Abstract
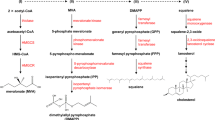
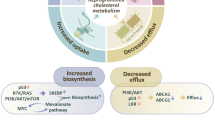
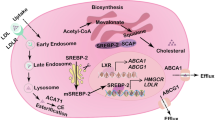
Cholesterol homeostasis is vital for proper cellular and systemic functions. Disturbed cholesterol balance underlies not only cardiovascular disease but also an increasing number of other diseases such as neurodegenerative diseases and cancers. The cellular cholesterol level reflects the dynamic balance between biosynthesis, uptake, export and esterification — a process in which cholesterol is converted to neutral cholesteryl esters either for storage in lipid droplets or for secretion as constituents of lipoproteins. In this Review, we discuss the latest advances regarding how each of the four parts of cholesterol metabolism is executed and regulated. The key factors governing these pathways and the major mechanisms by which they respond to varying sterol levels are described. Finally, we discuss how these pathways function in a concerted manner to maintain cholesterol homeostasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Luo, J., Jiang, L. Y., Yang, H. Y. & Song, B. L. Intracellular cholesterol transport by sterol transfer proteins at membrane contact sites. Trends Biochem. Sci. 44, 273–292 (2019).
Wong, L. H., Gatta, A. T. & Levine, T. P. Lipid transfer proteins: the lipid commute via shuttles, bridges and tubes. Nat. Rev. Mol. Cell Biol. 20, 85–101 (2019).
Liscum, L. & Munn, N. J. Intracellular cholesterol transport. Biochim. Biophys. Acta 1438, 19–37 (1999).
Sezgin, E., Levental, I., Mayor, S. & Eggeling, C. The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 18, 361–374 (2017).
Luu, W., Sharpe, L. J., Capell-Hattam, I., Gelissen, I. C. & Brown, A. J. Oxysterols: old tale, new twists. Annu. Rev. Pharmacol. Toxicol. 56, 447–467 (2016).
Porter, J. A., Young, K. E. & Beachy, P. A. Cholesterol modification of Hedgehog signaling proteins in animal development. Science 274, 255–259 (1996).
Xiao, X. et al. Cholesterol modification of smoothened is required for Hedgehog signaling. Mol. Cell 66, 154–162 (2017).
Shibuya, Y., Chang, C. C. Y. & Chang, T. Y. ACAT1/SOAT1 as a therapeutic target for Alzheimer’s disease. Future Med. Chem. 7, 2451–2467 (2015).
Kuzu, O. F., Noory, M. A. & Robertson, G. P. The role of cholesterol in cancer. Cancer Res. 76, 2063–2070 (2016).
Silvente-Poirot, S. & Poirot, M. Cholesterol and cancer, in the balance. Science 343, 1445–1446 (2014).
Altmann, S. W. et al. Niemann–Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science 303, 1201–1204 (2004). This work shows that NPC1L1 is highly expressed in the small intestine and is critical for dietary cholesterol absorption. NPC1L1-deficient mice absorb much less cholesterol and are insensitive to ezetimibe, a cholesterol absorption inhibitor.
Goldstein, J. L. & Brown, M. S. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 29, 431–438 (2009).
Maxfield, F. R. & van Meer, G. Cholesterol, the central lipid of mammalian cells. Curr. Opin. Cell Biol. 22, 422–429 (2010).
Phillips, M. C. Molecular mechanisms of cellular cholesterol efflux. J. Biol. Chem. 289, 24020–24029 (2014).
Chang, T. Y., Li, B. L., Chang, C. C. & Urano, Y. Acyl-coenzyme A:cholesterol acyltransferases. Am. J. Physiol. Endocrinol. Metab. 297, E1–E9 (2009).
Schmitz, G. & Grandl, M. The molecular mechanisms of HDL and associated vesicular trafficking mechanisms to mediate cellular lipid homeostasis. Arterioscler. Thromb. Vasc. Biol. 29, 1718–1722 (2009).
Luo, J., Jiang, L., Yang, H. & Song, B. L. Routes and mechanisms of post-endosomal cholesterol trafficking: a story that never ends. Traffic 18, 209–217 (2017).
Repa, J. J. & Mangelsdorf, D. J. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu. Rev. Cell Dev. Biol. 16, 459–481 (2000).
Brown, M. S. & Goldstein, J. L. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89, 331–340 (1997).
Horton, J. D., Goldstein, J. L. & Brown, M. S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 109, 1125–1131 (2002).
Vergnes, L. et al. SREBP-2-deficient and hypomorphic mice reveal roles for SREBP-2 in embryonic development and SREBP-1c expression. J. Lipid Res. 57, 410–421 (2016).
Gong, X. et al. Complex structure of the fission yeast SREBP–SCAP binding domains reveals an oligomeric organization. Cell Res. 26, 1197–1211 (2016).
Brown, M. S., Radhakrishnan, A. & Goldstein, J. L. Retrospective on cholesterol homeostasis: the central role of Scap. Annu. Rev. Biochem. 87, 783–807 (2018). This article provides a historical overview and the latest theory on SCAP and the SREBP pathway.
Radhakrishnan, A., Goldstein, J. L., McDonald, J. G. & Brown, M. S. Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: a delicate balance. Cell Metab. 8, 512–521 (2008). This work determines that in Chinese hamster ovary cells the ER cholesterol, exceeding 5% of total membrane lipids, will block SREBP activation.
Radhakrishnan, A., Ikeda, Y., Kwon, H. J., Brown, M. S. & Goldstein, J. L. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proc. Natl Acad. Sci. USA 104, 6511–6518 (2007).
Yabe, D., Xia, Z. P., Adams, C. M. & Rawson, R. B. Three mutations in sterol-sensing domain of SCAP block interaction with insig and render SREBP cleavage insensitive to sterols. Proc. Natl Acad. Sci. USA 99, 16672–16677 (2002).
Yang, T. et al. Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell 110, 489–500 (2002). This work shows that the ER protein INSIG1 binds to the SCAP–SREBP complex in the presence of sterols and mediates its negative feedback regulation of cholesterol synthesis.
Feramisco, J. D. et al. Intramembrane aspartic acid in SCAP protein governs cholesterol-induced conformational change. Proc. Natl Acad. Sci. USA 102, 3242–3247 (2005).
Gong, Y. et al. Sterol-regulated ubiquitination and degradation of Insig-1 creates a convergent mechanism for feedback control of cholesterol synthesis and uptake. Cell Metab. 3, 15–24 (2006).
Lee, J. N., Song, B., DeBose-Boyd, R. A. & Ye, J. Sterol-regulated degradation of Insig-1 mediated by the membrane-bound ubiquitin ligase gp78. J. Biol. Chem. 281, 39308–39315 (2006).
Horton, J. D. et al. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl Acad. Sci. USA 100, 12027–12032 (2003). This study identifies direct SREBP targets through systematically analysing genes differentially expressed in mice overexpressing Srebp1a or Srebp2, or lacking Scap.
Yabe, D., Brown, M. S. & Goldstein, J. L. Insig-2, a second endoplasmic reticulum protein that binds SCAP and blocks export of sterol regulatory element-binding proteins. Proc. Natl Acad. Sci. USA 99, 12753–12758 (2002).
Huber, M. D., Vesely, P. W., Datta, K. & Gerace, L. Erlins restrict SREBP activation in the ER and regulate cellular cholesterol homeostasis. J. Cell Biol. 203, 427–436 (2013).
Zhang, L. et al. Inhibition of cholesterol biosynthesis through RNF145-dependent ubiquitination of SCAP. eLife 6, e28766 (2017).
Irisawa, M., Inoue, J., Ozawa, N., Mori, K. & Sato, R. The sterol-sensing endoplasmic reticulum (ER) membrane protein TRC8 hampers ER to Golgi transport of sterol regulatory element-binding protein-2 (SREBP-2)/SREBP cleavage-activated protein and reduces SREBP-2 cleavage. J. Biol. Chem. 284, 28995–29004 (2009).
Du, X. M., Kristiana, I., Wong, J. & Brown, A. J. Involvement of Akt in ER-to-Golgi transport of SCAP/SREBP: a link between a key cell proliferative pathway and membrane synthesis. Mol. Biol. Cell 17, 2735–2745 (2006).
Xu, D. Q. et al. PAQR3 modulates cholesterol homeostasis by anchoring Scap/SREBP complex to the Golgi apparatus. Nat. Commun. 6, 8100 (2015).
Kuan, Y. C. et al. Heat shock protein 90 modulates lipid homeostasis by regulating the stability and function of sterol regulatory element-binding protein (SREBP) and SREBP cleavage-activating protein. J. Biol. Chem. 292, 3016–3028 (2017).
Peterson, T. R. et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 146, 408–420 (2011).
Eid, W. et al. mTORC1 activates SREBP-2 by suppressing cholesterol trafficking to lysosomes in mammalian cells. Proc. Natl Acad. Sci. USA 114, 7999–8004 (2017).
Zhang, D. Q. et al. Lipogenic transcription factor ChREBP mediates fructose-induced metabolic adaptations to prevent hepatotoxicity. J. Clin. Invest. 127, 2855–2867 (2017).
Sundqvist, A. et al. Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCFFbw7. Cell Metab. 1, 379–391 (2005).
Giandomenico, V., Simonsson, M., Gronroos, E. & Ericsson, J. Coactivator-dependent acetylation stabilizes members of the SREBP family of transcription factors. Mol. Cell. Biol. 23, 2587–2599 (2003).
Walker, A. K. et al. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 24, 1403–1417 (2010).
Rodgers, J. T. & Puigserver, P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc. Natl Acad. Sci. USA 104, 12861–12866 (2007).
Kotzka, J. et al. Insulin-activated Erk-mitogen-activated protein kinases phosphorylate sterol regulatory element-binding protein-2 at serine residues 432 and 455 in vivo. J. Biol. Chem. 279, 22404–22411 (2004).
Li, Y. et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 13, 376–388 (2011).
Hirano, Y., Murata, S., Tanaka, K., Shimizu, M. & Sato, R. Sterol regulatory element-binding proteins are negatively regulated through SUMO-1 modification independent of the ubiquitin/26S proteasome pathway. J. Biol. Chem. 278, 16809–16819 (2003).
Sato, R. et al. Sterol-dependent transcriptional regulation of sterol regulatory element-binding protein-2. J. Biol. Chem. 271, 26461–26464 (1996).
Tao, R. Y., Xiong, X. W., DePinho, R. A., Deng, C. X. & Dong, X. C. Hepatic SREBP-2 and cholesterol biosynthesis are regulated by FoxO3 and Sirt6. J. Lipid Res. 54, 2745–2753 (2013).
Tao, R. Y., Xiong, X. W., DePinho, R. A., Deng, C. X. & Dong, X. C. FoxO3 transcription factor and Sirt6 deacetylase regulate low density lipoprotein (LDL)-cholesterol homeostasis via control of the proprotein convertase subtilisin/kexin type 9 (Pcsk9) gene expression. J. Biol. Chem. 288, 29252–29259 (2013).
Liscum, L. et al. Domain structure of 3-hydroxy-3-methylglutaryl coenzyme A reductase, a glycoprotein of the endoplasmic reticulum. J. Biol. Chem. 260, 522–530 (1985).
Goldstein, J. L. & Brown, M. S. Regulation of the mevalonate pathway. Nature 343, 425–430 (1990).
Nakanishi, M., Goldstein, J. L. & Brown, M. S. Multivalent control of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Mevalonate-derived product inhibits translation of mRNA and accelerates degradation of enzyme. J. Biol. Chem. 263, 8929–8937 (1988).
Sever, N. et al. Insig-dependent ubiquitination and degradation of mammalian 3-hydroxy-3-methylglutaryl-CoA reductase stimulated by sterols and geranylgeraniol. J. Biol. Chem. 278, 52479–52490 (2003).
Song, B. L., Javitt, N. B. & DeBose-Boyd, R. A. Insig-mediated degradation of HMG CoA reductase stimulated by lanosterol, an intermediate in the synthesis of cholesterol. Cell Metab. 1, 179–189 (2005).
Chen, L. et al. Endogenous sterol intermediates of the mevalonate pathway regulate HMG-CoA reductase degradation and SREBP-2 processing. J. Lipid Res. 60, 1765–1775 (2019).
Song, B. L. & DeBose-Boyd, R. A. Insig-dependent ubiquitination and degradation of 3-hydroxy-3-methylglutaryl coenzyme A reductase stimulated by δ- and γ-tocotrienols. J. Biol. Chem. 281, 25054–25061 (2006).
Song, B. L., Sever, N. & DeBose-Boyd, R. A. gp78, a membrane-anchored ubiquitin ligase, associates with Insig-1 and couples sterol-regulated ubiquitination to degradation of HMG CoA reductase. Mol. Cell 19, 829–840 (2005).
Jo, Y., Lee, P. C. W., Sguigna, P. V. & DeBose-Boyd, R. A. Sterol-induced degradation of HMG CoA reductase depends on interplay of two Insigs and two ubiquitin ligases, gp78 and Trc8. Proc. Natl Acad. Sci. USA 108, 20503–20508 (2011).
Jiang, L. Y. et al. Ring finger protein 145 (RNF145) is a ubiquitin ligase for sterol-induced degradation of HMG-CoA reductase. J. Biol. Chem. 293, 4047–4055 (2018).
Sever, N., Yang, T., Brown, M. S., Goldstein, J. L. & DeBose-Boyd, R. A. Accelerated degradation of HMG CoA reductase mediated by binding of Insig-1 to its sterol-sensing domain. Mol. Cell 11, 25–33 (2003).
Ikeda, Y. et al. Regulated endoplasmic reticulum-associated degradation of a polytopic protein p97 recruits proteasomes to Insig-1 before extraction from membranes. J. Biol. Chem. 284, 34889–34900 (2009).
Morris, L. L., Hartman, I. Z., Jun, D. J., Seemann, J. & DeBose-Boyd, R. A. Sequential actions of the AAA-ATPase valosin-containing protein (VCP)/p97 and the proteasome 19S regulatory particle in sterol-accelerated, endoplasmic reticulum (ER)-associated degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J. Biol. Chem. 289, 19053–19066 (2014).
Cao, J. et al. Ufd1 is a cofactor of gp78 and plays a key role in cholesterol metabolism by regulating the stability of HMG-CoA reductase. Cell Metab. 6, 115–128 (2007).
Jiang, S. Y. et al. Schnyder corneal dystrophy-associated UBIAD1 mutations cause corneal cholesterol accumulation by stabilizing HMG-CoA reductase. PLOS Genet. 15, e1008289 (2019).
Schumacher, M. M., Elsabrouty, R., Seemann, J., Jo, Y. & DeBose-Boyd, R. A. The prenyltransferase UBIAD1 is the target of geranylgeraniol in degradation of HMG CoA reductase. eLife 4, e05560 (2015).
Schumacher, M. M., Jun, D. J., Jo, Y., Seemann, J. & DeBose-Boyd, R. A. Geranylgeranyl-regulated transport of the prenyltransferase UBIAD1 between membranes of the ER and Golgi. J. Lipid Res. 57, 1286–1299 (2016).
Jo, Y. et al. Schnyder corneal dystrophy-associated UBIAD1 inhibits ER-associated degradation of HMG CoA reductase in mice. eLife 8, e44396 (2019).
Lee, P. C. W., Sever, N. & DeBose-Boyd, R. A. Isolation of sterol-resistant Chinese hamster ovary cells with genetic deficiencies in both Insig-1 and Insig-2. J. Biol. Chem. 280, 25242–25249 (2005).
Hwang, S. et al. Hypoxia-inducible factor 1α activates insulin-induced gene 2 (Insig-2) transcription for degradation of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase in the liver. J. Biol. Chem. 292, 9382–9393 (2017).
Nguyen, A. D., McDonald, J. G., Bruick, R. K. & DeBose-Boyd, R. A. Hypoxia stimulates degradation of 3-hydroxy-3-methylglutaryl-coenzyme a reductase through accumulation of lanosterol and hypoxia-inducible factor-mediated induction of Insigs. J. Biol. Chem. 282, 27436–27446 (2007).
Yabe, D., Komuro, R., Liang, G., Goldstein, J. L. & Brown, M. S. Liver-specific mRNA for Insig-2 down-regulated by insulin: implications for fatty acid synthesis. Proc. Natl Acad. Sci. USA 100, 3155–3160 (2003).
Horton, J. D., Bashmakov, Y., Shimomura, I. & Shimano, H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc. Natl Acad. Sci. USA 95, 5987–5992 (1998). This work shows that expression of SREBP1 and SREBP2 is downregulated by fasting and upregulated by refeeding in the mouse liver.
Lee, J. P. et al. The TRC8 ubiquitin ligase is sterol regulated and interacts with lipid and protein biosynthetic pathways. Mol. Cancer Res. 8, 93–106 (2010).
Lee, J. N., Gong, Y., Zhang, X. Y. & Ye, J. Proteasomal degradation of ubiquitinated Insig proteins is determined by serine residues flanking ubiquitinated lysines. Proc. Natl Acad. Sci. USA 103, 4958–4963 (2006).
Liu, T. F. et al. Ablation of gp78 in liver improves hyperlipidemia and insulin resistance by inhibiting SREBP to decrease lipid biosynthesis. Cell Metab. 16, 213–225 (2012).
Schoebel, S. et al. Cryo-EM structure of the protein-conducting ERAD channel Hrd1 in complex with Hrd3. Nature 548, 352–355 (2017).
Menzies, S. A. et al. The sterol-responsive RNF145 E3 ubiquitin ligase mediates the degradation of HMG-CoA reductase together with gp78 and Hrd1. eLife 7, e40009 (2018).
Istvan, E. S., Palnitkar, M., Buchanan, S. K. & Deisenhofer, J. Crystal structure of the catalytic portion of human HMG-CoA reductase: insights into regulation of activity and catalysis. EMBO J. 19, 819–830 (2000).
Sato, R., Goldstein, J. L. & Brown, M. S. Replacement of serine-871 of hamster 3-hydroxy-3-methylglutaryl-CoA reductase prevents phosphorylation by AMP-activated kinase and blocks inhibition of sterol synthesis induced by ATP depletion. Proc. Natl Acad. Sci. USA 90, 9261–9265 (1993).
Clarke, P. R. & Hardie, D. G. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 9, 2439–2446 (1990).
Soto-Acosta, R., Bautista-Carbajal, P., Cervantes-Salazar, M., Angel-Ambrocio, A. H. & del Angel, R. M. DENV up-regulates the HMG-CoA reductase activity through the impairment of AMPK phosphorylation: a potential antiviral target. PLOS Pathog. 13, e1006257 (2017).
Zhang, X. J. et al. Thyroid-stimulating hormone decreases HMG-CoA reductase phosphorylation via AMP-activated protein kinase in the liver. J. Lipid Res. 56, 963–971 (2015).
Min, H. K. et al. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 15, 665–674 (2012).
Gill, S., Stevenson, J., Kristiana, I. & Brown, A. J. Cholesterol-dependent degradation of squalene monooxygenase, a control point in cholesterol synthesis beyond HMG-CoA reductase. Cell Metab. 13, 260–273 (2011). This work identifies that squalene monooxygenase is another rate-limiting enzyme besides HMGCR in cholesterol synthesis and is subjected to cholesterol-induced degradation.
Laden, B. P., Tang, Y. Z. & Porter, T. D. Cloning, heterologous expression, and enzymological characterization of human squalene monooxygenase. Arch. Biochem. Biophys. 374, 381–388 (2000).
Howe, V., Chua, N. K., Stevenson, J. & Brown, A. J. The regulatory domain of squalene monooxygenase contains a re-entrant loop and senses cholesterol via a conformational change. J. Biol. Chem. 290, 27533–27544 (2015).
Padyana, A. K. et al. Structure and inhibition mechanism of the catalytic domain of human squalene epoxidase. Nat. Commun. 10, 97 (2019).
Nagai, M., Sakakibara, J., Nakamura, Y., Gejyo, F. & Ono, T. SREBP-2 and NF-Y are involved in the transcriptional regulation of squalene epoxidase. Biochem. Biophys. Res. Commun. 295, 74–80 (2002).
Howe, V., Sharpe, L. J., Prabhu, A. V. & Brown, A. J. New insights into cellular cholesterol acquisition: promoter analysis of human HMGCR and SQLE, two key control enzymes in cholesterol synthesis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1862, 647–657 (2017).
Chua, N. K., Howe, V., Jatana, N., Thukral, L. & Brown, A. J. A conserved degron containing an amphipathic helix regulates the cholesterol-mediated turnover of human squalene monooxygenase, a rate-limiting enzyme in cholesterol synthesis. J. Biol. Chem. 292, 19959–19973 (2017).
Zelcer, N. et al. The E3 ubiquitin ligase MARCH6 degrades squalene monooxygenase and affects 3-hydroxy-3-methyl-glutaryl coenzyme a reductase and the cholesterol synthesis pathway. Mol. Cell. Biol. 34, 1262–1270 (2014).
Tan, J. M. E. et al. Differential use of E2 ubiquitin conjugating enzymes for regulated degradation of the rate-limiting enzymes HMGCR and SQLE in cholesterol biosynthesis. Atherosclerosis 281, 137–142 (2018).
Chua, N. K., Hart-Smith, G. & Brown, A. J. Non-canonical ubiquitination of the cholesterol-regulated degron of squalene monooxygenase. J. Biol. Chem. 294, 8134–8147 (2019).
Sharpe, L. J. et al. Cholesterol increases protein levels of the E3 ligase MARCH6 and thereby stimulates protein degradation. J. Biol. Chem. 294, 2436–2448 (2019).
Loregger, A. et al. A MARCH6 and IDOL E3 ubiquitin ligase circuit uncouples cholesterol synthesis from lipoprotein uptake in hepatocytes. Mol. Cell. Biol. 36, 285–294 (2016).
Davies, J. P., Levy, B. & Ioannou, Y. A. Evidence for a Niemann–Pick C (NPC) gene family: identification and characterization of NPC1L1. Genomics 65, 137–145 (2000).
Wang, J. et al. Membrane topology of human NPC1L1, a key protein in enterohepatic cholesterol absorption. J. Lipid Res. 50, 1653–1662 (2009).
Zhang, J. H. et al. The N-terminal domain of NPC1L1 protein binds cholesterol and plays essential roles in cholesterol uptake. J. Biol. Chem. 286, 25088–25097 (2011).
Kwon, H. J., Palnitkar, M. & Deisenhofer, J. The structure of the NPC1L1 N-terminal domain in a closed conformation. PLOS ONE 6, e18722 (2011).
Weinglass, A. B. et al. Extracellular loop C of NPC1L1 is important for binding to ezetimibe. Proc. Natl Acad. Sci. USA 105, 11140–11145 (2008).
Li, P. S. et al. The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1. Nat. Med. 20, 80–86 (2014). This study identifies an endocytic motif YVNxxF at the C-terminal tail of NPC1L1. Cholesterol binds to the N-terminal domain of NPC1L1 and induces the dissociation of YVNxxF from the plasma membrane, allowing it to be recognized and bound by NUMB. NUMB further recruits AP2–clathrin to initiate endocytosis.
Zhang, Y. Y. et al. A LIMA1 variant promotes low plasma LDL cholesterol and decreases intestinal cholesterol absorption. Science 360, 1087–1092 (2018). This work identifies that a LIMA1 variant is associated with low LDL-c in humans and that LIMA1 critically regulates intestinal cholesterol absorption by promoting NPC1L1 trafficking from endocytic recycling compartments to the plasma membrane.
Ge, L. et al. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 7, 508–519 (2008). This study shows that NPC1L1 mediates cholesterol uptake through clathrin-mediated vesicular endocytosis. Ezetimibe inhibits cholesterol uptake by blocking the endocytosis of NPC1L1.
Yu, L. et al. Cholesterol-regulated translocation of NPC1L1 to the cell surface facilitates free cholesterol uptake. J. Biol. Chem. 281, 6616–6624 (2006).
Ge, L. et al. Flotillins play an essential role in Niemann–Pick C1-like 1-mediated cholesterol uptake. Proc. Natl Acad. Sci. USA 108, 551–556 (2011).
Nihei, W. et al. NPC1L1-dependent intestinal cholesterol absorption requires ganglioside GM3 in membrane microdomains. J. Lipid Res. 59, 2181–2187 (2018).
Xie, C., Li, N., Chen, Z. J., Li, B. L. & Song, B. L. The small GTPase Cdc42 interacts with Niemann–Pick C1-like 1 (NPC1L1) and controls its movement from endocytic recycling compartment to plasma membrane in a cholesterol-dependent manner. J. Biol. Chem. 286, 35933–35942 (2011).
Chu, B. B. et al. Requirement of myosin Vb.Rab11a.Rab11–FIP2 complex in cholesterol-regulated translocation of NPC1L1 to the cell surface. J. Biol. Chem. 284, 22481–22490 (2009).
Xie, C. et al. Ezetimibe blocks the internalization of NPC1L1 and cholesterol in mouse small intestine. J. Lipid Res. 53, 2092–2101 (2012).
Xie, P. et al. Genetic demonstration of intestinal NPC1L1 as a major determinant of hepatic cholesterol and blood atherogenic lipoprotein levels. Atherosclerosis 237, 609–617 (2014).
Wei, J. et al. The clathrin adaptor proteins ARH, Dab2, and numb play distinct roles in Niemann–Pick C1-Like 1 versus low density lipoprotein receptor-mediated cholesterol uptake. J. Biol. Chem. 289, 33689–33700 (2014).
Myocardial Infarction Genetics Consortium Investigators. et al. Inactivating mutations in NPC1L1 and protection from coronary heart disease. N. Engl. J. Med. 371, 2072–2082 (2014).
Alrefai, W. A. et al. Modulation of human Niemann–Pick C1-like 1 gene expression by sterol: role of sterol regulatory element binding protein 2. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G369–G376 (2007).
Pramfalk, C. et al. HNF1α and SREBP2 are important regulators of NPC1L1 in human liver. J. Lipid Res. 51, 1354–1362 (2010).
Kim, Y. C. et al. Small heterodimer partner and fibroblast growth factor 19 inhibit expression of NPC1L1 in mouse intestine and cholesterol absorption. Gastroenterology 156, 1052–1065 (2019).
Davis, H. R. et al. Niemann–Pick C1 like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 279, 33586–33592 (2004).
Kawase, A., Araki, Y., Ueda, Y., Nakazaki, S. & Iwaki, M. Impact of a high-cholesterol diet on expression levels of Niemann–Pick C1-like 1 and intestinal transporters in rats and mice. Eur. J. Drug Metab. Pharmacokinet. 41, 457–463 (2016).
Iwayanagi, Y., Takada, T. & Suzuki, H. HNF4α is a crucial modulator of the cholesterol-dependent regulation of NPC1L1. Pharm. Res. 25, 1134–1141 (2008).
Iwayanagi, Y. et al. Human NPC1L1 expression is positively regulated by PPARα. Pharm. Res. 28, 405–412 (2011).
Kikuchi, T. et al. Intestinal CREBH overexpression prevents high-cholesterol diet-induced hypercholesterolemia by reducing Npc1l1 expression. Mol. Metab. 5, 1092–1102 (2016).
Malhotra, P. et al. d-Glucose modulates intestinal Niemann–Pick C1-like 1 (NPC1L1) gene expression via transcriptional regulation. Am. J. Physiol. Gastroinest. Liver Physiol. 304, G203–G210 (2013).
Duval, C. et al. Niemann–Pick C1 like 1 gene expression is down-regulated by LXR activators in the intestine. Biochem. Biophys. Res. Commun. 340, 1259–1263 (2006).
Hagita, S. et al. Transcriptional control of intestinal cholesterol absorption, adipose energy expenditure and lipid handling by Sortilin. Sci. Rep. 8, 9006 (2018).
Malhotra, P. et al. Mechanisms of Niemann–Pick type C1 Like 1 protein degradation in intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 316, C559–C566 (2019).
Jeon, H. & Blacklow, S. C. Structure and physiologic function of the low-density lipoprotein receptor. Annu. Rev. Biochem. 74, 535–562 (2005).
Lopez, D., Abisambra Socarras, J. F., Bedi, M. & Ness, G. C. Activation of the hepatic LDL receptor promoter by thyroid hormone. Biochim. Biophys. Acta 1771, 1216–1225 (2007).
Wijers, M., Kuivenhoven, J. A. & van de Sluis, B. The life cycle of the low-density lipoprotein receptor: insights from cellular and in-vivo studies. Curr. Opin. Lipidol. 26, 82–87 (2015).
Garcia, C. K. et al. Autosomal recessive hypercholesterolemia caused by mutations in a putative LDL receptor adaptor protein. Science 292, 1394–1398 (2001). This study identifies that mutations in the ARH gene cause a different form of hypercholesterolaemia from that caused by LDLR deficiency in humans.
Morris, S. M. & Cooper, J. A. Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic 2, 111–123 (2001).
Rudenko, G. et al. Structure of the LDL receptor extracellular domain at endosomal pH. Science 298, 2353–2358 (2002).
Bartuzi, P. et al. CCC- and WASH-mediated endosomal sorting of LDLR is required for normal clearance of circulating LDL. Nat. Commun. 7, 10961 (2016).
Fedoseienko, A. et al. The COMMD family regulates plasma LDL levels and attenuates atherosclerosis through stabilizing the CCC complex in endosomal LDLR trafficking. Circ. Res. 122, 1648–1660 (2018).
Li, J. & Pfeffer, S. R. Lysosomal membrane glycoproteins bind cholesterol and contribute to lysosomal cholesterol export. eLife 5, e21635 (2016).
Kwon, H. J. et al. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell 137, 1213–1224 (2009). This paper provides structural evidence showing that cholesterol binds directly to the N-terminal domain of NPC1. Based on the structures, the authors proposed that NPC1 receives cholesterol from NPC2 and inserts it into the lysosomal membrane.
Chu, B. B. et al. Cholesterol transport through lysosome–peroxisome membrane contacts. Cell 161, 291–306 (2015).
Xiao, J. et al. Cholesterol transport through the peroxisome–ER membrane contacts tethered by PI(4,5)P2 and extended synaptotagmins. Sci. China Life Sci. 62, 1117–1135 (2019).
Yang, H. Extended synaptotagmins, peroxisome–endoplasmic reticulum contact and cholesterol transport. Sci. China Life Sci. 62, 1266–1269 (2019).
Du, X. et al. A role for oxysterol-binding protein-related protein 5 in endosomal cholesterol trafficking. J. Cell Biol. 192, 121–135 (2011).
Zhao, K. & Ridgway, N. D. Oxysterol-binding protein-related protein 1L regulates cholesterol egress from the endo-lysosomal system. Cell Rep. 19, 1807–1818 (2017).
Wang, H. et al. ORP2 delivers cholesterol to the plasma membrane in exchange for phosphatidylinositol 4, 5-bisphosphate (PI(4,5)P2. Mol. Cell 73, 458–473 (2019).
Infante, R. E. & Radhakrishnan, A. Continuous transport of a small fraction of plasma membrane cholesterol to endoplasmic reticulum regulates total cellular cholesterol. eLife 6, e25466 (2017).
Adi, D. et al. IDOL G51S variant is associated with high blood cholesterol and increases low-density lipoprotein receptor degradation. Arterioscler. Thromb. Vasc. Biol. 39, 2468–2479 (2019).
Zelcer, N., Hong, C., Boyadjian, R. & Tontonoz, P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science 325, 100–104 (2009). This work identifies that IDOL is an LXR target gene and that the IDOL protein promotes ubiquitylation and degradation of LDLR in the extrahepatic tissues in mice.
Hong, C. et al. The E3 ubiquitin ligase IDOL induces the degradation of the low density lipoprotein receptor family members VLDLR and ApoER2. J. Biol. Chem. 285, 19720–19726 (2010).
Calkin, A. C. et al. FERM-dependent E3 ligase recognition is a conserved mechanism for targeted degradation of lipoprotein receptors. Proc. Natl Acad. Sci. USA 108, 20107–20112 (2011).
Sorrentino, V. et al. Distinct functional domains contribute to degradation of the low density lipoprotein receptor (LDLR) by the E3 ubiquitin ligase inducible degrader of the LDLR (IDOL). J. Biol. Chem. 286, 30190–30199 (2011).
Zhang, L. et al. The IDOL–UBE2D complex mediates sterol-dependent degradation of the LDL receptor. Genes Dev. 25, 1262–1274 (2011).
Scotti, E. et al. IDOL stimulates clathrin-independent endocytosis and multivesicular body-mediated lysosomal degradation of the low-density lipoprotein receptor. Mol. Cell. Biol. 33, 1503–1514 (2013).
Sorrentino, V. et al. The LXR–IDOL axis defines a clathrin-, caveolae-, and dynamin-independent endocytic route for LDLR internalization and lysosomal degradation. J. Lipid Res. 54, 2174–2184 (2013).
Hong, C. et al. The LXR–Idol axis differentially regulates plasma LDL levels in primates and mice. Cell Metab. 20, 910–918 (2014). This study shows that IDOL is differentially expressed in mice and non-human primates, and that activation of the LXR–IDOL pathway reduces hepatic LDLR protein and elevates plasma LDL levels in non-human primates.
Scotti, E. et al. Targeted disruption of the Idol gene alters cellular regulation of the low-density lipoprotein receptor by sterols and liver X receptor agonists. Mol. Cell. Biol. 31, 1885–1893 (2011).
Nelson, J. K. et al. Deubiquitylase inhibition reveals liver X receptor-independent transcriptional regulation of the E3 ubiquitin ligase IDOL and lipoprotein uptake. J. Biol. Chem. 291, 4813–4825 (2016).
Nelson, J. K. et al. The deubiquitylase USP2 regulates the LDLR pathway by counteracting the E3-ubiquitin ligase IDOL. Circ. Res. 118, 410–419 (2016).
Seidah, N. G. & Prat, A. The biology and therapeutic targeting of the proprotein convertases. Nat. Rev. Drug Discov. 11, 367–383 (2012).
Cunningham, D. et al. Structural and biophysical studies of PCSK9 and its mutants linked to familial hypercholesterolemia. Nat. Struct. Mol. Biol. 14, 413–419 (2007).
Horton, J. D., Cohen, J. C. & Hobbs, H. H. PCSK9: a convertase that coordinates LDL catabolism. J. Lipid Res. 50, S172–S177 (2009).
Lagace, T. A. PCSK9 and LDLR degradation: regulatory mechanisms in circulation and in cells. Curr. Opin. Lipidol. 25, 387–393 (2014).
Kwon, H. J., Lagace, T. A., McNutt, M. C., Horton, J. D. & Deisenhofer, J. Molecular basis for LDL receptor recognition by PCSK9. Proc. Natl Acad. Sci. USA 105, 1820–1825 (2008).
Zhang, D. W. et al. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J. Biol. Chem. 282, 18602–18612 (2007). Together with reference 160, this work provides structural and biochemical evidence showing that PCSK9 directly binds to the EGF-A domain of LDLR.
Gustafsen, C. et al. Heparan sulfate proteoglycans present PCSK9 to the LDL receptor. Nat. Commun. 8, 503 (2017).
Lagace, T. A. et al. Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice. J. Clin. Invest. 116, 2995–3005 (2006). This study uses parabiotic mice to show that PCSK9 secreted in plasma can degrade LDLR on the surface of hepatocytes.
Wang, Y., Huang, Y., Hobbs, H. H. & Cohen, J. C. Molecular characterization of proprotein convertase subtilisin/kexin type 9-mediated degradation of the LDLR. J. Lipid Res. 53, 1932–1943 (2012).
Tveten, K. et al. Interaction between the ligand-binding domain of the LDL receptor and the C-terminal domain of PCSK9 is required for PCSK9 to remain bound to the LDL receptor during endosomal acidification. Hum. Mol. Genet. 21, 1402–1409 (2012).
Zhang, D. W., Garuti, R., Tang, W. J., Cohen, J. C. & Hobbs, H. H. Structural requirements for PCSK9-mediated degradation of the low-density lipoprotein receptor. Proc. Natl Acad. Sci. USA 105, 13045–13050 (2008).
Poirier, S. et al. Dissection of the endogenous cellular pathways of PCSK9-induced low density lipoprotein receptor degradation: evidence for an intracellular route. J. Biol. Chem. 284, 28856–28864 (2009).
Li, H. et al. Hepatocyte nuclear factor 1α plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine. J. Biol. Chem. 284, 28885–28895 (2009).
Ai, D. et al. Regulation of hepatic LDL receptors by mTORC1 and PCSK9 in mice. J. Clin. Invest. 122, 1262–1270 (2012).
Seidah, N. G., Awan, Z., Chretien, M. & Mbikay, M. PCSK9: a key modulator of cardiovascular health. Circ. Res. 114, 1022–1036 (2014).
Seidah, N. G., Chretien, M. & Mbikay, M. The ever-expanding saga of the proprotein convertases and their roles in body homeostasis: emphasis on novel proprotein convertase subtilisin kexin number 9 functions and regulation. Curr. Opin. Lipidol. 29, 144–150 (2018).
Glerup, S., Schulz, R., Laufs, U. & Schluter, K. D. Physiological and therapeutic regulation of PCSK9 activity in cardiovascular disease. Basic Res. Cardiol. 112, 32 (2017).
Naeli, P., Azad, F. M., Malakootian, M., Seidah, N. G. & Mowla, S. J. Post-transcriptional regulation of PCSK9 by miR-191, miR-222, and miR-224. Front. Genet. 8, 189 (2017).
Fitzgerald, M. L., Mujawar, Z. & Tamehiro, N. ABC transporters, atherosclerosis and inflammation. Atherosclerosis 211, 361–370 (2010).
Attie, A. D. ABCA1: at the nexus of cholesterol, HDL and atherosclerosis. Trends Biochem. Sci. 32, 172–179 (2007).
Rosenson, R. S. et al. Cholesterol efflux and atheroprotection advancing the concept of reverse cholesterol transport. Circulation 125, 1905–1919 (2012).
Gelissen, I. C. et al. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler. Thromb. Vasc. Biol. 26, 534–540 (2006). This study shows that ABCA1-mediated cholesterol export generates nascent HDLs that serve as substrates for ABCG1-mediated cholesterol export.
Quazi, F. & Molday, R. S. Differential phospholipid substrates and directional transport by ATP-binding cassette proteins ABCA1, ABCA7, and ABCA4 and disease-causing mutants. J. Biol. Chem. 288, 34414–34426 (2013).
Gulshan, K. et al. PI(4,5)P2 is translocated by ABCA1 to the cell surface where it mediates apolipoprotein A1 binding and nascent HDL assembly. Circ. Res. 119, 827–838 (2016).
Qian, H. et al. Structure of the human lipid exporter ABCA1. Cell 169, 1228–1239 (2017).
Nagata, K. O., Nakada, C., Kasai, R. S., Kusumi, A. & Ueda, K. ABCA1 dimer–monomer interconversion during HDL generation revealed by single-molecule imaging. Proc. Natl Acad. Sci. USA 110, 5034–5039 (2013).
Ishigami, M. et al. Temporary sequestration of cholesterol and phosphatidylcholine within extracellular domains of ABCA1 during nascent HDL generation. Sci. Rep. 8, 6170 (2018).
Phillips, M. C. Is ABCA1 a lipid transfer protein? J. Lipid Res. 59, 749–763 (2018).
Wang, S. H., Gulshan, K., Brubaker, G., Hazen, S. L. & Smith, J. D. ABCA1 mediates unfolding of apolipoprotein AI N terminus on the cell surface before lipidation and release of nascent high-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 33, 1197–1205 (2013).
Boadu, E., Nelson, R. C. & Francis, G. A. ABCA1-dependent mobilization of lysosomal cholesterol requires functional Niemann–Pick C2 but not Niemann–Pick C1 protein. Biochim. Biophys. Acta 1821, 396–404 (2012).
Takahashi, Y. & Smith, J. D. Cholesterol efflux to apolipoprotein AI involves endocytosis and resecretion in a calcium-dependent pathway. Proc. Natl Acad. Sci. USA 96, 11358–11363 (1999).
Yokoyama, S. et al. Calpain-mediated ABCA1 degradation: post-translational regulation of ABCA1 for HDL biogenesis. Biochim. Biophys. Acta 1821, 547–551 (2012).
Costet, P., Luo, Y., Wang, N. & Tall, A. R. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J. Biol. Chem. 275, 28240–28245 (2000).
Kemmerer, M., Wittig, I., Richter, F., Brune, B. & Namgaladze, D. AMPK activates LXRα and ABCA1 expression in human macrophages. Int. J. Biochem. Cell Biol. 78, 1–9 (2016).
Li, C. H. et al. Puerarin promotes ABCA1-mediated cholesterol efflux and decreases cellular lipid accumulation in THP-1 macrophages. Eur. J. Pharmacol. 811, 74–86 (2017).
Sallam, T. et al. Transcriptional regulation of macrophage cholesterol efflux and atherogenesis by a long noncoding RNA. Nat. Med. 24, 304–312 (2018). This work identifies an LXR-responsive lncRNA, MeXis, and shows that it enhances ABCA1 expression and macrophage cholesterol efflux.
Moon, S. H. et al. p53 represses the mevalonate pathway to mediate tumor suppression. Cell 176, 564–580 (2019). This study shows that p53 can repress the mevalonate pathway through transactivating expression of ABCA1, which, in addition to mediating cholesterol efflux, promotes sterol transport from the plasma membrane to the ER and, thus, inhibits proteolytic activation of SREBP2.
Yamauchi, Y. et al. Deficiency in the lipid exporter ABCA1 impairs retrograde sterol movement and disrupts sterol sensing at the endoplasmic reticulum. J. Biol. Chem. 290, 23464–23477 (2015).
Horie, T. et al. microRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc. Natl Acad. Sci. USA 107, 17321–17326 (2010).
Rayner, K. J. et al. miR-33 contributes to the regulation of cholesterol homeostasis. Science 328, 1570–1573 (2010). This work identifies miR-33 as an intronic microRNA that is co-transcribed with Srebp2 and negatively regulates human ABCA1 expression and murine ABCA1 and ABCG1 expression, thereby inhibiting cholesterol efflux.
Marquart, T. J., Allen, R. M., Ory, D. S. & Baldan, A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc. Natl Acad. Sci. USA 107, 12228–12232 (2010).
Oram, J. F. & Heinecke, J. W. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol. Rev. 85, 1343–1372 (2005).
Cavelier, C., Lorenzi, I., Rohrer, L. & von Eckardstein, A. Lipid efflux by the ATP-binding cassette transporters ABCA1 and ABCG1. Biochim. Biophys. Acta 1761, 655–666 (2006).
Rotllan, N., Price, N., Pati, P., Goedeke, L. & Fernandez-Hernando, C. microRNAs in lipoprotein metabolism and cardiometabolic disorders. Atherosclerosis 246, 352–360 (2016).
Kennedy, M. A. et al. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 1, 121–131 (2005).
Out, R. et al. Coexistence of foam cells and hypocholesterolemia in mice lacking the ABC transporters A1 and G1. Circ. Res. 102, 113–120 (2008).
Westerterp, M. et al. Deficiency of ATP-binding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ. Res. 112, 1456–1465 (2013).
Wang, N., Lan, D. B., Chen, W. G., Matsuura, F. & Tall, A. R. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl Acad. Sci. USA 101, 9774–9779 (2004).
Kobayashi, A. et al. Efflux of sphingomyelin, cholesterol, and phosphatidylcholine by ABCG1. J. Lipid Res. 47, 1791–1802 (2006).
Terasaka, N., Wang, N., Yvan-Charvet, L. & Tall, A. R. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc. Natl Acad. Sci. USA 104, 15093–15098 (2007).
Tarling, E. J. & Edwards, P. A. ATP binding cassette transporter G1 (ABCG1) is an intracellular sterol transporter. Proc. Natl Acad. Sci. USA 108, 19719–19724 (2011).
Tarling, E. J. & Edwards, P. A. Intracellular localization of endogenous mouse ABCG1 is mimicked by both ABCG1-L550 and ABCG1-P550-brief report. Arterioscler. Thromb. Vasc. Biol. 36, 1323–1327 (2016).
Sano, O. et al. ABCA1, ABCG1, and ABCG4 are distributed to distinct membrane meso-domains and disturb detergent-resistant domains on the plasma membrane. PLOS ONE 9, e109886 (2014).
Wang, N., Ranalletta, M., Matsuura, F., Peng, F. & Tall, A. R. LXR-induced redistribution of ABCG1 to plasma membrane in macrophages enhances cholesterol mass efflux to HDL. Arterioscler. Thromb. Vasc. Biol. 26, 1310–1316 (2006).
Neufeld, E. B. et al. Cellular localization and trafficking of the human ABCG1 transporter. Biology 3, 781–800 (2014).
Pandzic, E. et al. The ATP binding cassette transporter, ABCG1, localizes to cortical actin filaments. Sci. Rep. 7, 42025 (2017).
Vaughan, A. M. & Oram, J. F. ABCG1 redistributes cell cholesterol to domains removable by high density lipoprotein but not by lipid-depleted apolipoproteins. J. Biol. Chem. 280, 30150–30157 (2005).
Sankaranarayanan, S. et al. Effects of acceptor composition and mechanism of ABCG1-mediated cellular free cholesterol efflux. J. Lipid Res. 50, 275–284 (2009).
Kennedy, M. A. et al. Characterization of the human ABCG1 gene—liver X receptor activates an internal promoter that produces a novel transcript encoding an alternative form of the protein. J. Biol. Chem. 276, 39438–39447 (2001).
Wang, D. L. et al. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ. Res. 111, 967–981 (2012).
Li, D. et al. Adenosine monophosphate-activated protein kinase induces cholesterol efflux from macrophage-derived foam cells and alleviates atherosclerosis in apolipoprotein E-deficient mice. J. Biol. Chem. 285, 33499–33509 (2010).
Goossens, P. et al. Membrane cholesterol efflux drives tumor-associated macrophage reprogramming and tumor progression. Cell Metab. 29, 1376–1389 (2019).
Graf, G. A. et al. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J. Biol. Chem. 278, 48275–48282 (2003).
Wang, J. et al. Relative roles of ABCG5/ABCG8 in liver and intestine. J. Lipid Res. 56, 319–330 (2015). This study shows that hepatic ABCG5 and ABCG8 mediates cholesterol excretion into bile and that intestinal ABCG5 and ABCG8 contributes to cholesterol efflux via the non-hepatobiliary route.
Wu, J. E. et al. Hepatic ABCG5 and ABCG8 overexpression increases hepatobiliary sterol transport but does not alter aortic atherosclerosis in transgenic mice. J. Biol. Chem. 279, 22913–22925 (2004).
Kosters, A. et al. Relation between hepatic expression of ATP-binding cassette transporters G5 and G8 and biliary cholesterol secretion in mice. J. Hepatol. 38, 710–716 (2003).
Jakulj, L. et al. Transintestinal cholesterol transport is active in mice and humans and controls ezetimibe-induced fecal neutral sterol excretion. Cell Metab. 24, 783–794 (2016). This work shows that trans-intestinal cholesterol excretion is active in humans and responsible for most ezetimibe-induced cholesterol efflux.
de Boer, J. F. et al. Intestinal farnesoid X receptor controls transintestinal cholesterol excretion in mice. Gastroenterology 152, 1126–1138 (2017).
Vrins, C. et al. The sterol transporting heterodimer ABCG5/ABCG8 requires bile salts to mediate cholesterol efflux. FEBS Lett. 581, 4616–4620 (2007).
Lee, J. Y. et al. Crystal structure of the human sterol transporter ABCG5/ABCG8. Nature 533, 561–564 (2016).
Small, D. M. Role of ABC transporters in secretion of cholesterol from liver into bile. Proc. Natl Acad. Sci. USA 100, 4–6 (2003).
Lu, K. et al. Two genes that map to the STSL locus cause sitosterolemia: genomic structure and spectrum of mutations involving sterolin-1 and sterolin-2 encoded by ABCG5 and ABCG8 respectively. Am. J. Hum. Genet. 69, 359–359 (2001).
Berge, K. E. et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 290, 1771–17751 (2000).
Remaley, A. T. et al. Comparative genome analysis of potential regulatory elements in the ABCG5–ABCG8 gene cluster. Biochem. Biophys. Res. Commun. 295, 276–282 (2002).
Freeman, L. A. et al. The orphan nuclear receptor LRH-1 activates the ABCG5/ABCG8 intergenic promoter. J. Lipid Res. 45, 1197–1206 (2004).
Sumi, K. et al. Cooperative interaction between hepatocyte nuclear factor 4α and GATA transcription factors regulates ATP-binding cassette sterol transporters ABCG5 and ABCG8. Mol. Cell. Biol. 27, 4248–4260 (2007).
Biddinger, S. B. et al. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat. Med. 14, 778–782 (2008).
Balasubramaniyan, N., Ananthanarayanan, M. & Suchy, F. J. Nuclear factor-κB regulates the expression of multiple genes encoding liver transport proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G618–G628 (2016).
Repa, J. J. et al. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors α and β. J. Biol. Chem. 277, 18793–18800 (2002).
Yu, L. Q. et al. Stimulation of cholesterol excretion by the liver X receptor agonist requires ATP-binding cassette transporters G5 and G8. J. Biol. Chem. 278, 15565–15570 (2003).
Back, S. S. et al. Cooperative transcriptional activation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 genes by nuclear receptors including Liver-X-Receptor. BMB Rep. 46, 322–327 (2013).
Li, T. et al. Overexpression of cholesterol 7α-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis. Hepatology 53, 996–1006 (2011).
Rogers, M. A. et al. Acyl-CoA:cholesterol acyltransferases (ACATs/SOATs): enzymes with multiple sterols as substrates and as activators. J. Steroid Biochem. Mol. Biol. 151, 102–107 (2015).
Joyce, C. W. et al. ACAT1 and ACAT2 membrane topology segregates a serine residue essential for activity to opposite sides of the endoplasmic reticulum membrane. Mol. Biol. Cell 11, 3675–3687 (2000).
Lin, S., Lu, X. H., Chang, C. C. Y. & Chang, T. Y. Human acyl-coenzyme A:cholesterol acyltransferase expressed in Chinese hamster ovary cells: membrane topology and active site location. Mol. Biol. Cell 14, 2447–2460 (2003).
Guo, Z. Y., Lin, S., Heinen, J. A., Chang, C. C. Y. & Chang, T. Y. The active site His-460 of human acyl-coenzyme A:cholesterol acyltransferase 1 resides in a hitherto undisclosed transmembrane domain. J. Biol. Chem. 280, 37814–37826 (2005).
Yu, C. J. et al. Human acyl-CoA:cholesterol acyltransferase-1 is a homotetrameric enzyme in intact cells and in vitro. J. Biol. Chem. 274, 36139–36145 (1999).
Yu, C. et al. Role of the N-terminal hydrophilic domain of acyl-coenzyme A:cholesterol acyltransferase 1 on the enzyme’s quaternary structure and catalytic efficiency. Biochemistry 41, 3762–3769 (2002).
Das, A., Davis, M. A. & Rudel, L. L. Identification of putative active site residues of ACAT enzymes. J. Lipid Res. 49, 1770–1781 (2008).
Sakashita, N. et al. Localization of human acyl-coenzyme A:cholesterol acyltransferase-1 (ACAT-1) in macrophages and in various tissues. Am. J. Pathol. 156, 227–236 (2000).
Chang, C. C. Y. et al. Immunological quantitation and localization of ACAT-1 and ACAT-2 in human liver and small intestine. J. Biol. Chem. 275, 28083–28092 (2000).
Miyazaki, A. et al. Expression of ACAT-1 protein in human atherosclerotic lesions and cultured human monocytes–macrophages. Arterioscler. Thromb. Vasc. Biol. 18, 1568–1574 (1998).
Fazio, S. et al. Increased atherosclerosis in LDL receptor-null mice lacking ACAT1 in macrophages. J. Clin. Invest. 107, 163–171 (2001).
Su, Y. R. et al. Reduced ABCA1-mediated cholesterol efflux and accelerated atherosclerosis in apolipoprotein E-deficient mice lacking macrophage-derived ACAT1. Circulation 111, 2373–2381 (2005).
Melton, E. M. et al. Myeloid Acat1/Soat1 KO attenuates pro-inflammatory responses in macrophages and protects against atherosclerosis in a model of advanced lesions. J. Biol. Chem. 294, 15836–15849 (2019).
Li, J. et al. Abrogating cholesterol esterification suppresses growth and metastasis of pancreatic cancer. Oncogene 35, 6378–6388 (2016).
Yue, S. H. et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 19, 393–406 (2014).
Yang, W. et al. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature 531, 651–655 (2016).
Chang, C. C. Y. et al. Recombinant acyl-CoA:cholesterol acyltransferase-1 (ACAT-1) purified to essential homogeneity utilizes cholesterol in mixed micelles or in vesicles in a highly cooperative manner. J. Biol. Chem. 273, 35132–35141 (1998).
Liu, J., Chang, C. C., Westover, E. J., Covey, D. F. & Chang, T. Y. Investigating the allosterism of acyl-CoA:cholesterol acyltransferase (ACAT) by using various sterols: in vitro and intact cell studies. Biochem. J. 391, 389–397 (2005).
Zhang, Y. et al. Cholesterol is superior to 7-ketocholesterol or 7α-hydroxycholesterol as an allosteric activator for acyl-coenzyme A:cholesterol acyltransferase 1. J. Biol. Chem. 278, 11642–11647 (2003).
Rogers, M. A. et al. Cellular pregnenolone esterification by acyl-CoA:cholesterol acyltransferase. J. Biol. Chem. 287, 17483–17492 (2012).
Li, B. L. et al. Human acyl-CoA:cholesterol acyltransferase-1 (ACAT-1) gene organization and evidence that the 4.3-kilobase ACAT-1 mRNA is produced from two different chromosomes. Biol. Chem. 274, 11060–11071 (1999).
Yang, J. B. et al. Synergistic transcriptional activation of human acyl-coenzyme A:cholesterol acyltransterase-1 gene by interferon-γ and all-trans-retinoic acid THP-1 cells. Biol. Chem. 276, 20989–20998 (2001).
Yang, L. et al. Enhancement of human ACAT1 gene expression to promote the macrophage-derived foam cell formation by dexamethasone. Cell Res. 14, 315–323 (2004).
Lei, L. et al. TNF-α stimulates the ACAT1 expression in differentiating monocytes to promote the CE-laden cell formation. J. Lipid Res. 50, 1057–1067 (2009).
Ge, J. et al. Insulin induces human acyl-coenzyme A:cholesterol acyltransferase1 gene expression via MAP kinases and CCAAT/enhancer-binding protein α. J. Cell. Biochem. 114, 2188–2198 (2013).
Parini, P. et al. ACAT2 is localized to hepatocytes and is the major cholesterol-esterifying enzyme in human liver. Circulation 110, 2017–2023 (2004).
Cases, S. et al. ACAT-2, a second mammalian acyl-CoA: cholesterol acyltransferase—its cloning, expression, and characterization. J. Biol. Chem. 273, 26755–26764 (1998).
Nguyen, T. M., Sawyer, J. K., Kelley, K. L., Davis, M. A. & Rudel, L. L. Cholesterol esterification by ACAT2 is essential for efficient intestinal cholesterol absorption: evidence from thoracic lymph duct cannulation. J. Lipid Res. 53, 95–104 (2012).
Buhman, K. K. et al. Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat. Med. 6, 1341–1347 (2000).
Repa, J. J., Buhman, K. K., Farese, R. V., Dietschy, J. M. & Turley, S. D. ACAT2 deficiency limits cholesterol absorption in the cholesterol-fed mouse: impact on hepatic cholesterol homeostasis. Hepatology 40, 1088–1097 (2004).
Willner, E. L. et al. Deficiency of acyl CoA:cholesterol acyltransferase 2 prevents atherosclerosis in apolipoprotein E-deficient mice. Proc. Natl Acad. Sci. USA 100, 1262–1267 (2003).
Lee, R. G. et al. Plasma cholesteryl esters provided by lecithin: cholesterol acyltransferase and acyl-coenzyme A:cholesterol acyltransferase 2 have opposite atherosclerotic potential. Circ. Res. 95, 998–1004 (2004).
Ohshiro, T. et al. Pyripyropene A, an acyl-coenzyme A:cholesterol acyltransferase 2-selective inhibitor, attenuates hypercholesterolemia and atherosclerosis in murine models of hyperlipidemia. Arterioscler. Thromb. Vasc. Biol. 31, 1108–1115 (2011).
Iqbal, J., Boutjdir, M., Rudel, L. L. & Hussain, M. M. Intestine-specific MTP and global ACAT2 deficiency lowers acute cholesterol absorption with chylomicrons and HDLs. J. Lipid Res. 55, 2261–2275 (2014).
Temel, R. E. et al. Intestinal cholesterol absorption is substantially reduced in mice deficient in both ABCA1 and ACAT2. J. Lipid Res. 46, 2423–2431 (2005).
Turley, S. D., Valasek, M. A., Repa, J. J. & Dietschy, J. M. Multiple mechanisms limit the accumulation of unesterified cholesterol in the small intestine of mice deficient in both ACAT2 and ABCA1. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G1012–G1022 (2010).
Cho, K. H. et al. Mass-production of human ACAT-1 and ACAT-2 to screen isoform-specific inhibitor: a different substrate specificity and inhibitory regulation. Biochem. Biophys. Res. Commun. 309, 864–872 (2003).
Temel, R. E., Gebre, A. K., Parks, J. S. & Rudel, L. L. Compared with acyl-CoA:cholesterol O-acyltransferase (ACAT) 1 and lecithin:cholesterol acyltransferase, ACAT2 displays the greatest capacity to differentiate cholesterol from sitosterol. J. Biol. Chem. 278, 47594–47601 (2003).
Nguyen, T. M. et al. ACAT2 and ABCG5/G8 are both required for efficient cholesterol absorption in mice: evidence from thoracic lymph duct cannulation. J. Lipid Res. 53, 1598–1609 (2012).
Pramfalk, C., Davis, M. A., Eriksson, M., Rudel, L. L. & Parini, P. Control of ACAT2 liver expression by HNF1. J. Lipid Res. 46, 1868–1876 (2005).
Pramfalk, C. et al. Control of ACAT2 liver expression by HNF4α lesson from MODY1 patients. Arterioscler. Thromb. Vasc. Biol. 29, 1235–1241 (2009).
Song, B. L. et al. Human acyl-CoA:cholesterol acyltransferase 2 gene expression in intestinal Caco-2 cells and in hepatocellular carcinoma. Biochem. J. 394, 617–626 (2006).
Wang, Y. J. et al. Cholesterol and fatty acids regulate cysteine ubiquitylation of ACAT2 through competitive oxidation. Nat. Cell Biol. 19, 808–819 (2017). This work shows that lipid overloading increases ROS that oxidizes ACAT2 on Cys277, thereby decreasing ubiquitylation of the protein. The increased ACAT2 converts toxic cholesterol to cholesteryl ester. This study suggests that competitive oxidation and ubiquitylation of Cys is a new mechanism of sensing ROS.
Widenmaier, S. B. et al. NRF1 is an ER membrane sensor that is central to cholesterol homeostasis. Cell 171, 1094–1109 (2017). This study shows that the ER-bound transcription factor NRF1 can sense and respond to high cholesterol levels by promoting cholesterol efflux and suppressing inflammation.
Zambrano, F., Fleischer, S. & Fleischer, B. Lipid composition of the Golgi apparatus of rat kidney and liver in comparison with other subcellular organelles. Biochim. Biophys. Acta 380, 357–369 (1975).
Najafi-Shoushtari, S. H. et al. microRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 328, 1566–1569 (2010).
Yang, C. D. et al. Sterol intermediates from cholesterol biosynthetic pathway as liver X receptor ligands. J. Biol. Chem. 281, 27816–27826 (2006).
Sallam, T. et al. Feedback modulation of cholesterol metabolism by the lipid-responsive non-coding RNA LeXis. Nature 534, 124–128 (2016). This work identifies another LXR-responsive lncRNA, LeXis, and shows that it represses SREBP2 expression and decreases cholesterol biosynthesis.
Jiang, Y. et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature 567, 257–261 (2019).
Ren, R. B. et al. Crystal structure of a mycobacterial Insig homolog provides insight into how these sensors monitor sterol levels. Science 349, 187–191 (2015).
Gong, X. et al. Structural insights into the Niemann–Pick C1 (NPC1)-mediated cholesterol transfer and Ebola infection. Cell 165, 1467–1478 (2016).
Gao, Y., Zhou, Y., Goldstein, J. L., Brown, M. S. & Radhakrishnan, A. Cholesterol-induced conformational changes in the sterol-sensing domain of the Scap protein suggest feedback mechanism to control cholesterol synthesis. J. Biol. Chem. 292, 8729–8737 (2017).
Schumacher, M. M., Jun, D. J., Johnson, B. M. & DeBose-Boyd, R. A. UbiA prenyltransferase domain-containing protein-1 modulates HMG-CoA reductase degradation to coordinate synthesis of sterol and nonsterol isoprenoids. J. Biol. Chem. 293, 312–323 (2018).
Cannon, C. P. et al. Ezetimibe added to statin therapy after acute coronary syndromes. N. Engl. J. Med. 372, 2387–2397 (2015).
Blom, D. J. et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N. Engl. J. Med. 370, 1809–1819 (2014).
Jiang, S. Y. et al. Discovery of a potent HMG-CoA reductase degrader that eliminates statin-induced reductase accumulation and lowers cholesterol. Nat. Commun. 9, 5138 (2018).
Vite, C. H. et al. Intracisternal cyclodextrin prevents cerebellar dysfunction and Purkinje cell death in feline Niemann–Pick type C1 disease. Sci. Transl. Med. 7, 276ra26 (2015).
Vance, J. E. & Karten, B. Niemann–Pick C disease and mobilization of lysosomal cholesterol by cyclodextrin. J. Lipid Res. 55, 1609–1621 (2014).
Maarup, T. J. et al. Intrathecal 2-hydroxypropyl-β-cyclodextrin in a single patient with Niemann–Pick C1. Mol. Genet. Metab. 116, 75–79 (2015).
Patterson, M. C. et al. in The Metabolic and Molecular Bases of Inherited Disease 8th edn (eds Scriver, C. R., Beaudet, A. L., Sly, W. S., & Valle, D.) 3611–3633 (McGraw-Hill, 2001).
Weiss, J. S. More on Schnyder corneal dystrophy. Ophthalmology 116, 2260 (2009).
Nowaczyk, M. J. M. & Irons, M. B. Smith–Lemli–Opitz syndrome: phenotype, natural history, and epidemiology. Am. J. Med. Genet. C 160c, 250–262 (2012).
Goldstein, J. L. & Brown, M. S. in The Metabolic and Molecular Bases of Inherited Disease 8th edn (eds Scriver, C. R., Beaudet, A. L., Sly, W. S., & Valle, D.) 2863–2901 (McGraw-Hill, 2001).
Henderson, R., O’Kane, M., McGilligan, V. & Watterson, S. The genetics and screening of familial hypercholesterolaemia. J. Biomed. Sci. 23, 39 (2016).
Kolovou, G. D., Mikhailidis, D. P., Anagnostopoulou, K. K., Daskalopoulou, S. S. & Cokkinos, D. V. Tangier disease four decades of research: a reflection of the importance of HDL. Curr. Med. Chem. 13, 771–782 (2006).
Escola-Gil, J. C. et al. Sitosterolemia: diagnosis, investigation, and management. Curr. Atheroscler. Rep. 16, 424 (2014).
Acknowledgements
The authors thank Lu-Yi Jiang and Yun-Feng Li for drafting the original figures. Work from the B.-L.S. laboratory is supported by grants from the National Natural Science Foundation of China (91753204, 31600651, 31690102, 91857000 and 31771568) and the Ministry of Science and Technology of China (2016YFA0500100 and 2018YFA0800700).
Author information
Authors and Affiliations
Contributions
J.L. and B.-L.S. wrote the Review and H.Y. revised it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Molecular Cell Biology thanks N. Ridgway and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Sterols
-
A subgroup of steroids with a hydroxyl group at the C-3 position of the A-ring. A steroid is a biologically active organic compound with four rings (A–D) arranged in a specific molecular configuration.
- Sphingolipids
-
A class of lipids with a polar head group and two non-polar tails. The core of a sphingolipid is an amino alcohol called sphingosine.
- Glycosylphosphatidylinositol-anchored proteins
-
Proteins with glycosylphosphatidylinositol (GPI) attached at the C-termini. The GPI anchor is a unique mode of protein binding to the plasma membrane.
- Oxysterols
-
The oxidized derivatives of cholesterol.
- Bile acids
-
The hydroxylated steroids which are amphipathic and synthesized from cholesterol in the liver. Bile acids are secreted into the intestine where they play an important role in emulsifying dietary lipids to facilitate their absorption.
- Niemann–Pick type C1
-
(NPC1). A large (1278 amino acids in humans), 13-pass transmembrane protein that binds cholesterol with the 3β-hydroxyl group and the tetracyclic ring of cholesterol buried and the iso-octyl side chain exposed via the N-terminal domain. NPC1 is ubiquitously expressed and localizes on lysosomal membrane. Mutations in NPC1 cause 95% of NPC cases.
- Chylomicrons
-
The triglyceride-rich lipid particles in the blood and lymph that are solely produced by the intestine. Chylomicrons deliver lipids to the liver and extrahepatic tissues. After depletion of their triglycerides by the extrahepatic tissues, chylomicrons become chylomicron remnants that are cleared by the liver.
- Very-low-density lipoproteins
-
(VLDLs). The triglyceride-rich lipid particles in the blood that are produced by the liver. VLDLs enable fats and cholesterol to move within the water-based bloodstream. VLDLs are converted to intermediate-density lipoproteins and low-density lipoproteins in the bloodstream.
- Low-density lipoproteins
-
(LDLs). The lipid particles enriched in cholesteryl esters. Each LDL particle contains a single apolipoprotein B-100 molecule and delivers lipids, mainly cholesterol, and vitamins to extrahepatic tissues, where it is taken up by an LDL receptor.
- Apolipoprotein
-
(apo). A protein that binds lipids to form lipoproteins, which then transport lipids and fat-soluble vitamins in circulation.
- COPII-coated vesicles
-
The membrane vesicles coated by coatomer II (COP II), which is a type of vesicle coat protein that facilitates the formation of transport vesicles from the endoplasmic reticulum (ER). COPII-coated vesicles exit from specialized regions of the ER membrane devoid of bound ribosomes, known as ‘ER exit sites’, and deliver their content to the Golgi.
- Insulin-induced gene (INSIG) proteins
-
INSIG proteins, including INSIG1 and INSIG2, are integral membrane proteins of the endoplasmic reticulum that mediate sterol regulation of sterol regulatory element-binding protein cleavage-activating protein (SCAP) and 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA reductase).
- RING-finger ubiquitin ligases
-
The largest type of E3 ubiquitin ligases with the RING (really interesting new gene) finger domains that bind two zinc ions in a unique ‘cross-brace’ arrangement through a defined motif of cysteine and histidine residues.
- SCF ubiquitin ligase complex
-
The complex that catalyses the ubiquitylation of proteins for degradation. The core components of the Skp, Cullin, F-box (SCF) complex include the scaffold protein Cul1, the RING-finger protein RBX1/ROC1 and the adaptor protein Skp1. The F-box protein (FBP) is the variable component determining substrate specificity. In most cases, FBPs recognize phosphorylated proteins.
- Sirtuin 1
-
(SIRT1). Member of a family of proteins that act predominantly as NAD-dependent deacetylases. There are seven sirtuins in mammals, SIRT1–SIRT7. Some sirtuins can remove various acyl lysine modifications from proteins.
- ERKs
-
Widely expressed protein-serine/threonine kinases that are activated via the phosphorylation of tyrosine. Activation of ERK can affect cell proliferation, survival, apoptosis, motility, metabolism and differentiation.
- AMPK
-
(AMP-activated protein kinase). A central regulator of energy homeostasis that is activated when the cellular ATP level is low. AMPK activation inhibits cholesterol and fatty acid synthesis.
- Mevalonate
-
A product generated from 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) by the action of HMG-CoA reductase. The mevalonate pathway in mammals leads to the synthesis of sterols, isoprenoids, dolichol, haeme, ubiquinione and so forth.
- Isoprenoids
-
A class of naturally occurring organic compounds that are composed of two or more units of isoprene. They are synthesized in the mevalonate pathway in mammals.
- Lanosterol
-
The first sterol intermediate in the mevalonate pathway consisting of 30 carbons. Lanosterol is synthesized by cyclization of squalene and can potently stimulate degradation of hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) without inhibiting the processing of sterol regulatory element-binding protein (SREBP).
- ER-associated degradation
-
(ERAD). A surveillance system that clears misfolded proteins in the endoplasmic reticulum (ER) via ubiquitylation and proteasomal degradation.
- Prenyltransferase
-
A class of enzymes that transfer prenyl moieties to acceptor molecules. They are responsible for menaquinone and ubiquinone biosynthesis, or protein modification called prenylation that is the covalent linkage of a lipid consisting of three or four isoprene units to a thiol of a cysteine side chain.
- Geranylgeraniol
-
A diterpene alcohol containing 20 carbons that is synthesized in the mevalonate pathway. Geranylgeraniol can be used to synthesize vitamins E and K, and to modify proteins in a process known as geranylgeranylation.
- E2 enzyme
-
Also known as ubiquitin-conjugating enzyme. E2 enzymes perform the second step in the ubiquitylation reaction. Through the series of reactions of E1, E2 and E3, cellular proteins are linked to ubiquitin.
- Bile canaliculi
-
Thin tubes formed by intercellular space between hepatocytes. They carry biles towards the interlobar bile ducts.
- Endocytic recycling compartment
-
(ERC). An intracellular cholesterol-rich site composed of a mixture of individual and interconnected vesicles and tubules near the microtubule-organizing centre. The ERC is RAB11a positive and regulates vesicular recycling to the plasma membrane.
- Flotillins
-
A family of two ubiquitously expressed, membrane-associated proteins, namely, flotillin 1 and flotillin 2. They play a role in forming cholesterol-rich membrane microdomains, endocytosis and signal transduction.
- Gangliosides
-
A species of plasma membrane-concentrated lipids. Each ganglioside molecule is composed of a glycosphingolipid linked to one or more sialic acid.
- Brush border
-
The apical plasma membrane consisting of an array of densely packed microvilli, which are tiny projections intended to increase the surface area for absorption.
- Liver X receptors
-
(LXRs). The sterol-sensitive transcription factors that belong to the nuclear receptor family and are activated by oxysterols and desmosterol. LXRs promote cholesterol efflux mainly by upregulating ATP-binding cassette (ABC) subfamily A member 1 (ABCA1) and ABC subfamily G member 1 (ABCG1), ABCG5 and ABCG8. They also increase fatty acid synthesis by elevating sterol regulatory element-binding protein 1c (SREBP1c) expression.
- Thyroid hormones
-
Two tyrosine-based, iodine-containing hormones produced by the thyroid gland. They participate in the regulation of metabolism and growth.
- ARH
-
(Autosomal recessive hypercholesterolaemia). An adaptor protein that binds low-density lipoprotein receptor and mediates its endocytosis in hepatocytes and lymphocytes. Mutations in ARH cause an autosomal recessive form of hypercholesterolaemia.
- NPC2
-
(Niemann–Pick type C2). A small (132 amino acids in humans), luminal protein that resides in late endosomes and lysosomes, and binds cholesterol with the iso-octyl side chain of cholesterol buried and the 3β-hydroxyl group exposed. Mutations in NPC2 cause 5% of NPC cases.
- ESCRT complexes
-
(Endosomal sorting complexes required for transport). These protein complexes comprise multiple cytosolic subunits. They transport ubiquitylated cargo to cellular vesicles by promoting membrane budding into the endosomes to form multivesicular bodies, which eventually fuse with lysosome and cause degradation of the cargo.
- Foam cells
-
Cells derived from macrophages that take up too much cholesterol from oxidized low-density lipoproteins and become laden with lipid droplets, giving them a foamy appearance. Foam cells promote the atherosclerotic plaque build-up and inflammation during atherosclerosis.
- Lecithin:cholesterol acyltransferase
-
(LCAT). A lipoprotein-associated enzyme that transfers the fatty acid from the sn-2 position of phosphatidylcholine (lecithin) to cholesterol to form a cholesteryl ester.
- Transintestinal cholesterol excretion pathway
-
A process of faecal excretion of plasma-derived cholesterol from the inside of enterocytes to the intestinal lumen.
- Micelles
-
The spherical assembly of amphiphilic molecules dispersed in water solvent.
- Immunological synapse
-
The interface formed between an antigen-presenting cell or target cell and a lymphocyte such as a T cell, B cell or natural killer cell.
- Sitosterol
-
A plant sterol with a chemical structure very similar to that of cholesterol. Sitosterol is poorly absorbed by healthy individuals and may help to lower cholesterol in humans.
- Retinoic acid
-
A metabolite of vitamin A1 (all-trans-retinol). Retinoic acid is a ligand of nuclear receptors RAR and RXR and regulates cell growth and differentiation.
Rights and permissions
About this article
Cite this article
Luo, J., Yang, H. & Song, BL. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol 21, 225–245 (2020). https://doi.org/10.1038/s41580-019-0190-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-019-0190-7
This article is cited by
-
Unlocking the mysteries of VLDL: exploring its production, intracellular trafficking, and metabolism as therapeutic targets
Lipids in Health and Disease (2024)
-
Geniposide alleviates cholesterol-induced endoplasmic reticulum stress and apoptosis in osteoblasts by mediating the GLP-1R/ABCA1 pathway
Journal of Orthopaedic Surgery and Research (2024)
-
Reprogramming of lipid metabolism in the tumor microenvironment: a strategy for tumor immunotherapy
Lipids in Health and Disease (2024)
-
The novel molecular mechanism of pulmonary fibrosis: insight into lipid metabolism from reanalysis of single-cell RNA-seq databases
Lipids in Health and Disease (2024)
-
Serum metabolism characteristics of patients with myocardial injury after noncardiac surgery explored by the untargeted metabolomics approach
BMC Cardiovascular Disorders (2024)