Abstract
Microglia are resident macrophages of the CNS that are involved in its development, homeostasis and response to infection and damage. Microglial activation is a common feature of neurological disorders, and although in some instances this activation can be damaging, protective and regenerative functions of microglia have been revealed. The most prominent example of the regenerative functions is a role for microglia in supporting regeneration of myelin after injury, a process that is critical for axonal health and relevant to numerous disorders in which loss of myelin integrity is a prevalent feature, such as multiple sclerosis, Alzheimer disease and motor neuron disease. Although drugs that are intended to promote remyelination are entering clinical trials, the mechanisms by which remyelination is controlled and how microglia are involved are not completely understood. In this Review, we discuss work that has identified novel regulators of microglial activation — including molecular drivers, population heterogeneity and turnover — that might influence their pro-remyelination capacity. We also discuss therapeutic targeting of microglia as a potential approach to promoting remyelination.
Key points
-
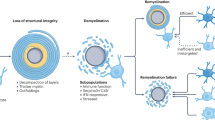
Microglia can support remyelination in the CNS after injury via clearance of debris, secretion of growth factors and cytokines and modulation of the extracellular matrix.
-
Novel drivers of pro-remyelination microglia activation and function have recently been identified.
-
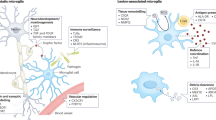
Microglia exhibit heterogeneity in their transcriptome, protein expression, proliferation and function within and between CNS regions, which might influence their pro-remyelination capacity.
-
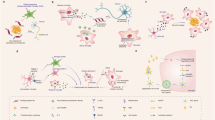
Active microglia turnover occurs under homeostatic conditions in both the human and rodent CNS; turnover is altered with disease and ageing and can be experimentally targeted to alter pathological outcome.
-
Although no microglia-specific drug targeting strategy currently exists, microglia might be targeted by pro-remyelination drugs that are currently in clinical trials.
-
Challenges in developing microglia-targeted drugs include understanding microglial heterogeneity, avoiding pro-inflammatory effects, developing microglia-specific drug delivery platforms, determining optimal timing of drug delivery and refining tools to monitor microglial responses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Aarum, J., Sandberg, K., Haeberlein, S. L. & Persson, M. A. Migration and differentiation of neural precursor cells can be directed by microglia. Proc. Natl Acad. Sci. USA 100, 15983–15988 (2003).
Ueno, M. et al. Layer V cortical neurons require microglial support for survival during postnatal development. Nat. Neurosci. 16, 543–551 (2013).
Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012).
Torres, L. et al. Dynamic microglial modulation of spatial learning and social behavior. Brain Behav. Immun. 55, 6–16 (2016).
Hagemeyer, N. et al. Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol. 134, 441–458 (2017).
Wlodarczyk, A. et al. A novel microglial subset plays a key role in myelinogenesis in developing brain. EMBO J. 36, 3292–3308 (2017).
Erblich, B., Zhu, L., Etgen, A. M., Dobrenis, K. & Pollard, J. W. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLOS ONE 6, e26317 (2011).
Parkhurst, C. N. et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155, 1596–1609 (2013).
Hickman, S. E. et al. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 16, 1896–1905 (2013).
Dagher, N. N. et al. Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg-AD mice. J. Neuroinflamm. 12, 139 (2015).
Spangenberg, E. E. et al. Eliminating microglia in Alzheimer’s mice prevents neuronal loss without modulating amyloid-beta pathology. Brain 139, 1265–1281 (2016).
Nissen, J. C., Thompson, K. K., West, B. L. & Tsirka, S. E. Csf1R inhibition attenuates experimental autoimmune encephalomyelitis and promotes recovery. Exp. Neurol. 307, 24–36 (2018).
Krasemann, S. et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47, 566–581 (2017).
Mildner, A., Huang, H., Radke, J., Stenzel, W. & Priller, J. P2Y12 receptor is expressed on human microglia under physiological conditions throughout development and is sensitive to neuroinflammatory diseases. Glia 65, 375–387 (2017).
Zrzavy, T. et al. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain 140, 1900–1913 (2017).
Butovsky, O. et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat. Neurosci. 17, 131–143 (2014).
Ajami, B. et al. Single-cell mass cytometry reveals distinct populations of brain myeloid cells in mouse neuroinflammation and neurodegeneration models. Nat. Neurosci. 21, 541–551 (2018).
Mrdjen, D. et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48, 380–395 (2018).
Holtman, I. R. et al. Induction of a common microglia gene expression signature by aging and neurodegenerative conditions: a co-expression meta-analysis. Acta Neuropathol. Commun. 3, 31 (2015).
Mathys, H. et al. Temporal tracking of microglia activation in neurodegeneration at single-cell resolution. Cell Rep. 21, 366–380 (2017).
Raj, D. et al. Increased white matter inflammation in aging- and Alzheimer’s disease brain. Front. Mol. Neurosci. 10, 206 (2017).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290 (2017).
Yin, Z. et al. Immune hyperreactivity of Abeta plaque-associated microglia in Alzheimer’s disease. Neurobiol. Aging 55, 115–122 (2017).
Butovsky, O. & Weiner, H. L. Microglial signatures and their role in health and disease. Nat. Rev. Neurosci. 19, 622–635 (2018).
Kanazawa, M. et al. Microglia preconditioned by oxygen-glucose deprivation promote functional recovery in ischemic rats. Sci. Rep. 7, 42582 (2017).
Stangel, M., Kuhlmann, T., Matthews, P. M. & Kilpatrick, T. J. Achievements and obstacles of remyelinating therapies in multiple sclerosis. Nat. Rev. Neurol. 13, 742–754 (2017).
Behrendt, G. et al. Dynamic changes in myelin aberrations and oligodendrocyte generation in chronic amyloidosis in mice and men. Glia 61, 273–286 (2013).
Franklin, R. J. M. & Ffrench-Constant, C. Regenerating CNS myelin - from mechanisms to experimental medicines. Nat. Rev. Neurosci. 18, 753–769 (2017).
Olah, M. et al. Identification of a microglia phenotype supportive of remyelination. Glia 60, 306–321 (2012).
Kotter, M. R., Li, W. W., Zhao, C. & Franklin, R. J. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J. Neurosci. 26, 328–332 (2006).
Rawji, K. S. et al. Deficient surveillance and phagocytic activity of myeloid cells within demyelinated lesions in aging mice visualized by ex vivo live multiphoton imaging. J. Neurosci. 38, 1973–1988 (2018).
Durafourt, B. A. et al. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia 60, 717–727 (2012).
Lampron, A. et al. Inefficient clearance of myelin debris by microglia impairs remyelinating processes. J. Exp. Med. 212, 481–495 (2015).
Neumann, H., Kotter, M. R. & Franklin, R. J. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain 132, 288–295 (2009).
Pu, A., Stephenson, E. L. & Yong, V. W. The extracellular matrix: Focus on oligodendrocyte biology and targeting CSPGs for remyelination therapies. Glia 66, 1809–1825 (2018).
Miron, V. E. Microglia-driven regulation of oligodendrocyte lineage cells, myelination, and remyelination. J. Leukoc. Biol. 101, 1103–1108 (2017).
Franklin, R. J. Why does remyelination fail in multiple sclerosis? Nat. Rev. Neurosci. 3, 705–714 (2002).
Patani, R., Balaratnam, M., Vora, A. & Reynolds, R. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol. Appl. Neurobiol. 33, 277–287 (2007).
Wolswijk, G. Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain 125, 338–349 (2002).
Voss, E. V. et al. Characterisation of microglia during de- and remyelination: can they create a repair promoting environment? Neurobiol. Dis. 45, 519–528 (2012).
Miron, V. E. et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 16, 1211–1218 (2013).
Hammond, T. R. et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50, 253–271 (2018).
Locatelli, G. et al. Mononuclear phagocytes locally specify and adapt their phenotype in a multiple sclerosis model. Nat. Neurosci. 21, 1196–1208 (2018).
Zabala, A. et al. P2X4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis. EMBO Mol. Med. 10, e8743 (2018).
Yu, Z. et al. MSX3 switches microglia polarization and protects from inflammation-induced demyelination. J. Neurosci. 35, 6350–6365 (2015).
Mei, F. et al. Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. eLife 5, e18246 (2016).
Vogel, D. Y. et al. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J. Neuroinflamm. 10, 35 (2013).
Peferoen, L. A. et al. Activation status of human microglia is dependent on lesion formation stage and remyelination in multiple sclerosis. J. Neuropathol. Exp. Neurol. 74, 48–63 (2015).
Bennett, F. C. et al. A combination of ontogeny and CNS environment establishes microglial identity. Neuron 98, 1170–1183 (2018).
Yamasaki, R. et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med. 211, 1533–1549 (2014).
Healy, L. M. et al. MerTK is a functional regulator of myelin phagocytosis by human myeloid cells. J. Immunol. 196, 3375–3384 (2016).
Greenhalgh, A. D. et al. Peripherally derived macrophages modulate microglial function to reduce inflammation after CNS injury. PLOS Biol. 16, e2005264 (2018).
Ruckh, J. M. et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 10, 96–103 (2012).
Olah, M. et al. A transcriptomic atlas of aged human microglia. Nat. Commun. 9, 539 (2018).
Galatro, T. F. et al. Transcriptomic analysis of purified human cortical microglia reveals age-associated changes. Nat. Neurosci. 20, 1162–1171 (2017).
Safaiyan, S. et al. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat. Neurosci. 19, 995–998 (2016).
Hill, R. A., Li, A. M. & Grutzendler, J. Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat. Neurosci. 21, 683–695 (2018).
Cantuti-Castelvetri, L. et al. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 359, 684–688 (2018).
Skripuletz, T. et al. Astrocytes regulate myelin clearance through recruitment of microglia during cuprizone-induced demyelination. Brain 136, 147–167 (2013).
Cantoni, C. et al. TREM2 regulates microglial cell activation in response to demyelination in vivo. Acta Neuropathol. 129, 429–447 (2015).
Poliani, P. L. et al. TREM2 sustains microglial expansion during aging and response to demyelination. J. Clin. Invest. 125, 2161–2170 (2015).
Beckmann, N. et al. Brain region-specific enhancement of remyelination and prevention of demyelination by the CSF1R kinase inhibitor BLZ945. Acta Neuropathol. Commun. 6, 9 (2018).
Bruce, K. D. et al. Lipoprotein lipase is a feature of alternatively-activated microglia and may facilitate lipid uptake in the CNS during demyelination. Front. Mol. Neurosci. 11, 57 (2018).
Laflamme, N. et al. mCSF-induced microglial activation prevents myelin loss and promotes its repair in a mouse model of multiple sclerosis. Front. Cell. Neurosci. 12, 178 (2018).
Church, J. S., Kigerl, K. A., Lerch, J. K., Popovich, P. G. & McTigue, D. M. TLR4 deficiency impairs oligodendrocyte formation in the injured spinal cord. J. Neurosci. 36, 6352–6364 (2016).
Natrajan, M. S. et al. Retinoid X receptor activation reverses age-related deficiencies in myelin debris phagocytosis and remyelination. Brain 138, 3581–3597 (2015).
Dillenburg, A. et al. Activin receptors regulate the oligodendrocyte lineage in health and disease. Acta Neuropathol. 135, 887–906 (2018).
Pasquini, L. A. et al. Galectin-3 drives oligodendrocyte differentiation to control myelin integrity and function. Cell Death Differ. 18, 1746–1756 (2011).
Arnett, H. A. et al. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat. Neurosci. 4, 1116–1122 (2001).
Hlavica, M. et al. Intrathecal insulin-like growth factor 1 but not insulin enhances myelin repair in young and aged rats. Neurosci. Lett. 648, 41–46 (2017).
Mason, J. L., Suzuki, K., Chaplin, D. D. & Matsushima, G. K. Interleukin-1beta promotes repair of the CNS. J. Neurosci. 21, 7046–7052 (2001).
Lalive, P. H. et al. TGF-beta-treated microglia induce oligodendrocyte precursor cell chemotaxis through the HGF-c-Met pathway. Eur. J. Immunol. 35, 727–737 (2005).
Dziembowska, M. et al. A role for CXCR4 signaling in survival and migration of neural and oligodendrocyte precursors. Glia 50, 258–269 (2005).
Nicholas, R. S., Wing, M. G. & Compston, A. Nonactivated microglia promote oligodendrocyte precursor survival and maturation through the transcription factor NF-kappa B. Eur. J. Neurosci. 13, 959–967 (2001).
Giera, S. et al. Microglial transglutaminase-2 drives myelination and myelin repair via GPR56/ADGRG1 in oligodendrocyte precursor cells. eLife 7, e33385 (2018).
Van Strien, M. E. et al. Tissue transglutaminase activity is involved in the differentiation of oligodendrocyte precursor cells into myelin-forming oligodendrocytes during CNS remyelination. Glia 59, 1622–1634 (2011).
Lawson, L. J., Perry, V. H., Dri, P. & Gordon, S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39, 151–170 (1990).
Grabert, K. et al. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 19, 504–516 (2016).
Ayata, P. et al. Epigenetic regulation of brain region-specific microglia clearance activity. Nat. Neurosci. 21, 1049–1060 (2018).
De Biase, L. M. et al. Local cues establish and maintain region-specific phenotypes of basal ganglia microglia. Neuron 95, 341–356 (2017).
Bottcher, C. et al. Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry. Nat. Neurosci. 22, 78–90 (2019).
Kondo, Y. & Duncan, I. D. Selective reduction in microglia density and function in the white matter of colony-stimulating factor-1-deficient mice. J. Neurosci. Res. 87, 2686–2695 (2009).
Remington, L. T., Babcock, A. A., Zehntner, S. P. & Owens, T. Microglial recruitment, activation, and proliferation in response to primary demyelination. Am. J. Pathol. 170, 1713–1724 (2007).
Matcovitch-Natan, O. et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 353, aad8670 (2016).
Li, Q. et al. Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron 101, 207–223 (2019).
Tay, T. L., Sagar, Dautzenberg, J., Grun, D. & Prinz, M. Unique microglia recovery population revealed by single-cell RNAseq following neurodegeneration. Acta Neuropathol. Commun. 6, 87 (2018).
Skripuletz, T. et al. Cerebellar cortical demyelination in the murine cuprizone model. Brain Pathol. 20, 301–312 (2010).
Askew, K. et al. Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Rep. 18, 391–405 (2017).
Tay, T. L. et al. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat. Neurosci. 20, 793–803 (2017).
Reu, P. et al. The lifespan and turnover of microglia in the human brain. Cell Rep. 20, 779–784 (2017).
Fuger, P. et al. Microglia turnover with aging and in an Alzheimer’s model via long-term in vivo single-cell imaging. Nat. Neurosci. 20, 1371–1376 (2017).
Lawson, L. J., Perry, V. H. & Gordon, S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience 48, 405–415 (1992).
Ajami, B., Bennett, J. L., Krieger, C., McNagny, K. M. & Rossi, F. M. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 14, 1142–1149 (2011).
Bruttger, J. et al. Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity 43, 92–106 (2015).
Elmore, M. R. et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380–397 (2014).
Huang, Y. et al. Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nat. Neurosci. 21, 530–540 (2018).
Hillmer, A. T. et al. Microglial depletion and activation: A [(11)C]PBR28 PET study in nonhuman primates. EJNMMI Res. 7, 59 (2017).
Zhang, Y. et al. Repopulating retinal microglia restore endogenous organization and function under CX3CL1-CX3CR1 regulation. Sci. Adv. 4, eaap8492 (2018).
Huang, Y. et al. Dual extra-retinal origins of microglia in the model of retinal microglia repopulation. Cell Discov. 4, 9 (2018).
Cronk, J. C. et al. Peripherally derived macrophages can engraft the brain independent of irradiation and maintain an identity distinct from microglia. J. Exp. Med. 215, 1627–1647 (2018).
Yao, Y. et al. Dynamics of spinal microglia repopulation following an acute depletion. Sci. Rep. 6, 22839 (2016).
Najafi, A. R. et al. A limited capacity for microglial repopulation in the adult brain. Glia 66, 2385–2396 (2018).
Rice, R. A. et al. Microglial repopulation resolves inflammation and promotes brain recovery after injury. Glia 65, 931–944 (2017).
Szalay, G. et al. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat. Commun. 7, 11499 (2016).
Jin, W. N. et al. Depletion of microglia exacerbates postischemic inflammation and brain injury. J. Cereb. Blood Flow Metab. 37, 2224–2236 (2017).
Rubino, S. J. et al. Acute microglia ablation induces neurodegeneration in the somatosensory system. Nat. Commun. 9, 4578 (2018).
Butowski, N. et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro-oncology 18, 557–564 (2016).
Elmore, M. R. P. et al. Replacement of microglia in the aged brain reverses cognitive, synaptic, and neuronal deficits in mice. Aging Cell 17, e12832 (2018).
Mosher, K. I. & Wyss-Coray, T. Microglial dysfunction in brain aging and Alzheimer’s disease. Biochem. Pharmacol. 88, 594–604 (2014).
Samanani, S. et al. Screening for inhibitors of microglia to reduce neuroinflammation. CNS Neurol. Disord. Drug Targets 12, 741–749 (2013).
Sarkar, S. et al. Therapeutic activation of macrophages and microglia to suppress brain tumor-initiating cells. Ann. Neurosci. 20, 154 (2013).
Doring, A. et al. Stimulation of monocytes, macrophages, and microglia by amphotericin B and macrophage colony-stimulating factor promotes remyelination. J. Neurosci. 35, 1136–1148 (2015).
Skihar, V. et al. Promoting oligodendrogenesis and myelin repair using the multiple sclerosis medication glatiramer acetate. Proc. Natl Acad. Sci. USA 106, 17992–17997 (2009).
Jackson, S. J., Giovannoni, G. & Baker, D. Fingolimod modulates microglial activation to augment markers of remyelination. J. Neuroinflamm. 8, 76 (2011).
Durafourt, B. A. et al. Differential responses of human microglia and blood-derived myeloid cells to FTY720. J. Neuroimmunol. 230, 10–16 (2011).
Li, W. W., Setzu, A., Zhao, C. & Franklin, R. J. Minocycline-mediated inhibition of microglia activation impairs oligodendrocyte progenitor cell responses and remyelination in a non-immune model of demyelination. J. Neuroimmunol. 158, 58–66 (2005).
Moller, T. et al. Critical data-based re-evaluation of minocycline as a putative specific microglia inhibitor. Glia 64, 1788–1794 (2016).
Green, A. J. et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet 390, 2481–2489 (2017).
Mei, F. et al. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat. Med. 20, 954–960 (2014).
Norenberg, W. et al. Clemastine potentiates the human P2X7 receptor by sensitizing it to lower ATP concentrations. J. Biol. Chem. 286, 11067–11081 (2011).
Apolloni, S., Fabbrizio, P., Parisi, C., Amadio, S. & Volonte, C. Clemastine confers neuroprotection and induces an anti-inflammatory phenotype in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Mol. Neurobiol. 53, 518–531 (2016).
Xiao, L. et al. Quetiapine facilitates oligodendrocyte development and prevents mice from myelin breakdown and behavioral changes. Mol. Psychiatry 13, 697–708 (2008).
Zhang, Y. et al. Quetiapine alleviates the cuprizone-induced white matter pathology in the brain of C57BL/6 mouse. Schizophr. Res. 106, 182–191 (2008).
Wang, H. et al. Quetiapine inhibits microglial activation by neutralizing abnormal STIM1-mediated intercellular calcium homeostasis and promotes myelin repair in a cuprizone-induced mouse model of demyelination. Front. Cell. Neurosci. 9, 492 (2015).
Zhang, M. et al. Thyroid hormone alleviates demyelination induced by cuprizone through its role in remyelination during the remission period. Exp. Biol. Med. (Maywood) 240, 1183–1196 (2015).
Lima, F. R. et al. Regulation of microglial development: a novel role for thyroid hormone. J. Neurosci. 21, 2028–2038 (2001).
Mori, Y. et al. Effects of 3,3′,5-triiodothyronine on microglial functions. Glia 63, 906–920 (2015).
Mullin, A. P. et al. rHIgM22 enhances remyelination in the brain of the cuprizone mouse model of demyelination. Neurobiol. Dis. 105, 142–155 (2017).
Zorina, Y., Stricker, J., Caggiano, A. O. & Button, D. C. Human IgM antibody rHIgM22 promotes phagocytic clearance of myelin debris by microglia. Sci. Rep. 8, 9392 (2018).
Durafourt, B. A., Moore, C. S., Blain, M. & Antel, J. P. Isolating, culturing, and polarizing primary human adult and fetal microglia. Methods Mol. Biol. 1041, 199–211 (2013).
Muffat, J. et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat. Med. 22, 1358–1367 (2016).
Abud, E. M. et al. iPSC-derived human microglia-like cells to study neurological diseases. Neuron 94, 278–293 (2017).
Haenseler, W. et al. A highly efficient human pluripotent stem cell microglia model displays a neuronal-co-culture-specific expression profile and inflammatory response. Stem Cell Rep. 8, 1727–1742 (2017).
Pandya, H. et al. Differentiation of human and murine induced pluripotent stem cells to microglia-like cells. Nat. Neurosci. 20, 753–759 (2017).
Bohlen, C. J. et al. Diverse requirements for microglial survival, specification, and function revealed by defined-medium cultures. Neuron 94, 759–773 (2017).
Gosselin, D. et al. An environment-dependent transcriptional network specifies human microglia identity. Science 356, eaal3222 (2017).
Ormel, P. R. et al. Microglia innately develop within cerebral organoids. Nat. Commun. 9, 4167 (2018).
Lewis, N., Hill, J., Juchem, K., Stefanopoulos, D. & Modis, L. RNA sequencing of microglia and monocyte-derived macrophages from mice with experimental autoimmune encephalomyelitis illustrates a changing phenotype with disease course. J. Neuroimmunol. 277, 26–38 (2014).
Brown, R. A., Narayanan, S. & Arnold, D. L. Imaging of repeated episodes of demyelination and remyelination in multiple sclerosis. Neuroimage Clin. 6, 20–25 (2014).
Cusick, M. F., Libbey, J. E., Patel, D. C., Doty, D. J. & Fujinami, R. S. Infiltrating macrophages are key to the development of seizures following virus infection. J. Virol. 87, 1849–1860 (2013).
Mizutani, M. et al. The fractalkine receptor but not CCR2 is present on microglia from embryonic development throughout adulthood. J. Immunol. 188, 29–36 (2012).
O’Koren, E. G., Mathew, R. & Saban, D. R. Fate mapping reveals that microglia and recruited monocyte-derived macrophages are definitively distinguishable by phenotype in the retina. Sci. Rep. 6, 20636 (2016).
Vainchtein, I. D. et al. In acute experimental autoimmune encephalomyelitis, infiltrating macrophages are immune activated, whereas microglia remain immune suppressed. Glia 62, 1724–1735 (2014).
Saederup, N. et al. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLOS ONE 5, e13693 (2010).
Goldmann, T. et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci. 16, 1618–1626 (2013).
Xue, J. et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40, 274–288 (2014).
Murray, P. J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 (2014).
Wang, J., Siffert, M., Spiliotis, M. & Gottstein, B. Repeated long-term DT application in the DEREG mouse induces a neutralizing anti-DT antibody response. J. Immunol. Res. 2016, 1450398 (2016).
Acknowledgements
V.E.M. is funded by a Career Development Award from the Medical Research Council and the United Kingdom Multiple Sclerosis Society (MR/M020827/1), as well as from funds from the Medical Research Council Centre for Reproductive Health (MR/N02256/1).
Reviewer information
Nature Reviews Neurology thanks D. Fitzgerald, S. Rivest, M. Stangel and V. W. Yong for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Both authors contributed to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
V.E.M. has received research funds related to remyelination within the past 5 years from Biogen, BVBioMed, Clene Nanomedicine, GlaxoSmithKline and Rewind Therapeutics.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Oligodendrocyte progenitor cells
-
(OPCs). A glial subtype derived from neuroepithelium that can differentiate into mature oligodendrocytes, astrocytes and Schwann cells.
- iNOS
-
(Inducible nitric oxide synthase). An enzyme that catalyses the generation of nitric oxide from l-arginine and competes with arginase 1 (ARG1) for the same substrate.
- TNF
-
(Tumour necrosis factor). A pro-inflammatory cytokine that can have deleterious and beneficial effects on the oligodendrocyte lineage.
- CD16–CD32
-
Low-affinity immunoglobulin Fc receptors FcγIIA/B (CD16) and FcγIIIA/B (CD32); involved in phagocytosis, microorganism toxicity, stimulation of cytokine production and antibody-dependent cell-mediated cytotoxicity.
- ARG1
-
(Arginase 1). An enzyme that catalyses the generation of ornithine and urea from l-arginine and competes with iNOS for the same substrate, thus indirectly reducing production of nitric oxide.
- IGF1
-
(Insulin-like growth factor 1). A growth factor that stimulates oligodendrocyte differentiation and remyelination.
- CD206
-
Also known as mannose receptor. A transmembrane glycoprotein that binds to mannosylated molecules and probably plays a role in clearance of pathogens.
- Repopulation
-
Re-establishment of a cell population following depletion or loss; microglia repopulation following robust depletion (80–99%) can occur within 1 week.
- Single-cell sequencing
-
A next-generation sequencing approach that allows transcriptomic profiling of individual cells, providing information regarding heterogeneity of a population.
- Induced pluripotent stem cells
-
(iPSCs). Pluripotent stem cells derived from reprogramming of somatic cells; iPSC-derived microglia react to inflammatory stimuli and phagocytose in a similar manner to primary microglia.
Rights and permissions
About this article
Cite this article
Lloyd, A.F., Miron, V.E. The pro-remyelination properties of microglia in the central nervous system. Nat Rev Neurol 15, 447–458 (2019). https://doi.org/10.1038/s41582-019-0184-2
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-019-0184-2
This article is cited by
-
Susceptibility to acute cognitive dysfunction in aged mice is underpinned by reduced white matter integrity and microgliosis
Communications Biology (2024)
-
Microglia regulation of central nervous system myelin health and regeneration
Nature Reviews Immunology (2024)
-
Prenatal Programming of Monocyte Chemotactic Protein-1 Signaling in Autism Susceptibility
Molecular Neurobiology (2024)
-
MFG-E8 Alleviates Cognitive Impairments Induced by Chronic Cerebral Hypoperfusion by Phagocytosing Myelin Debris and Promoting Remyelination
Neuroscience Bulletin (2024)
-
Serum LDL Promotes Microglial Activation and Exacerbates Demyelinating Injury in Neuromyelitis Optica Spectrum Disorder
Neuroscience Bulletin (2024)