzheimers Res. Ther. 7, 56 (2015).
Kim, C. C., Nakamura, M. C. & Hsieh, C. L. Brain trauma elicits non-canonical macrophage activation states. J. Neuroinflammation 13, 117 (2016).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290.e17 (2017).
Jay, T. R., von Saucken, V. E. & Landreth, G. E. TREM2 in neurodegenerative diseases. Mol. Neurodegeneration https://doi.org/10.1186/s13024-017-0197-5 (2017).
Galatro, T. F. et al. Transcriptomic analysis of purified human cortical microglia reveals age-associated changes. Nat. Neurosci. 20, 1162–1171 (2017).
Mathys, H. et al. Temporal tracking of microglia activation in neurodegeneration at single-cell resolution. Cell Rep. 21, 366–380 (2017).
Friedman, B. A. et al. Diverse brain myeloid expression profiles reveal distinct microglial activation states and aspects of Alzheimer’s disease not evident in mouse models. Cell Rep. 22, 832–847 (2018).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Olah, M. et al. A transcriptomic atlas of aged human microglia. Nat. Commun. https://doi.org/10.1038/s41467-018-02926-5 (2018).
Tan, Y. L., Yuan, Y. & Tian, L. Microglial regional heterogeneity and its role in the brain. Mol. Psychiatry 25, 351–367 (2020).
Masuda, T. et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392 (2019).
Grabert, K. et al. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 19, 504–516 (2016).
Mastroeni, D. et al. Laser-captured microglia in the Alzheimer’s and Parkinson’s brain reveal unique regional expression profiles and suggest a potential role for hepatitis B in the Alzheimer’s brain. Neurobiol. Aging 63, 12–21 (2018).
Prokop, S. et al. Impact of TREM2 risk variants on brain region-specific immune activation and plaque microenvironment in Alzheimer’s disease patient brain samples. Acta Neuropathol. 138, 613–630 (2019).
Lee, C. Y. D. et al. Elevated TREM2 gene dosage reprograms microglia responsivity and ameliorates pathological phenotypes in Alzheimer’s disease models. Neuron 97, 1032 (2018).
Krasemann, S. et al. The TREM2–APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47, 566 (2017).
Wang, Y. et al. TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J. Exp. Med. 213, 667–675 (2016).
Jay, T. R. et al. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. J. Exp. Med. 212, 287–295 (2015).
Lian, H. et al. NFκB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer’s disease. Neuron 85, 101–115 (2015).
Lian, H. et al. Astrocyte–microglia cross talk through complement activation modulates amyloid pathology in mouse models of Alzheimer’s disease. J. Neurosci. 36, 577–589 (2016).
Simon, E., Obst, J. & Gomez-Nicola, D. The evolving dialogue of microglia and neurons in Alzheimer’s disease: microglia as necessary transducers of pathology. Neuroscience 405, 24–34 (2019).
Walker, D. G., Dalsing-Hernandez, J. E., Campbell, N. A. & Lue, L. F. Decreased expression of CD200 and CD200 receptor in Alzheimer’s disease: a potential mechanism leading to chronic inflammation. Exp. Neurol. 215, 5–19 (2009).
Holtman, I. R. et al. Induction of a common microglia gene expression signature by aging and neurodegenerative conditions: a co-expression meta-analysis. Acta Neuropathol. Commun. 3, 31 (2015).
Swardfager, W. et al. A meta-analysis of cytokines in Alzheimer’s disease. Biol. Psychiatry 68, 930–941 (2010).
El Kadmiri, N., Said, N., Slassi, I., El Moutawakil, B. & Nadifi, S. Biomarkers for Alzheimer disease: classical and novel candidates’ review. Neuroscience 370, 181–190 (2018).
Olsson, B. et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 15, 673–684 (2016).
Baldacci, F., Lista, S., Cavedo, E., Bonuccelli, U. & Hampel, H. Diagnostic function of the neuroinflammatory biomarker YKL-40 in Alzheimer’s disease and other neurodegenerative diseases. Expert Rev. Proteom. 14, 285–299 (2017).
Sutphen, C. L. et al. Longitudinal cerebrospinal fluid biomarker changes in preclinical Alzheimer disease during middle age. JAMA Neurol. 72, 1029–1042 (2015).
Alcolea, D. et al. Amyloid precursor protein metabolism and inflammation markers in preclinical Alzheimer disease. Neurology 85, 626–633 (2015).
Edison, P. & Brooks, D. J. Role of neuroinflammation in the trajectory of Alzheimer’s disease and in vivo quantification using PET. J. Alzheimers Dis. 64, S339–S351 (2018).
Venneti, S., Lopresti, B. J. & Wiley, C. A. The peripheral benzodiazepine receptor (Translocator protein 18 kDa) in microglia: from pathology to imaging. Prog. Neurobiol. 80, 308–322 (2006).
Diorio, D., Welner, S. A., Butterworth, R. F., Meaney, M. J. & Suranyi-Cadotte, B. E. Peripheral benzodiazepine binding sites in Alzheimer’s disease frontal and temporal cortex. Neurobiol. Aging 12, 255–258 (1991).
Junck, L. et al. PET imaging of human gliomas with ligands for the peripheral benzodiazepine binding-site. Ann. Neurol. 26, 752–758 (1989).
Alam, M. M., Lee, J. & Lee, S. Y. Recent progress in the development of TSPO PET Ligands for neuroinflammation imaging in neurological diseases. Nucl. Med. Mol. Imaging 51, 283–296 (2017).
Owen, D. R. J. et al. Mixed-affinity binding in humans with 18-kDa translocator protein ligands. J. Nucl. Med. 52, 24–32 (2011).
Kreisl, W. C. et al. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J. Cereb. Blood Flow. Metab. 33, 53–58 (2013).
Fan, Z. et al. Can studies of neuroinflammation in a TSPO genetic subgroup (HAB or MAB) be applied to the entire AD cohort? J. Nucl. Med. 56, 707–713 (2015).
Lavisse, S. et al. Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. J. Neurosci. 32, 10809–10818 (2012).
Ji, B. et al. Imaging of peripheral benzodiazepine receptor expression as biomarkers of detrimental versus beneficial glial responses in mouse models of Alzheimer’s and other CNS pathologies. J. Neurosci. 28, 12255–12267 (2008).
Rojas, S. et al. Imaging brain inflammation with [11C]PK11195 by PET and induction of the peripheral-type benzodiazepine receptor after transient focal ischemia in rats. J. Cereb. Blood Flow. Metab. 27, 1975–1986 (2007).
Venneti, S., Wang, G., Nguyen, J. & Wiley, C. A. The positron emission tomography ligand DAA1106 binds with high affinity to activated microglia in human neurological disorders. J. Neuropathol. Exp. Neurol. 67, 1001–1010 (2008).
Janssen, B., Vugts, D. J., Windhorst, A. D. & Mach, R. H. PET imaging of microglial activation-beyond targeting TSPO. Molecules https://doi.org/10.3390/molecules23030607 (2018).
Narayanaswami, V. et al. Emerging PET radiotracers and targets for imaging of neuroinflammation in neurodegenerative diseases: outlook beyond TSPO. Mol. Imaging 17, 1536012118792317 (2018).
Beaino, W. et al. Purinergic receptors P2Y12R and P2X7R: potential targets for PET imaging of microglia phenotypes in multiple sclerosis. J. Neuroinflammation 14, 259 (2017).
Mcgeer, P. L., Itagaki, S., Tago, H. & Mcgeer, E. G. Reactive microglia in patients with senile dementia of Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci. Lett. 79, 195–200 (1987).
Tooyama, I., Kimura, H., Akiyama, H. & Mcgeer, P. L. Reactive microglia express class-I and class-II major histocompatibility complex antigens in Alzheimers disease. Brain Res. 523, 273–280 (1990).
Hayes, A., Thaker, U., Iwatsubo, T., Pickering-Brown, S. M. & Mann, D. M. Pathological relationships between microglial cell activity and tau and amyloid β protein in patients with Alzheimer’s disease. Neurosci. Lett. 331, 171–174 (2002).
Dani, M. et al. Microglial activation correlates in vivo with both tau and amyloid in Alzheimer’s disease. Brain 141, 2740–2754 (2018).
Kitazawa, M., Yamasaki, T. R. & LaFerla, F. M. Microglia as a potential bridge between the amyloid β-peptide and tau. Ann. N.Y. Acad. Sci. 1035, 85–103 (2004).
McGeer, P. L. & McGeer, E. G. The amyloid cascade-inflammatory hypothesis of Alzheimer disease: implications for therapy. Acta Neuropathol. 126, 479–497 (2013).
Delbo, R., Angeretti, N., Lucca, E., Desimoni, M. G. & Forloni, G. Reciprocal control of inflammatory cytokines, IL-1 and IL-6, and β-amyloid production in cultures. Neurosci. Lett. 188, 70–74 (1995).
Akiyama, H. et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 21, 383–421 (2000).
Hanisch, U. K. Microglia as a source and target of cytokines. Glia 40, 140–155 (2002).
Yang, T., Li, S. M., Xu, H. X., Walsh, D. M. & Selkoe, D. J. Large soluble oligomers of amyloid β-protein from Alzheimer brain are far less neuroactive than the smaller oligomers to which they dissociate. J. Neurosci. 37, 152–163 (2017).
Venegas, C. & Heneka, M. T. Danger-associated molecular patterns in Alzheimer’s disease. J. Leukoc. Biol. 101, 87–98 (2017).
Liu, S. et al. TLR2 is a primary receptor for Alzheimer’s amyloid β peptide to trigger neuroinflammatory activation. J. Immunol. 188, 1098–1107 (2012).
Murgas, P., Godoy, B. & von Bernhardi, R. Aβ potentiates inflammatory activation of glial cells induced by scavenger receptor ligands and inflammatory mediators in culture. Neurotox. Res. 22, 69–78 (2012).
Alawieyah Syed Mortadza, S., Sim, J. A., Neubrand, V. E. & Jiang, L. H. A critical role of TRPM2 channel in Aβ42-induced microglial activation and generation of tumor necrosis factor-α. Glia 66, 562–575 (2018).
Husemann, J., Loike, J. D., Kodama, T. & Silverstein, S. C. Scavenger receptor class B type I (SR-BI) mediates adhesion of neonatal murine microglia to fibrillar β-amyloid. J. Neuroimmunol. 114, 142–150 (2001).
Koenigsknecht, J. & Landreth, G. Microglial phagocytosis of fibrillar β-amyloid through a β1 integrin-dependent mechanism. J. Neurosci. 24, 9838–9846 (2004).
Malko, P., Syed Mortadza, S. A., McWilliam, J. & Jiang, L.-H. TRPM2 channel in microglia as a new player in neuroinflammation associated with a spectrum of central nervous system pathologies. Front. Pharmacol. 10, 239 (2019).
Strowig, T., Henao-Mejia, J., Elinav, E. & Flavell, R. Inflammasomes in health and disease. Nature 481, 278–286 (2012).
Heneka, M. T. et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493, 674 (2013).
White, C. S., Lawrence, C. B., Brough, D. & Rivers-Auty, J. Inflammasomes as therapeutic targets for Alzheimer’s disease. Brain Pathol. 27, 223–234 (2017).
Doens, D. & Fernandez, P. L. Microglia receptors and their implications in the response to amyloid-β for Alzheimer’s disease pathogenesis. J. Neuroinflammation 11, 48 (2014).
Guerreiro, R. & Hardy, J. Genetics of Alzheimer’s disease. Neurotherapeutics 11, 732–737 (2014).
Ulland, T. K. et al. TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell 170, 649–663.e13 (2017).
Paresce, D. M., Chung, H. Y. & Maxfield, F. R. Slow degradation of aggregates of the Alzheimer’s disease amyloid β-protein by microglial cells. J. Biol. Chem. 272, 29390–29397 (1997).
Cho, M. H. et al. Autophagy in microglia degrades extracellular β-amyloid fibrils and regulates the NLRP3 inflammasome. Autophagy 10, 1761–1775 (2014).
Plaza-Zabala, A., Sierra-Torre, V. & Sierra, A. Autophagy and microglia: novel partners in neurodegeneration and aging. Int. J. Mol. Sci. 18, 598 (2017).
Pan, X.-D. et al. Microglial phagocytosis induced by fibrillar β-amyloid is attenuated by oligomeric β-amyloid: implications for Alzheimer’s disease. Mol. Neurodegener. 6, 45 (2011).
Hellwig, S. et al. Forebrain microglia from wild-type but not adult 5xFAD mice prevent amyloid-β plaque formation in organotypic hippocampal slice cultures. Sci. Rep. 5, 14624 (2015).
Spangenberg, E. E. & Green, K. N. Inflammation in Alzheimer’s disease: lessons learned from microglia-depletion models. Brain Behav. Immun. 61, 1–11 (2017).
Raha-Chowdhury, R. et al. Erythromyeloid-derived TREM2: a major determinant of Alzheimer’s disease pathology in Down syndrome. J. Alzheimers Dis. 61, 1143–1162 (2018).
Streit, W. J., Sammons, N. W., Kuhns, A. J. & Sparks, D. L. Dystrophic microglia in the aging human brain. Glia 45, 208–212 (2004).
Streit, W. J. Microglial senescence: does the brain’s immune system have an expiration date? Trends Neurosci. 29, 506–510 (2006).
Hawcroft, G., Gardner, S. H. & Hull, M. A. Activation of peroxisome proliferator-activated receptor gamma does not explain the antiproliferative activity of the nonsteroidal anti-inflammatory drug indomethacin on human colorectal cancer cells. J. Pharmacol. Exp. Ther. 305, 632–637 (2003).
Chen, C. H. et al. Increased NF-κB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int. J. Neuropsychopharmacol. 15, 77–90 (2012).
Venegas, C. et al. Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease. Nature 552, 355–361 (2017).
Morales, I., Jimenez, J. M., Mancilla, M. & Maccioni, R. B. Tau oligomers and fibrils induce activation of microglial cells. J. Alzheimers Dis. 37, 849–856 (2013).
Wes, P. D. et al. Tau overexpression impacts a neuroinflammation gene expression network perturbed in Alzheimer’s disease. PLoS ONE 9, e106050 (2014).
Bolos, M. et al. Direct evidence of internalization of tau by microglia in vitro and in vivo. J. Alzheimers Dis. 50, 77–87 (2016).
Streit, W. J. et al. Microglial activation occurs late during preclinical Alzheimer’s disease. Glia 66, 2550–2562 (2018).
Asai, H. et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 18, 1584–1593 (2015).
Felsky, D. et al. Neuropathological correlates and genetic architecture of microglial activation in elderly human brain. Nat. Commun. 10, 409 (2019).
Ising, C. et al. NLRP3 inflammasome activation drives tau pathology. Nature 575, 669–673 (2019).
Chen, W. et al. Increased tauopathy drives microglia-mediated clearance of β-amyloid. Acta Neuropathol. Commun. 4, 63 (2016).
Sekiya, M. et al. Integrated biology approach reveals molecular and pathological interactions among Alzheimer’s Aβ42, Tau, TREM2, and TYROBP in Drosophila models. Genome Med. https://doi.org/10.1186/s13073-018-0530-9 (2018).
Takahashi, H. et al. Opposing effects of progranulin deficiency on amyloid and tau pathologies via microglial TYROBP network. Acta Neuropathol. 133, 785–807 (2017).
Lee, S. et al. Opposing effects of membrane-anchored CX3CL1 on amyloid and tau pathologies via the p38 MAPK pathway. J. Neurosci. 34, 12538–12546 (2014).
Bolos, M. et al. Absence of CX3CR1 impairs the internalization of tau by microglia. Mol. Neurodegeneration https://doi.org/10.1186/s13024-017-0200-1 (2017).
Hamelin, L. et al. Early and protective microglial activation in Alzheimer’s disease: a prospective study using 18F-DPA-714 PET imaging. Brain 139, 1252–1264 (2016).
Fan, Z., Brooks, D. J., Okello, A. & Edison, P. An early and late peak in microglial activation in Alzheimer’s disease trajectory. Brain 140, 792–803 (2017).
Parbo, P. et al. Does inflammation precede tau aggregation in early Alzheimer’s disease? A PET study. Neurobiol. Dis. 117, 211–216 (2018).
Dunn, N., Mullee, M., Perry, V. H. & Holmes, C. Association between dementia and infectious disease — evidence from a case-control study. Alzheimers Dis. Assoc. Disord. 19, 91–94 (2005).
t’ Veld, B. A. et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N. Engl. J. Med. 345, 1515–1521 (2001).
Etminan, M., Gill, S. & Samii, A. Effect of non-steroidal anti-inflammatory drugs on risk of Alzheimer’s disease: systematic review and meta-analysis of observational studies. Brit. Med. J. 327, 128 (2003).
Johnson, V. E. et al. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136, 28–42 (2013).
Hanzel, C. E. et al. Neuronal driven pre-plaque inflammation in a transgenic rat model of Alzheimer’s disease. Neurobiol. Aging 35, 2249–2262 (2014).
Okello, A. et al. Microglial activation and amyloid deposition in mild cognitive impairment. A PET study. Neurology 72, 56–62 (2009).
Femminella, G. D. et al. Microglial activation in early Alzheimer trajectory is associated with higher gray matter volume. Neurology 92, e1331–e1343 (2019).
Dani, M. et al. Tau aggregation correlates with amyloid deposition in both mild cognitive impairment and Alzheimer’s disease subjects. J. Alzheimers Dis. 70, 455–465 (2019).
Hamelin, L. et al. Early and protective microglial activation in Alzheimer’s disease: a prospective study using 18F-DPA-714 PET imaging. Brain 139, 1252–1264 (2016).
Kreisl, W. C., Henter, I. D. & Innis, R. B. Imaging translocator protein as a biomarker of neuroinflammation in dementia. Adv. Pharmacol. 82, 163–185 (2018).
Kreisl, W. C. et al. 11C-PBR28 binding to translocator protein increases with progression of Alzheimer’s disease. Neurobiol. Aging 44, 53–61 (2016).
Philippens, I. H. et al. Acceleration of amyloidosis by inflammation in the amyloid-β marmoset monkey model of Alzheimer’s disease. J. Alzheimers Dis. 55, 101–113 (2017).
Hollingworth, P. et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat. Genet. 43, 429 (2011).
Lambert, J. C. et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 45, 1452–U1206 (2013).
Perry, V. H. & Holmes, C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 10, 217–224 (2014).
Femminella, G. D. et al. Does microglial activation influence hippocampal volume and neuronal function in Alzheimer’s disease and Parkinson’s disease dementia? J. Alzheimers Dis. 51, 1275–1289 (2016).
Fan, Z. et al. Influence of microglial activation on neuronal function in Alzheimer’s and Parkinson’s disease dementia. Alzheimers Dement. 11, 608–621.e7 (2015).
Yokoi, T. et al. Involvement of the precuneus/posterior cingulate cortex is significant for the development of Alzheimer’s disease: a PET (THK5351, PiB) and resting fMRI study. Front. Aging Neurosci. 10, 304 (2018).
Passamonti, L. et al. Neuroinflammation and functional connectivity in Alzheimer’s disease: interactive influences on cognitive performance. J. Neurosci. 39, 7218–7226 (2019).
Melah, K. E. et al. Cerebrospinal fluid markers of Alzheimer’s disease pathology and microglial activation are associated with altered white matter microstructure in asymptomatic adults at risk for Alzheimer’s disease. J. Alzheimers Dis. 50, 873–886 (2016).
Edison, P. et al. Microglia, amyloid, and cognition in Alzheimer’s disease: an [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol. Dis. 32, 412–419 (2008).
Yokokura, M. et al. In vivo changes in microglial activation and amyloid deposits in brain regions with hypometabolism in Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 38, 343–351 (2011).
Kreisl, W. C. et al. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain 136, 2228–2238 (2013).
Combs, C. K., Karlo, J. C., Kao, S. C. & Landreth, G. E. β-Amyloid stimulation of microglia and monocytes results in TNFα-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J. Neurosci. 21, 1179–1188 (2001).
Floden, A. M., Li, S. & Combs, C. K. β-amyloid-stimulated microglia induce neuron death via synergistic stimulation of tumor necrosis factor α and NMDA receptors. J. Neurosci. 25, 2566–2575 (2005).
Martin, E., Boucher, C., Fontaine, B. & Delarasse, C. Distinct inflammatory phenotypes of microglia and monocyte-derived macrophages in Alzheimer’s disease models: effects of aging and amyloid pathology. Aging Cell 16, 27–38 (2017).
Neniskyte, U., Neher, J. J. & Brown, G. C. Neuronal death induced by nanomolar amyloid β is mediated by primary phagocytosis of neurons by microglia. J. Biol. Chem. 286, 39904–39913 (2011).
Shi, Q. Q. et al. Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aaf6295 (2017).
Serrano-Pozo, A., Betensky, R. A., Frosch, M. P. & Hyman, B. T. Plaque-associated local toxicity increases over the clinical course of Alzheimer disease. Am. J. Pathol. 186, 375–384 (2016).
Raj, A., Kuceyeski, A. & Weiner, M. A network diffusion model of disease progression in dementia. Neuron 73, 1204–1215 (2012).
Braak, H. & Del Tredici, K. The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain 138, 2814–2833 (2015).
Fan, Z., Okello, A. A., Brooks, D. J. & Edison, P. Longitudinal influence of microglial activation and amyloid on neuronal function in Alzheimer’s disease. Brain 138, 3685–3698 (2015).
Hamelin, L. et al. Distinct dynamic profiles of microglial activation are associated with progression of Alzheimer’s disease. Brain 141, 1855–1870 (2018).
Cagnin, A. et al. In-vivo measurement of activated microglia in dementia. Lancet 358, 461–467 (2001).
Wiley, C. A. et al. Carbon 11-labeled Pittsburgh compound B and carbon 11-labeled (R)-PK11195 positron emission tomographic imaging in Alzheimer disease. Arch. Neurol. 66, 60–67 (2009).
Lopez-Picon, F. R. et al. Neuroinflammation appears early on PET imaging and then plateaus in a mouse model of Alzheimer disease. J. Nucl. Med. 59, 509–515 (2018).
Yokokura, M. et al. Depiction of microglial activation in aging and dementia: positron emission tomography with [11C]DPA713 versus [11C](R)PK11195. J. Cereb. Blood Flow Metab. 37, 877–889 (2017).
Lyoo, C. H. et al. Cerebellum can serve as a pseudo-reference region in Alzheimer disease to detect neuroinflammation measured with PET radioligand binding to translocator protein. J. Nucl. Med. 56, 701–706 (2015).
Yaqub, M. et al. Optimization of supervised cluster analysis for extracting reference tissue input curves in (R)-[11C]PK11195 brain PET studies. J. Cereb. Blood Flow Metab. 32, 1600–1608 (2012).
Bradburn, S., Murgatroyd, C. & Ray, N. Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: a meta-analysis. Ageing Res. Rev. 50, 1–8 (2019).
Miguel-Alvarez, M. et al. Non-steroidal anti-inflammatory drugs as a treatment for Alzheimer’s disease: a systematic review and meta-analysis of treatment effect. Drugs Aging 32, 139–147 (2015).
Elewa, H. F., Hilali, H., Hess, D. C., Machado, L. S. & Fagan, S. C. Minocycline for short-term neuroprotection. Pharmacotherapy 26, 515–521 (2006).
Garcez, M. L. et al. Minocycline reduces inflammatory parameters in the brain structures and serum and reverses memory impairment caused by the administration of amyloid β (1-42) in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 77, 23–31 (2017).
Howard, R. et al. Minocycline at 2 different dosages vs placebo for patients with mild Alzheimer disease: a randomized clinical trial. JAMA Neurol. 77, 164–174 (2020).
Munoz, L. & Ammit, A. J. Targeting p38 MAPK pathway for the treatment of Alzheimer’s disease. Neuropharmacology 58, 561–568 (2010).
Thawkar, B. S. & Kaur, G. Inhibitors of NF-κB and P2X7/NLRP3/caspase 1 pathway in microglia: novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer’s disease. J. Neuroimmunol. 326, 62–74 (2019).
Mandrekar-Colucci, S., Karlo, J. C. & Landreth, G. E. Mechanisms underlying the rapid peroxisome proliferator-activated receptor-gamma-mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer’s disease. J. Neurosci. 32, 10117–10128 (2012).
Flores, J. et al. Caspase-1 inhibition alleviates cognitive impairment and neuropathology in an Alzheimer’s disease mouse model. Nat. Commun. 9, 3916 (2018).
Steeland, S. et al. Counteracting the effects of TNF receptor-1 has therapeutic potential in Alzheimer’s disease. EMBO Mol. Med. 10, e8300 (2018).
Shi, J. Q. et al. Anti-TNF-α reduces amyloid plaques and tau phosphorylation and induces CD11c-positive dendritic-like cell in the APP/PS1 transgenic mouse brains. Brain Res. 1368, 239–247 (2011).
Tobinick, E. L. & Gross, H. Rapid improvement in verbal fluency and aphasia following perispinal etanercept in Alzheimer’s disease. BMC Neurol. 8, 27 (2008).
Butchart, J. et al. Etanercept in Alzheimer disease: a randomized, placebo-controlled, double-blind, phase 2 trial. Neurology 84, 2161–2168 (2015).
Kitazawa, M. et al. Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal β-catenin pathway function in an Alzheimer’s disease model. J. Immunol. 187, 6539–6549 (2011).
Grimaldi, L. M. et al. A pilot study on the use of interferon β1a in early Alzheimer’s disease subjects. J. Neuroinflammation 11, 30 (2014).
Moussa, C. et al. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflammation 14, 1 (2017).
Alves, S. et al. Interleukin-2 improves amyloid pathology, synaptic failure and memory in Alzheimer’s disease mice. Brain 140, 826–842 (2017).
Kiyota, T. et al. CNS expression of anti-inflammatory cytokine interleukin-4 attenuates Alzheimer’s disease-like pathogenesis in APP+PS1 bigenic mice. FASEB J. 24, 3093–3102 (2010).
Fu, A. K. et al. IL-33 ameliorates Alzheimer’s disease-like pathology and cognitive decline. Proc. Natl Acad. Sci. USA 113, E2705–E2713 (2016).
Zheng, C., Zhou, X. W. & Wang, J. Z. The dual roles of cytokines in Alzheimer’s disease: update on interleukins, TNF-α, TGF-β and IFN-γ. Transl. Neurodegener. 5, 7 (2016).
Mandrekar-Colucci, S. & Landreth, G. E. Nuclear receptors as therapeutic targets for Alzheimer’s disease. Expert Opin. Ther. Targets 15, 1085–1097 (2011).
Escribano, L. et al. Rosiglitazone rescues memory impairment in Alzheimer’s transgenic mice: mechanisms involving a reduced amyloid and tau pathology. Neuropsychopharmacology 35, 1593–1604 (2010).
Yamanaka, M. et al. PPARγ/RXRα-induced and CD36-mediated microglial amyloid-β phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J. Neurosci. 32, 17321–17331 (2012).
Gold, M. et al. Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement. Geriatr. Cogn. Disord. 30, 131–146 (2010).
Yin, J. et al. NLRP3 inflammasome inhibitor ameliorates amyloid pathology in a mouse model of Alzheimer’s disease. Mol. Neurobiol. 55, 1977–1987 (2018).
Perry, V. H. & Teeling, J. Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin. Immunopathol. 35, 601–612 (2013).
Dias, H. K., Brown, C. L., Polidori, M. C., Lip, G. Y. & Griffiths, H. R. LDL-lipids from patients with hypercholesterolaemia and Alzheimer’s disease are inflammatory to microvascular endothelial cells: mitigation by statin intervention. Clin. Sci. 129, 1195–1206 (2015).
Verdile, G. et al. Inflammation and oxidative stress: the molecular connectivity between insulin resistance, obesity, and Alzheimer’s disease. Mediators Inflamm. 2015, 105828 (2015).
Chen, H. et al. Folic acid supplementation mitigates Alzheimer’s disease by reducing inflammation: a randomized controlled trial. Mediators Inflamm. 2016, 5912146 (2016).
Vedin, I. et al. Effects of docosahexaenoic acid-rich σ-3 fatty acid supplementation on cytokine release from blood mononuclear leukocytes: the OmegAD study. Am. J. Clin. Nutr. 87, 1616–1622 (2008).
Andrieu, S. et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 16, 377–389 (2017).
Ngandu, T. et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385, 2255–2263 (2015).
Kim, S. et al. Protocol for a pragmatic randomised controlled trial of body brain life-general practice and a lifestyle modification programme to decrease dementia risk exposure in a primary care setting. BMJ Open 8, e019329 (2018).
Rosenberg, A., Mangialasche, F., Ngandu, T., Solomon, A. & Kivipelto, M. Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: from FINGER to World-Wide FINGERS. J. Prev. Alzheimers Dis. 7, 29–36 (2020).
Jack, C. R. et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12, 207–216 (2013).
Acknowledgements
F.L. is sponsored by the China Scholarship Council to undertake postgraduate research.
Author information
Authors and Affiliations
Contributions
F.L. contributed to writing of the manuscript. P.E. made substantial contributions to discussions of the content and revision of the manuscript. Both authors researched data for the manuscript.
Corresponding author
Ethics declarations
Competing interests
P.E. declares that he was formerly funded by the Medical Research Council and now by the Higher Education Funding Council for England (HEFCE), that he has received grants from Alzheimer’s Drug Discovery Foundation, Alzheimer’s Research UK, Alzheimer’s Society UK, GE Healthcare, Novo Nordisk and Piramal Life Sciences, and that he has acted as a consultant to Novo Nordisk and Pfizer. The other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Leng, F., Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here?. Nat Rev Neurol 17, 157–172 (2021). https://doi.org/10.1038/s41582-020-00435-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-020-00435-y
This article is cited by
-
Exercise mimetics: a novel strategy to combat neuroinflammation and Alzheimer’s disease
Journal of Neuroinflammation (2024)
-
Comparative effect of selected caloric and non-caloric sweeteners on some neuroinflammatory indices in brain cortex and hippocampus of scopolamine-induced rat
Nutrire (2024)
-
Modulation of hippocampal protein expression by a brain penetrant biologic TNF-α inhibitor in the 3xTg Alzheimer’s disease mice
Journal of Translational Medicine (2024)
-
Single-cell RNA sequencing reveals roles of unique retinal microglia types in early diabetic retinopathy
Diabetology & Metabolic Syndrome (2024)
-
Profiling of long non-coding RNAs in hippocampal–entorhinal system subfields: impact of RN7SL1 on neuroimmune response modulation in Alzheimer’s disease
Journal of Neuroinflammation (2024)