Abstract
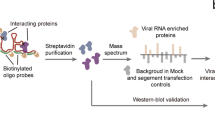
Flaviviruses, including dengue virus (DENV) and Zika virus (ZIKV), cause severe human disease. Co-opting cellular factors for viral translation and viral genome replication at the endoplasmic reticulum is a shared replication strategy, despite different clinical outcomes. Although the protein products of these viruses have been studied in depth, how the RNA genomes operate inside human cells is poorly understood. Using comprehensive identification of RNA-binding proteins by mass spectrometry (ChIRP-MS), we took an RNA-centric viewpoint of flaviviral infection and identified several hundred proteins associated with both DENV and ZIKV genomic RNA in human cells. Genome-scale knockout screens assigned putative functional relevance to the RNA–protein interactions observed by ChIRP-MS. The endoplasmic-reticulum-localized RNA-binding proteins vigilin and ribosome-binding protein 1 directly bound viral RNA and each acted at distinct stages in the life cycle of flaviviruses. Thus, this versatile strategy can elucidate features of human biology that control the pathogenesis of clinically relevant viruses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The raw and processed sequencing data can be found at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE109194.
References
Bhatt, S. et al. The global distribution and burden of dengue. Nature 496, 504–507 (2013).
Shepard, D. S., Undurraga, E. A., Halasa, Y. A. & Stanaway, J. D. The global economic burden of dengue: a systematic analysis. Lancet Infect. Dis. 16, 935–941 (2016).
Kaufmann, S. H. E., Dorhoi, A., Hotchkiss, R. S. & Bartenschlager, R. Host-directed therapies for bacterial and viral infections. Nat. Rev. Drug Discov. 17, 35–56 (2018).
Puschnik, A. S. et al. A small-molecule oligosaccharyltransferase inhibitor with pan-flaviviral activity. Cell Rep. 21, 3032–3039 (2017).
Apte-Sengupta, S., Sirohi, D. & Kuhn, R. J. Coupling of replication and assembly in flaviviruses. Curr. Opin. Virol. 9, 134–142 (2014).
Fernandez-Garcia, M. D., Mazzon, M., Jacobs, M. & Amara, A. Pathogenesis of flavivirus infections: using and abusing the host cell. Cell Host Microbe 5, 318–328 (2009).
Walsh, D. & Mohr, I. Viral subversion of the host protein synthesis machinery. Nat. Rev. Microbiol. 9, 860–875 (2011).
Garcia-Blanco, M. A., Vasudevan, S. G., Bradrick, S. S. & Nicchitta, C. Flavivirus RNA transactions from viral entry to genome replication. Antivir. Res. 134, 244–249 (2016).
Chu, C. et al. Systematic discovery of Xist RNA binding proteins. Cell 161, 404–416 (2015).
Chen, C. K. et al. Xist recruits the X chromosome to the nuclear lamina to enable chromosome-wide silencing. Science 354, 468–472 (2016).
Balinsky, C. A. et al. IRAV (FLJ11286), an interferon-stimulated gene with antiviral activity against dengue virus, interacts with MOV10. J. Virol. 91, e01606-16 (2017).
Paranjape, S. M. & Harris, E. Y box-binding protein-1 binds to the dengue virus 3′-untranslated region and mediates antiviral effects. J. Biol. Chem. 282, 30497–30508 (2007).
Umareddy, I. et al. Dengue virus regulates type I interferon signalling in a strain-dependent manner in human cell lines. J. Gen. Virol. 89, 3052–3062 (2008).
Lei, Y. et al. Functional interaction between cellular p100 and the dengue virus 3′ UTR. J. Gen. Virol. 92, 796–806 (2011).
Hentze, M. W., Castello, A., Schwarzl, T. & Preiss, T. A brave new world of RNA-binding proteins. Nat. Rev. Mol. Cell Biol. 19, 327–341 (2018).
Marceau, C. D. et al. Genetic dissection of Flaviviridae host factors through genome-scale CRISPR screens. Nature 535, 159–163 (2016).
Savidis, G. et al. Identification of Zika virus and dengue virus dependency factors using functional genomics. Cell Rep. 16, 232–246 (2016).
Zhang, R. et al. A CRISPR screen defines a signal peptide processing pathway required by flaviviruses. Nature 535, 164–168 (2016).
Lin, D. L. et al. Dengue virus hijacks a noncanonical oxidoreductase function of a cellular oligosaccharyltransferase complex. mBio 8, e00939-17 (2017).
Li, J.et al. A short hairpin RNA screen of interferon-stimulated genes identifies a novel negative regulator of the cellular antiviral response. mBio 4, e00385-13 (2013).
Cui, X. A., Zhang, H. & Palazzo, A. F. p180 promotes the ribosome-independent localization of a subset of mRNA to the endoplasmic reticulum. PLoS Biol. 10, e1001336 (2012).
Mobin, M. B. et al. The RNA-binding protein vigilin regulates VLDL secretion through modulation of Apob mRNA translation. Nat. Commun. 7, 12848 (2016).
Losfeld, M. E., Soncin, F., Ng, B. G., Singec, I. & Freeze, H. H. A sensitive green fluorescent protein biomarker of N-glycosylation site occupancy. FASEB J. 26, 4210–4217 (2012).
Reid, D. W. et al. Dengue virus selectively annexes endoplasmic reticulum-associated translation machinery as a strategy for co-opting host cell protein synthesis. J. Virol. 92, e01766-17 (2018).
Zarnegar, B. J. et al. irCLIP platform for efficient characterization of protein–RNA interactions. Nat. Methods 13, 489–492 (2016).
Savitz, A. J. & Meyer, D. I. Identification of a ribosome receptor in the rough endoplasmic reticulum. Nature 346, 540–544 (1990).
Eliseev, B. et al. Structure of a human cap-dependent 48S translation pre-initiation complex. Nucleic Acids Res. 46, 2678–2689 (2018).
Belanger, L., Roy, S. & Allard, D. New albumin gene 3′ adjacent to the α1-fetoprotein locus. J. Biol. Chem. 269, 5481–5484 (1994).
Shelness, G. S., Ingram, M. F., Huang, X. F. & DeLozier, J. A. Apolipoprotein B in the rough endoplasmic reticulum: translation, translocation and the initiation of lipoprotein assembly. J. Nutr. 129, 456S–462S (1999).
Darnell, J. C. et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146, 247–261 (2011).
Chen, Y. L., Yokokawa, F. & Shi, P. Y. The search for nucleoside/nucleotide analog inhibitors of dengue virus. Antivir. Res. 122, 12–19 (2015).
Cheng, M. H. & Jansen, R. P. A jack of all trades: the RNA-binding protein vigilin. WIRES RNA 8, e1448 (2017).
Cui, X. A. & Palazzo, A. F. Localization of mRNAs to the endoplasmic reticulum. WIRES RNA 5, 481–492 (2014).
Cunningham, K. S., Dodson, R. E., Nagel, M. A., Shapiro, D. J. & Schoenberg, D. R. Vigilin binding selectively inhibits cleavage of the vitellogenin mRNA 3′-untranslated region by the mRNA endonuclease polysomal ribonuclease 1. Proc. Natl Acad. Sci. USA 97, 12498–12502 (2000).
Hyde, M., Block-Alper, L., Felix, J., Webster, P. & Meyer, D. I. Induction of secretory pathway components in yeast is associated with increased stability of their mRNA. J. Cell Biol. 156, 993–1001 (2002).
Reid, D. W. & Nicchitta, C. V. Diversity and selectivity in mRNA translation on the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 16, 221–231 (2015).
Ueno, T., Kaneko, K., Sata, T., Hattori, S. & Ogawa-Goto, K. Regulation of polysome assembly on the endoplasmic reticulum by a coiled-coil protein, p180. Nucleic Acids Res. 40, 3006–3017 (2012).
Kruse, C. et al. The multi-KH protein vigilin associates with free and membrane-bound ribosomes. Cell. Mol. Life Sci. 60, 2219–2227 (2003).
Batlle, M., Marsellach, F. X., Huertas, D. & Azorin, F. Drosophila vigilin, DDP1, localises to the cytoplasm and associates to the rough endoplasmic reticulum. Biochim. Biophys. Acta 1809, 46–55 (2011).
Frey, S., Pool, M. & Seedorf, M. Scp160p, an RNA-binding, polysome-associated protein, localizes to the endoplasmic reticulum of Saccharomyces cerevisiae in a microtubule-dependent manner. J. Biol. Chem. 276, 15905–15912 (2001).
Hirschmann, W. D. et al. Scp160p is required for translational efficiency of codon-optimized mRNAs in yeast. Nucleic Acids Res. 42, 4043–4055 (2014).
Phillips, S. L., Soderblom, E. J., Bradrick, S. S. & Garcia-Blanco, M. A. Identification of proteins bound to dengue viral RNA in vivo reveals new host proteins important for virus replication. mBio 7, e01865-15 (2016).
Viktorovskaya, O. V., Greco, T. M., Cristea, I. M. & Thompson, S. R. Identification of RNA binding proteins associated with dengue virus RNA in infected cells reveals temporally distinct host factor requirements. PLoS Negl. Trop. Dis. 10, e0004921 (2016).
Schoggins, J. W. et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472, 481–485 (2011).
Carette, J. E. et al. Ebola virus entry requires the cholesterol transporter Niemann–Pick C1. Nature 477, 340–343 (2011).
Mlera, L., Meade-White, K., Saturday, G., Scott, D. & Bloom, M. E. Modeling Powassan virus infection in Peromyscus leucopus, a natural host. PLoS Negl. Trop. Dis. 11, e0005346 (2017).
Shan, C. et al. An infectious cDNA clone of Zika virus to study viral virulence, mosquito transmission, and antiviral inhibitors. Cell Host Microbe 19, 891–900 (2016).
Lanke, K. H. et al. GBF1, a guanine nucleotide exchange factor for Arf, is crucial for coxsackievirus B3 RNA replication. J. Virol. 83, 11940–11949 (2009).
Metsalu, T. & Vilo, J. ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 43, W566–W570 (2015).
Teo, G. et al. SAINTq: scoring protein-protein interactions in affinity purification - mass spectrometry experiments with fragment or peptide intensity data. Proteomics 16, 2238–2245 (2016).
Sanjana, N. E., Shalem, O. & Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783–784 (2014).
Li, W. et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 15, 554 (2014).
Huang da, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Carette, J. E. et al. Global gene disruption in human cells to assign genes to phenotypes by deep sequencing. Nat. Biotechnol. 29, 542–546 (2011).
Acknowledgements
We would like to thank A. Rubin, O. Botvinnik, M. R. Corces and A. Za for their helpful discussions on statistical and computational analyses; J. Ule, J. Zmrzlikar and N. Haberman for help with the iCount peak-calling software; L. Zhang and the Elias lab for assistance running samples for mass spectrometry analysis; J. Coller, X. Ji, D. Wagh and the Stanford Functional Genomics Facility for assistance with running samples for deep sequencing. We also thank the Stanford shared FACS facility and its former director M. Bigos for technical assistance. We acknowledge the NIH Biodefense and Emerging Infections Research Repository (BEI Resources) and the NIAID, NIH for providing the multiple DENV and ZIKV strains mentioned in Methods. We thank S. Braun and the members of the Carette, Kirkegaard and Sarnow labs for their helpful discussions and critical reading of the manuscript. The work was funded in part by the NIH (grant nos DP2 AI104557, R01 AI141970 and U19 AI109662 to J.E.C.; R37 AI047365 and R01 AI069000 to P.S. and R01 AI051622 to C.R.B.), the Burroughs Wellcome Investigators in the Pathogenesis of Infectious Disease (J.E.C.), the David and Lucile Packard Foundation (J.E.C.), the Stanford Dean’s Fellowship (Y.S.O.), the Child Health Research Institute Stanford (K.M.), the Damon Runyon Cancer Research Foundation (R.A.F.), the NSF-GRF (A.G.J. and C.D.M.) and the Howard Hughes Medical Institute (C.R.B.). C.D.M., J.G., L.M. and M.E.B. were supported by the Intramural Research Program of the NIAID.
Author information
Authors and Affiliations
Contributions
Y.S.O., K.M. and R.A.F. were responsible for the design and execution of experiments, data analysis and manuscript preparation. M.A.M. and P.S. performed and analysed the northern blot assays. J.D. carried out the MAGECK analysis for all CRISPR screening results. J.K.L. and N.R. assisted with the proteomic experiments. N.V.B. and K.K. were responsible for the immunofluorescence and fluorescent in situ hybridization targeting assays. A.G.J. assisted with the preparation of manuscript. A.S.P. and C.D.M. helped with the preparation of reagents, cell lines and viruses. L.M., J.M.G. and M.E.B. were responsible for the infection assays and analyses involving POWV. J.E.C. and C.R.B. supervised the research, acquired funding, interpreted data and prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9 and legends for Supplementary Tables.
Supplementary Table 1
Oligonucleotides used in this article.
Supplementary Table 2
Proteins identified by ChIRP-MS of DENV or ZIKV.
Supplementary Table 3
Overlap of ChIRP-MS hits with annotated ER-associated proteins.
Supplementary Table 4
RNA-seq of uninfected Huh7.5.1 cells or DENV- or ZIKV-infected cells.
Supplementary Table 5
Proteins identified by ChIRP-MS of RV.
Supplementary Table 6
Host genes identified in genome-scale KO screens for DENV or ZIKV.
Supplementary Table 7
RT stop counts of genes bound to RRBP1 as identified by irCLIP.
Supplementary Table 8
RT stop counts of genes bound to vigilin as identified by irCLIP.
Supplementary Table 9
Comparison of flavivirus host factors with published screens.
Supplementary Table 10
Intersection of ChIRP-MS with published work.
Rights and permissions
About this article
Cite this article
Ooi, Y.S., Majzoub, K., Flynn, R.A. et al. An RNA-centric dissection of host complexes controlling flavivirus infection. Nat Microbiol 4, 2369–2382 (2019). https://doi.org/10.1038/s41564-019-0518-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-019-0518-2
This article is cited by
-
Differences in proteome perturbations caused by the Wolbachia strain wAu suggest multiple mechanisms of Wolbachia-mediated antiviral activity
Scientific Reports (2023)
-
HDLBP binds ER-targeted mRNAs by multivalent interactions to promote protein synthesis of transmembrane and secreted proteins
Nature Communications (2022)
-
Comparison of viral RNA–host protein interactomes across pathogenic RNA viruses informs rapid antiviral drug discovery for SARS-CoV-2
Cell Research (2022)
-
Cotranslational prolyl hydroxylation is essential for flavivirus biogenesis
Nature (2021)
-
The SARS-CoV-2 RNA–protein interactome in infected human cells
Nature Microbiology (2020)