Abstract
The staphylococcal bi-component leukocidins Panton–Valentine leukocidin (PVL) and γ-haemolysin CB (HlgCB) target human phagocytes. Binding of the toxins’ S-components to human complement C5a receptor 1 (C5aR1) contributes to cellular tropism and human specificity of PVL and HlgCB. To investigate the role of both leukocidins during infection, we developed a human C5aR1 knock-in (hC5aR1KI) mouse model. HlgCB, but unexpectedly not PVL, contributed to increased bacterial loads in tissues of hC5aR1KI mice. Compared to humans, murine hC5aR1KI neutrophils showed a reduced sensitivity to PVL, which was mediated by the toxin’s F-component LukF-PV. By performing a genome-wide CRISPR–Cas9 screen, we identified CD45 as a receptor for LukF-PV. The human-specific interaction between LukF-PV and CD45 provides a molecular explanation for resistance of hC5aR1KI mouse neutrophils to PVL and probably contributes to the lack of a PVL-mediated phenotype during infection in these mice. This study demonstrates an unsuspected role of the F-component in driving the sensitivity of human phagocytes to PVL.
Similar content being viewed by others
Main
Staphylococcus aureus is a major bacterial pathogen in humans and is responsible for a diverse disease spectrum, ranging from superficial skin and soft tissue infections to severe invasive disease. Severe infections with S. aureus have a poor prognosis1. Treatment is further complicated by the emergence of methicillin-resistant S. aureus (MRSA) strains2 and by a lack of advancements in vaccine development3. A better understanding of the host–pathogen interaction during infection with S. aureus is essential to develop new therapeutic approaches.
Phagocytes have a pivotal role in the containment of S. aureus early after infection4. To counteract elimination by phagocytes, S. aureus secretes an arsenal of virulence factors. Among these are the leukocidins, a family of bi-component pore-forming toxins that target and kill phagocytes5,6. Human S. aureus isolates secrete up to five different leukocidins7: Panton–Valentine leukocidin (PVL; also known as LukSF-PV), γ-haemolysin AB and CB (HlgAB and HlgCB, respectively), leukocidin ED (LukED) and leukocidin AB (LukAB; also known as LukGH). Chromatography elution profiles differentiate the leukocidin protein components into S-migrating (slow) and F-migrating (fast) components, which are, with the exception of LukAB, secreted as inactive monomers5. Each canonical leukocidin combination consists of S- and F-components that hetero-oligomerize into an octameric membrane-spanning pore5,7. The leukocidins show structural and functional resemblance to the single-component pore-forming toxin of S. aureus, α-toxin (haemolysin-α (Hla))8, but the biological rationale for a bi-component system remains unresolved. Functional interactions by formation of non-canonical combinations of S- and F-components that are active (as for PVL and HlgCB9,10,11) or inactive (as for PVL and LukED12) suggest that the contribution of leukocidins to pathogenesis differs when expressed simultaneously. However, the contribution of the leukocidins to infection is incompletely understood5,7.
Specificity for human phagocytes and resistance of murine phagocytes to the majority of leukocidins hinder investigation during infection7. Recently, proteinaceous receptors have been identified for all leukocidins7,13,14,15,16,17,18,19,20. These receptors are targeted by the S-components in a species-specific manner. For the S-components of PVL and HlgCB, LukS-PV and HlgC, respectively, the human complement C5a receptor 1 (hC5aR1) was identified as the major receptor14,16. The identification of hC5aR1 as a shared receptor for LukS-PV and HlgC explains the specificity for human phagocytes as both toxins are incompatible with the murine C5aR1 orthologue14,16. Although differences exist in the interaction between LukS-PV and HlgC with hC5aR1 (refs 21,22), the necessity for S. aureus to secrete apparently redundant toxins is incompletely appreciated7. Even though receptors have been identified for all leukocidin S-components, it remains to be established whether the F-components also have host receptors. A recent study on the equine-specific leukocidin of S. aureus (LukPQ) suggests that the F-component might be involved in determining host tropism17.
Owing to the human specificity of both toxins, studies addressing the role of PVL and HlgCB during infection have proven to be challenging in mice6,23,24. In rabbits, PVL contributes to necrotizing pneumonia25, osteomyelitis26 and modestly enhances early stages of bacteraemic spread in a bloodstream infection model27. The contribution of PVL to skin infections in rabbits remains controversial28,29, although the presence of the genes encoding PVL is epidemiologically linked to severe skin and soft tissue infections in humans30. More recently, non-obese diabetic (NOD)/severe combined immune deficiency (SCID)/IL2R-γ-null (NSG) mice engrafted with primary human haematopoietic cells were found to be more susceptible to skin lesions31 and pneumonia32 than control mice in a PVL-dependent manner. These reports have focused on PVL exclusively. To the best of our knowledge, no studies have reported on the role of HlgCB during infection in vivo5. To understand the mechanisms of pore formation during infection and to assess both the contribution of each individual hC5aR1-targeting leukocidin and the contribution of the leukocidins as a group, a humanized in vivo model is needed.
Here, we report on the development of a hC5aR1 knock-in (hC5aR1KI) mouse to investigate the role of PVL and HlgCB during infection with S. aureus and on the subsequent identification of CD45 as a receptor for LukF-PV, the F-component of PVL.
Results
hC5aR1 increases bacterial loads during S. aureus infection
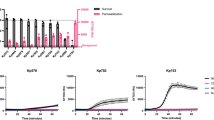
To investigate the contribution of HlgCB and PVL to infection, we developed a hC5aR1KI mouse. Quantification of hC5aR1 expression in hC5aR1KI mice recapitulated expression levels on human leukocytes33 (Fig. 1a). Compared to wild-type (WT) murine phagocytes, hC5aR1KI phagocytes signalled normally in response to murine and human C5a (Supplementary Fig. 1).
a, The expression of hC5aR1 on leukocytes from humans and hC5aR1KI mice shown as the antibody-binding capacity of an anti-hC5aR1 monoclonal antibody. The mean is shown, with n = 2 biologically independent samples. b, Bacterial loads after intraperitoneal infection with S. aureus WT or an isogenic ∆lukSF-PV ∆hlgACB-mutant strain in hC5aR1KI and WT mice. Sample sizes for hC5aR1KI and WT mice, respectively: n = 25 and n = 23 for S. aureus WT; n = 11 and n = 5 for S. aureus ∆lukSF-PV ∆hlgACB. c, Bacterial loads in the spleen and the kidneys after infection with S. aureus WT ST80 or an isogenic ∆lukSF-PV- or ∆hlgACB-mutant strain. Sample sizes for hC5aR1KI and WT mice, respectively: n = 25 and n = 23 for S. aureus WT; n = 6 and n = 6 for S. aureus ∆lukSF-PV; n = 6 and n = 6 for S. aureus ∆hlgACB. d, Bacterial loads in the spleen and the kidneys after infection of hC5aR1KI mice with S. aureus ∆hlgACB complemented with a plasmid encoding hlgAB (pAB) or hlgCB (pCB). Sample sizes: n = 9 for S. aureus ∆hlgACB; n = 10 for S. aureus ∆hlgACBpAB; n = 11 for S. aureus ∆hlgACBpCB. e, Bacterial loads in the skin recovered after subcutaneous infection with S. aureus WT or an isogenic ∆lukSF-PV ∆hlgACB strain. Sample sizes: n = 12 for all groups. f, Bacterial loads in the skin after infection with S. aureus WT ST80 or an isogenic ∆lukSF-PV- or ∆hlgACB-mutant strain. Sample sizes for hC5aR1KI and WT mice, respectively: n = 12 and n = 12 for S. aureus WT; n = 14 and n = 14 for S. aureus ∆lukSF; n = 12 and n = 12 for S. aureus ∆hlgACB. For panels b–f, mice were distributed over independent experiments. The solid horizontal lines express the geometric means; the horizontal dashed lines indicate the detection threshold. Significance is displayed: *P < 0.05, **P < 0.01, ***P < 0.001 and NS for not significant, and was calculated using ANOVA with Bonferroni post-test correction for multiple comparison. Exact P values are provided in Supplementary Table 3. See also Supplementary Fig. 1.
Next, a WT MRSA strain (ST80, which harbours the genes encoding PVL, and HlgAB and HlgCB34) was injected intraperitoneally into hC5aR1KI and WT mice (Fig. 1b). Twenty-four hours after infection, hC5aR1KI mice displayed 10–100-fold higher bacterial loads in the spleen and the kidneys than WT mice infected with the same WT S. aureus strain (Fig. 1b). The hC5aR1-dependent increase in bacterial burden was also observed in the peritoneal cavity. Bacterial loads in the peripheral blood showed a similar trend. These data demonstrate that hC5aR1 expression on phagocytes results in increased bacterial loads during infection with an S. aureus strain producing both PVL and HlgCB.
Leukocidins promote susceptibility of hC5aR1KI mice to S. aureus infection
To investigate whether the observed differences between WT and hC5aR1KI mice are due to hC5aR1-targeting leukocidins, we infected hC5aR1KI and WT mice with an isogenic ∆lukSF-PV ∆hlgACB double mutant S. aureus strain. No differences were observed between hC5aR1KI or WT mice in any of the cultured compartments after infection with the double mutant S. aureus strain (Fig. 1b). However, compared to the WT S. aureus strain, infection in hC5aR1KI mice with the double mutant S. aureus strain resulted in a 10–100-fold reduction in bacterial burdens in the spleen and the kidneys, with a similar trend in the peritoneal cavity and peripheral blood (Fig. 1b). In WT mice, no differences were observed in any of the cultured compartments after infection with the WT or double mutant S. aureus strain (Fig. 1b). Thus, these results confirm that the increased susceptibility of hC5aR1KI mice for WT S. aureus is mediated by at least one of the two hC5aR1-targeting leukocidins.
HlgCB, but not PVL, contributes to S. aureus pathophysiology in hC5aR1KI mice
To assess the individual involvement of HlgCB and PVL in hC5aR1-dependent S. aureus pathogenesis, hC5aR1KI mice were challenged with WT and isogenic single mutant S. aureus strains lacking either hlgACB (∆hlgACB) or lukSF-PV (∆lukSF-PV). Infection of hC5aR1KI mice with ∆lukSF-PV or WT bacteria resulted in similar bacterial loads in the spleen and the kidneys when compared to animals infected with WT bacteria (Fig. 1c). However, hC5aR1KI mice infected with S. aureus ∆hlgACB showed decreased bacterial loads in the spleen and the kidneys compared to WT bacteria (Fig. 1c). These results demonstrate that the increased susceptibility of hC5aR1KI mice to WT S. aureus is mediated by hlgACB.
The hlgACB gene cluster encodes two functional pore-forming toxins (HlgAB and HlgCB), of which HlgCB but not HlgAB targets hC5aR1 (refs 7,16). To confirm that the differences in bacterial loads recovered from hC5aR1KI mice infected with WT versus ∆hlgACB bacteria are mediated by HlgCB and not by HlgAB, hC5aR1KI mice were challenged with ∆hlgACB bacteria complemented with a plasmid encoding hlgAB or hlgCB (Fig. 1d). Infection of hC5aR1KI mice with S. aureus ∆hlgACB reconstituted with hlgCB but not hlgAB showed an increased number of colony-forming units (c.f.u.) in the spleen and the kidneys (Fig. 1d), demonstrating that HlgCB promotes S. aureus pathogenicity in hC5aR1KI mice during systemic infection.
To further assess the role of HlgCB and PVL, we infected hC5aR1KI and WT mice subcutaneously. Similar to the systemic infection model, mice expressing hC5aR1 displayed increased bacterial loads in the skin after infection with an S. aureus strain that produces both PVL and HlgCB (Fig. 1e). Furthermore, susceptibility of hC5aR1KI mice for WT S. aureus was again mediated by at least one of the two hC5aR1-targeting leukocidins as infection with the double mutant completely annulled the phenotype (Fig. 1e). In addition, infection of hC5aR1KI mice with S. aureus ∆lukSF-PV did not affect the bacterial loads, whereas hC5aR1KI mice infected with S. aureus ∆hlgACB showed a 100-fold decrease in the bacterial loads recovered from the skin compared to WT bacteria (Fig. 1f).
Taken together, our investigations demonstrate a hC5aR1-dependent contribution of HlgCB during infection with S. aureus. However, the absence of a role for PVL was unexpected25,26,27,28,31,32 and prompted us to investigate whether leukocytes of hC5aR1KI mice lack another factor that may be involved in the human-specific cytotoxicity of PVL.
PVL and HlgCB differentially target hC5aR1KI murine neutrophils in an F-component-specific manner
Bone-marrow-derived hC5aR1KI murine neutrophils were isolated and compared to human neutrophils for susceptibility to HlgCB and PVL at concentrations for which neutrophils of WT mice are fully resistant14,16. No differences in susceptibility to HlgCB-induced pore formation between human neutrophils and hC5aR1KI murine neutrophils were observed (Fig. 2a). However, hC5aR1KI murine neutrophils showed a decreased sensitivity to PVL compared to human neutrophils (Fig. 2a).
a, Susceptibility of bone-marrow-derived neutrophils from hC5aR1KI mice (n = 4) and human neutrophils isolated from healthy donors (n = 3) after exposure to canonical and non-canonical toxin combinations at the indicated concentrations. Cell permeability was determined by flow cytometry using propidium iodide at 30 minutes of post-toxin treatment. Dashed horizontal lines indicate the EC50, which is also expressed as a separate graph for statistical comparison. For all graphs, the mean ± s.d. is shown. Significance was calculated using a two-sided Student’s t-test. b, Bone-marrow-derived neutrophils from hC5aR1KI mice and human neutrophils were treated with LukF-PV at the indicated concentrations. Binding was subsequently determined by flow cytometry. The mean ± s.d. is shown, with n = 3. Significance was calculated using ANOVA with Bonferroni post-test correction for multiple comparison. For all panels, significance is displayed: **P < 0.01, ***P < 0.001 and NS for not significant. Exact P values are provided in Supplementary Table 3. See also Supplementary Fig. 2.
As hC5aR1 expression on hC5aR1KI murine neutrophils reflected that of human neutrophils (Fig. 1a), we questioned whether reduced susceptibility of hC5aR1KI murine neutrophils for PVL was due to a species-specific interaction of the cells with the toxin’s S-component or F-component. Non-canonical pairing of the S- and F-components of PVL and HlgCB enables the formation of functional pores in human phagocytes9,10,11. hC5aR1KI murine neutrophils were as susceptible to LukS-PV/HlgB as human neutrophils (Fig. 2a), indicating that reduced susceptibility of hC5aR1KI murine neutrophils to PVL is not due to a compromised interaction of LukS-PV with the cells. Correspondingly, we observed comparable binding of LukS-PV to hC5aR1KI murine and human neutrophils (Supplementary Fig. 2). However, hC5aR1KI murine neutrophils were less susceptible to HlgC/LukF-PV than human neutrophils (Fig. 2a). This finding indicates that the species-specific phenotype of PVL on hC5aR1KI murine neutrophils is mediated by the F-component, LukF-PV. Indeed, reduced binding of LukF-PV to hC5aR1KI murine neutrophils was observed when compared to human neutrophils (Fig. 2b).
These observations demonstrate that the observed reduced susceptibility of hC5aR1KI murine neutrophils to PVL is mediated by its F-component and imply the involvement of a host factor that displays human-specific interaction with LukF-PV.
PVL targets CD45
To identify additional host factors involved in PVL-mediated cytotoxicity, a genome-wide clustered regularly interspaced short palindromic repeats (CRISPR)–CRISPR-associated protein 9 (Cas9) screen for PVL resistance was set up in human U937-hC5aR1-SpCas9 cells. Focusing on cell surface proteins, the gene encoding C5aR1, C5AR1, was identified as a top hit, illustrating the validity of the screening method (Fig. 3a and Supplementary Tables 1 and 2). The other most enriched gene, which encodes a predicted surface protein, was PTPRC (Fig. 3a and Supplementary Tables 1 and 2). PTPRC encodes protein tyrosine phosphatase receptor type C (PTPRC; also known as CD45). Expression levels of C5aR1 and CD45 on the surface of U937-hC5aR1-SpCas9 cells (Fig. 3b) were in the same order of magnitude as on human neutrophils (Supplementary Fig. 3a).
a, Cellular components that are crucial for PVL-mediated killing identified by the introduction of a genome-wide sgRNA library in U937-hC5aR1-SpCas9 cells coupled to deep sequencing. The top 20 most significantly enriched genes, as calculated by the MaGeCK ‘positive enrichment score’, are visualized, with the two surface proteins C5aR1 and CD45 highlighted. b, Validation of receptor expression in U937-hC5aR1-SpCas9 cell lines transduced with two different sgRNAs for C5aR1 (hC5aR1− CD45+) or CD45 (hC5aR1+ CD45−) to generate single-gene knockout cell lines. Receptor expression is demonstrated as the antibody-binding capacity of an anti-hC5aR1 or anti-hCD45 monoclonal antibody. As a control, WT U937 cells (WT; hC5aR1− CD45+) and U937-hC5aR1-SpCas9 cells transduced with a non-targeting control (NTC; hC5aR1+ CD45+) were used. The dashed horizontal line indicates the detection threshold. The mean ± s.d. is shown, with n = 3. Significance was calculated using ANOVA with Bonferroni post-test correction for multiple comparison. c, Validation of the genome-wide CRISPR–Cas9 screen for PVL resistance in U937-hC5aR1-SpCas9 cells. Cells were exposed to PVL (7.5 nM) or HlgCB (2.5 nM). As a readout for cell permeability, internalization of DAPI was tested at 30 minutes post-toxin treatment on a monochromator-based microplate reader and expressed in relation to the maximal area under the curve for U937-hC5aR1-SpCas9 cells transduced with a NTC. Two guide RNAs were tested for C5aR1 and CD45. The mean ± s.d. is shown, with n = 3. Significance was calculated using a two-sided Student’s t-test. For all panels, significance is displayed: *P < 0.05, **P < 0.01, ***P < 0.001 and NS for not significant. Exact P values are provided in Supplementary Table 3. See also Supplementary Tables 1 and 2 and Supplementary Figs 3 and 4.
To validate the involvement of CD45 in PVL susceptibility, single knockout cells were generated. Mutant cells specifically lacked expression of C5aR1 or CD45 (Fig. 3b). Subsequently, cells were challenged with PVL or HlgCB at approximately half-maximum effective concentration (EC50) of toxin. As expected, C5aR1− CD45+ cells were resistant to both PVL and HlgCB (Fig. 3c). C5aR1+ CD45− cells were resistant to pore formation induced by PVL (Fig. 3c). Lactate dehydrogenase release confirmed that PVL induces actual cell lysis in a CD45-dependent manner (Supplementary Fig. 3b). Notably, the absence of CD45 on the cellular surface did not affect susceptibility to HlgCB toxicity (Fig. 3c). These findings show that PVL, but not HlgCB, targets CD45 to induce cell lysis, thereby providing evidence that PVL and HlgCB are functionally different toxins.
PVL targets CD45 in an F-component-specific manner
To further investigate the role of CD45 in cellular susceptibility to PVL, mutant cells were incubated with different concentrations of PVL and HlgCB. The absence of CD45 resulted in an increased EC50 for PVL, but not for HlgCB (Fig. 4a). Activation of C5aR1 by its ligand C5a was not affected by knocking out PTPRC, indicating that the reduced susceptibility of C5aR1+ CD45− cells to PVL toxicity is not due to an interplay between C5aR1 and CD45 (Supplementary Fig. 4a). These results indicate that CD45 is directly involved in cell susceptibility to PVL but not HlgCB.
a, Susceptibility of WT U937 cells (WT; hC5aR1− CD45+), U937-hC5aR1-SpCas9 cells transduced with a NTC (hC5aR1+ CD45+), a sgRNA for C5AR1 (hC5aR1− CD45+) or a sgRNA for PTPRC (hC5aR1+ CD45−) to canonical and non-canonical toxin combinations. As a readout for cell permeability, internalization of DAPI was tested at 30 minutes post-toxin treatment on a monochromator-based microplate reader and expressed in relation to the maximal area under the curve for NTC U937-hC5aR1-SpCas9 cells. b, Binding of LukF-PV to cells as detected by flow cytometry, expressed as the fold increased binding related to the background binding. In addition to WT U937 and NTC U937-hC5aR1-SpCas9 cells, two U937-hC5aR1-SpCas9 cell lines transduced with a vector containing a sgRNA for PTPRC (both hC5aR1+ CD45−) were tested. c, Pore formation of cells after pre-incubation with LukF-PV, followed by a washing step and the subsequent addition of LukS-PV (0.64 µg ml−1). Internalization of DAPI was tested at 30 minutes after the addition of LukS-PV on a monochromator-based microplate reader and expressed in relation to the maximal area under the curve for NTC U937-hC5aR1-SpCas9. d, Binding of LukF-PV to human neutrophils after pre-incubation with monoclonal anti-CD45 antibodies (10 µg ml−1). Binding is expressed in relation to buffer-only treated cells, as detected by flow cytometry. e, Pore formation of human neutrophils after pre-incubation with monoclonal antibodies (10 µg ml−1). Cell permeability was determined by flow cytometry using DAPI at 30 minutes post-toxin treatment. For panels a and e, the dashed horizontal lines indicate the EC50, which is also expressed as a separate graph for statistical comparison. For all panels, the mean ± s.d. is shown, with n = 3. Significance was calculated using a two-sided Student’s t-test for panel a and using ANOVA with Bonferroni post-test correction for multiple comparison for panels b–e. Significance is displayed: *P < 0.05, **P < 0.01, ***P < 0.001 and NS for not significant. Exact P values are provided in Supplementary Table 3. See also Supplementary Fig. 4b.
To test whether the resistance of CD45− cells to PVL, but not HlgCB, results from a disturbed interaction with the toxin’s S-component or F-component, non-canonical toxin combinations of PVL and HlgCB were subsequently tested. A comparable EC50 for LukS-PV/HlgB was observed for CD45− and CD45+ cells (Fig. 4a). However, an increase of the EC50 in CD45− cells, similar to that for PVL, was observed for HlgC/LukF-PV (Fig. 4a). These findings demonstrate that the resistance of CD45− cells to PVL depends on LukF-PV, suggesting that CD45 acts as a receptor for LukF-PV.
To study the interaction between CD45 and LukF-PV, we tested the binding of LukF-PV to the cell surface. In CD45− cells, LukF-PV binding was reduced compared to CD45+ cells irrespective of co-expression of C5aR1 (Fig. 4b and Supplementary Fig. 4b,c). Despite non-specific background binding in CD45− cells, CD45-dependent binding sites of LukF-PV could be saturated, indicating a specific interaction between CD45 and LukF-PV (Fig. 4b and Supplementary Fig. 4b,c). Pre-incubation of cells with LukF-PV followed by a washing step and the subsequent addition of LukS-PV showed a CD45-dependent susceptibility of cells to pore formation (Fig. 4c), demonstrating that CD45-dependent binding of LukF-PV specifically contributes to pore formation.
If CD45 is a receptor for LukF-PV, CD45 neutralization could interfere with LukF-PV binding and PVL cytotoxicity. Pre-treatment of human neutrophils with a monoclonal antibody against CD45 (clone 4B2) reduced the binding of LukF-PV (Fig. 4d). A small shift was observed for the EC50 of PVL in cells pre-treated with the antibody against CD45 (Fig. 4e and Supplementary Fig. 4d), providing further evidence for the interaction between LukF-PV and CD45.
CD45 is a receptor for LukF-PV
CD45 has multiple isoforms due to alternative splicing of exons encoding the distal part of the extracellular domain35 (Supplementary Fig. 5a). To investigate whether the isotype affects the susceptibility to PVL, the PVL–CD45 interaction was further investigated by expression of the shortest and longest CD45 isoforms (R0 and RABC, respectively) in C5aR1+ CD45− U937 cells (Fig. 5a and Supplementary Fig. 5). The expression of both CD45 isoforms restored PVL susceptibility, indicating that the distal extracellular domain of the receptor is not required for interaction with LukF-PV (Fig. 5a). To confirm that LukF-PV directly binds to CD45, surface plasmon resonance (SPR) was performed using recombinant CD45 or C5aR1+ U937 cells expressing human CD45 (hCD45). LukF-PV bound to the recombinant receptor and cells with a dissociation constant (Kd) of 1.2 µM and 1.4 µM, respectively (Table 1).
Susceptibility to PVL of U937-hC5aR1-SpCas9 cells transduced with a sgRNA targeting PTPRC (hC5aR1+ CD45−) and subsequently transduced with a plasmid containing either the hCD45 isoform R0 (phCD45-R0) or RABC (phCD45-RABC) (a), the murine CD45 isoform R0 (pmCD45-R0) or RABC (pmCD45-RABC) (b), or an empty plasmid (pEmpty). As a readout for cell permeability, internalization of DAPI was tested at 30 minutes post-toxin treatment on a monochromator-based microplate reader and expressed in relation to the maximal area under the curve for U937-hC5aR1-SpCas9 cells transduced with a NTC (hC5aR1+ CD45+). Dashed horizontal lines indicate the EC50, which is also expressed as a separate graph for statistical comparison. For all panels, the mean ± s.d. is shown, with n = 3. Significance is displayed: ***P < 0.001 and NS for not significant, and was calculated using ANOVA with Bonferroni post-test correction for multiple comparison. Exact P values are provided in Supplementary Table 3. See also Supplementary Figs 5 and 6.
Thus, these data not only identify CD45 as a receptor for PVL but also highlight CD45 as an F-component-specific leukocidin receptor.
PVL targets CD45 in a human-specific manner
As neutrophils express CD45 (ref. 35) but hC5aR1KI murine neutrophils showed a reduced F-component-dependent sensitivity to PVL, we hypothesized that LukF-PV interacts with CD45 in a species-specific manner and expressed the murine CD45 isoforms R0 or RABC in C5aR1+ CD45− U937 cells (Fig. 5b and Supplementary Fig. 5). Neither murine CD45 isoforms were capable of restoring PVL susceptibility (Fig. 5b). Next, we expressed hCD45 in hC5aR1KI murine macrophages. The expression of hCD45 in hC5aR1KI murine macrophages enhanced the susceptibility to PVL-induced pore formation (Supplementary Fig. 6), mirroring the phenotype observed in U937 cells (Fig. 5a). SPR using recombinant murine CD45 or C5aR1+ U937 cells expressing murine CD45 revealed a Kd for binding of LukF-PV of 14.9 µM and 14.2 µM, respectively (Table 1). Thus, the affinity of LukF-PV for murine CD45 is ±10-fold lower than the human receptor.
These results provide a molecular explanation for the species-specific interaction between LukF-PV and its receptor CD45. Incompatibility of PVL with murine CD45 probably explains the LukF-PV-dependent reduced sensitivity to PVL that was observed in hC5aR1KI murine neutrophils.
Discussion
The S-components of PVL and HlgCB target C5aR1 in a human-specific manner14,16. Human specificity has hindered in vivo studies of the role of these leukocidins. We developed a hC5aR1KI mouse to investigate the role of PVL and HlgCB during infection with S. aureus. Although HlgCB contributed to increased bacterial loads in hC5aRKI mice, no contribution of PVL was observed. The unexpected lack of a PVL-dependent phenotype during infection in these mice urged us to screen for additional host factors targeted by PVL. We show that PVL targets CD45 in a human-specific and LukF-PV-dependent manner, thereby demonstrating that LukF-PV and CD45 are specifically involved in pore formation. CD45 is expressed on all nucleated haematopoietic cells and is an abundant cell surface protein35. Our data indicate that reduced susceptibility of C5aR1+ CD45− cells to PVL toxicity is not due to affected signalling of C5aR1, but by a decreased binding of LukF-PV to CD45− cells.
The current model for leukocidin targeting of host cells proposes initial S-component binding followed by subsequent recruitment of the F-component5. We demonstrate that specific binding of LukF-PV is CD45 dependent and occurs independent of the S-component, indicating that the established model needs to be revised. The identification of a receptor for the F-component supports our understanding of the biological importance of two-component pore-forming systems. By targeting CD45 via its F-component in addition to C5aR1 via its S-component, PVL deploys a two-step control mechanism over phagocyte tropism and host species specificity. Furthermore, identification of CD45 as a receptor for LukF-PV supports the notion that PVL and HlgCB are non-redundant toxins on both a molecular and a functional level. Future investigations will have to identify putative receptors for F-components of other leukocidins.
The effects of CD45 expression on the susceptibility of C5aR1+ cells to PVL pore formation are moderate in terms of EC50. This moderate effect is reflected in the micromolar affinity of LukF-PV for CD45, which is significantly lower than the affinity of LukS-PV for C5aR1 (ref. 14). Murine CD45 could not restore the susceptibility of C5aR1+ CD45− cells to PVL, and the affinity of LukF-PV to murine CD45 is lower than the affinity to hCD45, suggesting a critical threshold that allows engagement of the toxin–receptor complex during pore formation. The extracellular domain shared by all CD45 isoforms is heavily glycosylated and contains a cysteine-rich region and three fibronectin type III repeats35 (Supplementary Fig. 5a). Although the overall organization of the extracellular domain is conserved, it is only 39% homologous between humans and mice35 (Supplementary Fig. 5c). Incompatibility of LukF-PV with murine CD45 may be dictated by multiple residues or post-translational modifications (Supplementary Fig. 5d).
The specificity of LukF-PV for hCD45 offers a molecular explanation for the observed F-component-dependent resistance of hC5aR1KI mouse neutrophils to PVL in vitro, which is supported by enhanced susceptibility of hC5aR1KI mouse macrophages expressing hCD45. The relative resistance of hC5aR1KI murine neutrophils to PVL probably contributes to the unexpected lack of a PVL-mediated phenotype during infection with S. aureus in these mice. As a result, functional interactions between PVL and HlgCB during infection in hC5aR1KI mice could not be investigated. Future options to assess the contribution of the leukocidins as a group are engineering of advanced genetically modified animal models7 or engrafting mice with primary human haematopoietic cells31,32. Owing to the small protective effects of monoclonal antibodies in vitro and the heterogeneity associated with human haematopoietic cells engraftment in mice31,32, we were unable to use this strategy in vivo to investigate the CD45–LukF-PV interaction during infection.
The mechanisms for the predisposition of otherwise healthy individuals to severe infections with S. aureus are poorly understood36,37. Human genetic factors might account for an unfavourable outcome38,39. CD45 deficiency was described in patients with SCID40,41, and abnormal splicing of PTPRC frequently occurs42. Variations in the PTPRC gene are probable candidates to explore the genetic predisposition to severe infections.
By taking advantage of the conserved susceptibility of hC5aR1KI mouse neutrophils to HlgCB, we show that HlgCB contributes to increased bacterial loads by using hC5aR1. Our data support the notion that leukocidins have an essential role in the pathogenicity of S. aureus15,16,20,27. Strategies aimed at protecting phagocytes from cytotoxicity by blocking the interaction between toxin and receptor offer avenues for therapeutic intervention. Receptor competition by means of monoclonal antibodies or small-molecule receptor antagonists confers protection against toxin-mediated pore formation in vitro18,20,21. The establishment of the role of hC5aR1 during infection and the identification of CD45 as a receptor for LukF-PV provide a rationale to further investigate the leukocidin receptors as candidate drug targets for severe S. aureus infections.
Methods
Construction and generation of hC5aR1KI mice
hC5aR1KI mice were generated as previously described43 at the Institut Clinique de la souris (Ilkirch-Graffenstaden, France) using standard knock-in techniques. Briefly, the targeting vector comprised a 4.5-kb region of mouse C57BL/6 genomic DNA upstream of the C5ar gene (gene ID: ENSMUSG00000049130) exon 2 (and ending with the murine ATG), exon 2 from human C5AR1 (gene ID: ENSMUST00000050770) encoding the full-length hC5aR1 from amino acid 2 to the stop codon of the protein in frame with ATG from the murine C5ar1 gene and a 3.5-kb region of the mouse C57BL/6 C5ar1 3′ untranslated region. The obtained vector was electroporated into C57BL/6 N embryonic stem cells. Embryonic stem cells containing the correctly targeted human C5AR1, as verified by Southern blot, were analysed by karyotyping before injection into blastocytes. Following verification of germline transmission, the LoxP-flanked neomycin selection cassette was deleted using deleter mice as previously described44. Mice homozygous for the hC5aR1 were generated and validated by genotyping PCR.
Construction of the CRISPR–Cas9 library
A genome-scale single guide RNA (sgRNA) library was designed, consisting of ±260,000 sgRNAs targeting every unique Refseq annotated (hg19) protein-coding isoform with up to 12 sgRNAs, plus 7,700 non-targeting control sequences. Where possible, the earliest possible coding exon of each transcript variant was targeted. All sgRNAs were designed to target the spCas9 recognition sequence (N)20NGG and must have passed the following off-targeting criteria: (1) the 11-base pair seed may not have an exact match to any other region in the human genome, and (2) if there is an exact off-target seed match, the remainder of the sgRNA sequence must have at least 7 mismatches with the potential off-target site. We selected up to 12 sgRNAs/transcripts for which the sequences are presented in Supplementary Table 2.
The designed 20-nucleotide target-specific sgRNA sequences were flanked by overhangs compatible with Gibson Assembly and synthesized as a pool on microarray surfaces (CustomArray). The synthesised sgRNA template sequences were of the format: 5′-GGAGAACCACCTTGTTGG-(N)20-GTTTAAGAGCTATGCTGGAAAC-3′. Template pools were PCR amplified by using Phusion Flash High-Fidelity PCR Master Mix (Thermo Fisher Scientific) according to the manufacturers protocol, with 1 ng µl−1 sgRNA template DNA, 1 µM forward primer (5′-GGAGAACCACCTTGTTGG-3′), 1 µM reverse primer (5′-GTTTCCAGCATAGCTCTTAAAC-3′) and the following cycle numbers: 1 cycle (98 °C for 3 min), 15 cycles (98 °C for 1 s, 55 °C for 15 s and 72 °C for 20 s) and 1 cycle (72 °C for 5 min). PCR products were purified using Minelute columns (Qiagen). The library vector sgLenti (MP-783) was prepared by restriction digest with AarI (Thermo Fisher) at 37 °C overnight, followed by extraction from 1% agarose gel of the digested band and purification via NucleoSpin columns (Macherey-Nagel). Using Gibson Assembly Master Mix (NEB), 1,000 ng digested sgLenti and 100 ng amplified sgRNA library insert were assembled in a total reaction volume of 200 µl. The reaction was purified using P-30 buffer exchange columns (Bio-Rad) that were equilibrated five times with H2O, and the total eluted volume was transformed into three vials of Electromax DH5-α (Thermo Fisher). Bacteria were recovered, cultured overnight in 500 ml LB (100 µg ml−1 ampicillin) and used for Maxiprep (Qiagen). In parallel, a fraction of the transformation reaction was plated and used to determine the total number of transformed clones. The library cloning coverage (that is, the number of bacteria colonies per sgRNA plasmid) was determined to be >100× to ensure even representation of the sgRNA sequences.
Cell lines and constructs
U937 human monocytic cells were obtained from the ATCC (American Type Culture Collection), cultured in RPMI medium supplemented with penicillin/streptomycin and 10% FCS, and tested for mycoplasma contamination. U937 cells were not authenticated. To sensitize the cells to PVL and HlgCB, hC5aR1 (CD88; NM_001736) was first stably expressed in U937 cells using a lentiviral expression system (U937-hC5aR1 cells). We cloned the C5AR1 cDNA in a dual promoter lentiviral vector (BIC-PGK-Zeo-T2a-mAmetrine; RP172), derived from no.2025.pCCLsin.PPT.pA.CTE.4 × -scrT.eGFP.mCMV.hPGK.NG-FR.pre (kindly provided by L. Naldini, San Raffaele Scientific Institute, Milan, Italy) as described elsewhere45. This lentiviral vector contains a human EF1A promoter to facilitate potent expression of the downstream cloned gene and expresses the fluorescent protein mAmetrine and the selection marker ZeoR from a different promoter (PGK). the virus was produced in 24-well plates using standard lentiviral production protocols and the third-generation packaging vectors pMD2G-VSVg, pRSV-REV and pMDL/RRE. Briefly, 0.25 µg lentiviral vector and 0.25 µg packaging vectors were co-transfected in 293T cells by using 1.5 µl Mirus LT1 tranfection reagent (Sopachem, Ochten). After 72 h, 100 µl unconcentrated viral supernatant adjusted to 8 mg ml−1 polybrene was used to infect ~50,000 U937 cells by spin infection at 1,000g for 2 h at 33 °C. U937-hC5aR1-expressing cells were selected by culturing in 400 µg ml−1 Zeocin.
To allow screening in U937-C5aR1 cells by using the genome-wide sgRNA library described below, the pSicoR-CRISPR-PuroR vector46 was altered to replace the PuroR for BlastR and remove the U6 promoter. This vector (pSicoR-SpCas9-BlastR; RP-613) expresses a human codon-optimized nuclear-localized Streptococcus pyogenes cas9 gene in the absence of a U6 promoter–sgRNA cassette. U937-hC5aR1 cells were transduced with the pSicoR-SpCas9-BlastR vector and were selected to purify with 20 µg ml−1 blasticidin, to generate U937-hC5aR1-SpCas9 cells.
U937-hC5aR1-SpCas9 cells were transduced by sgRNA-expressing vectors to generate knockout cell lines to enable genome-wide CRISPR–Cas9 library screening (described below) or to generate single-gene knockout cell lines. For this, a lentiviral vector was generated consisting of the pSicoR vector expressing PuroR-T2A-mCherry expressed from the EF1A promoter and a U6 promoter driving the expression of a sgRNA sequence (sgLenti, MP-783). To generate knockout cells for C5AR1 or PTPRC, the following sgRNA sequences were cloned in sgLenti: C5aR1_1 TCATCATAGTGCCCATAATC; C5aR1_2 GATGGCATTGATGGTCCGCT; CD45_1 TCACACTTATACTCATGTTC; CD45_2 ATTCTGTGTATCACAAGTAA. Upon virus production, as described above, U937-hC5aR1-SpCas9 cells were transduced with the sgRNA expression viruses and selected for purification by puromycin treatment (2 µg ml−1) to enrich for CD45− cells.
For cDNA rescue experiments, we cloned an anti-CD45 sgRNA (GAAACTTGCTGAACACCCGC) in the pSicoR-CRISPR-PuroR vector46, which co-expresses SpCas9 and puroR. Upon knock-out of PTPRC from U937-C5aR1 cells, cDNA expression vectors were introduced to express the human and mouse CD45-R0 and CD45-RABC genes. For this, the coding regions of hCD45-R0 (NM_080921.3), hCD45-RABC (NM_002838.4) and mouse CD45-R0 (NM_011210.3) were amplified from cDNA vectors purchased from Sino Biologicals and cloned in a dual promoter lentiviral vector derived from no.2025.pCCLsin.PPT.pA.CTE.4 × -scrT.eGFP.mCMV.hPGK.NGFR.pre (kindly provided by L. Naldini, San Raffaele Scientific Institute, Milan, Italy). This vector was altered to express the BlastR gene downstream of the PGK promoter and the PTPRC genes downstream of the EF1A promoter (RP-138). To prevent targeting of the hCD45 isoforms by the anti-hCD45 sgRNA present in the U937-hC5aR1-SpCas9 CD45-knockout cells, silent mutations were engineered in the sgRNA target sequence in the coding region of both hCD45-R0 and hCD45-RABC. The ABC region of mCD45-RABC (NM_001111316) was ordered as a human codon-optimized sequence as a gBlock (Integrated DNA Technologies) and cloned in between the codons that code for amino acids 30 and 31 in the mCD45-R0 vector (described above) by overlapping extension PCR. Human and mouse CD45-R0 and CD45-RABC were transduced in U937-hC5aR1-SpCas9 CD45-knockout cells and were selected to purity by blasticidin selection.
hC5aR1KI bone-marrow-derived macrophages were immortalized, as previously described, by transducing primary bone marrow cells with J2 virus at day 3 post-isolation47.
Genome-wide CRISPR–Cas9 library screen with PVL in U937-hC5aR1 cells
Approximately 600 × 106 U937-hC5aR1-SpCas9 cells were transduced with the genome-wide sgRNA expression library by spin infection at 1,000g for 90 min at 33 °C in the presence of 4 μg ml−1 polybrene. Approximately 15% of the cells were transduced, resulting in a ~350-fold overrepresentation of the library. Transduced cells were selected to purity with 2.0 μg ml−1 puromycin initiated at 2 days post-transduction. Twelve days post-transduction, 2 × 108 cells were incubated with 31 nM PVL for 30 min at 37 °C, which resulted in depletion of >99.5% of the cells. Cells were washed to remove the toxin and allowed to recover in complete RPMI for 15 days to enrich for viable cells. In parallel, an untreated control sample of sgRNA-tranduced cells was maintained at high complexity (>2 × 108 cells) throughout this time period. Genomic DNA was isolated from 5 × 107 outgrowing cells and 1 × 108 untreated control cells by standard phenol–chloroform extraction. sgRNA inserts were subsequently PCR amplified for 16 cycles with primers 5′- GGCTTGGATTTCTATAACTTCGTATAGCA-3′ and 5′-CGGGGACTGTGGGCGATGTG-3′ using the Titanium Taq PCR kit (Clontech). The PCR products were pooled and amplified using primers containing Illumina adapter sequences and a unique index for 15 cycles using the forward primer 5′-AATGATACGGCGACCACCGAGATCCACAAAAGG-AAACTCACCCTAAC-3′ and the reverse primer 5′-CAAGCAGAAGACGGCATACGAGAT-AGTCTCGTGACTGGAGTTCAGACGTG-3′ (RO-1479) for the treated sample or 5′- CAAGCAGAAGACGGCATACGAGATTGTCAGGTGACTGGAGTTCAGACGTG-3′ (RO-1478) for the untreated control sample. The 344-base pair PCR products were purified from 2% agarose gel using a PCR purification kit (Qiagen), and the DNA yield and quality were assessed by Bioanalyzer and Qubit analysis. PCR products were subsequently pooled in equimolar ratios and subjected to deep sequencing using the Illumina NextSeq500 platform. Sequences were aligned to the sgRNA library by using Bowtie2 (PMID: 22388286) and the counts per sgRNA were calculated. We used the MaGeCk package (PMID: 25476604) (available from https://sourceforge.net/projects/mageck/) as a computational tool to identify genes that were significantly enriched in the screens by comparing sgRNA read counts of control cells to PVL-incubated cells. The genes, including the significance for enrichment as calculated by the MaGeCK ‘positive enrichment score’, are presented in Supplementary Table 1.
Complementation of hC5aR1KI murine macrophages
Primary hC5aR1KI bone marrow cells were transduced with lentiviruses at day 3 post-isolation. Lentiviruses were added onto hC5aR1KI bone marrow cells (2 × 106 cells per well of a 6-well plate) at a multiplicity of infection of 100:1, as determined by titration on 293T human embryonic kidney cells. Transduction was promoted by a 2,000g spinoculation during 2 h at room temperature. After 6 h of incubation at 37 °C, 2 ml of fresh medium were added. Cells were carefully washed the next day and further incubated for 3 days before analysis. Adherent macrophages were collected by washing the plate once with PBS and incubating it with Versene (Thermo Fisher Scientific) for 5 min at room temperature.
Isolation of human and murine leukocytes
Cells were isolated as described elsewhere14,16. Briefly, the bone marrow of mice was harvested and immune cells were collected. Human leukocytes were isolated by Ficoll/Histopaque centrifugation. When required, hypotonic lysis of residual erythrocytes was performed by a 30-s incubation in sterile water followed by the addition of a large volume of PBS. All in vitro experiments with cells were performed with RPMI (Invitrogen) supplemented with 0.05% HSA (Sanquin) unless specified otherwise.
Bacterial strains and culture conditions
S. aureus strains used for this study are as follows: S. aureus strain USA300 clone SF8300 is a minimally passed representative PVL-positive community-associated MRSA isolate from the United States27, of which the isogenic ∆hlgACB mutant and the complemented strains were described elsewhere16,48. The S. aureus strain ST80 is a European community-associated MRSA isolate49, of which the isogenic ∆lukSF-PV mutant was previously reported on48. The ST80 isogenic ∆hlgACB mutant and the ∆lukSF-PV ∆hlgACB double mutant strains were generated as described elsewhere16. All strains were cultured in brain heart infusion. For in vivo experiments, mid-exponential subcultures were washed extensively in PBS.
Recombinant protein production and purification
LukS-PV, LukF-PV, HlgC and HlgB used for this study were cloned and expressed as described elsewhere14,16,48. Fluorescein isothiocyanate (FITC)-labelled LukS-PV and LukF-PV, used for binding studies in murine and human neutrophils, were previously described50,51. For binding studies in U937 cells, random Alexa Fluor-647-labelled LukF-PV was used. Alexa Fluor-647 NHS ester (also known as succinimidyl ester) was acquired from Molecular Probes/Thermo Fisher Scientific. The reactive dyes were dissolved in dimethylsulfoxide to a concentration of 10 mg ml−1. An amount of 100 µg purified toxin was labelled with 10 µg reactive dye in a total volume of 100 µl PBS containing 0.1 M sodium carbonate pH 8.4 for 90 min at room temperature protected from light. Subsequently, the labelled protein was separated from free non-reacted dye using a protein desalting spin column. Protein concentration was determined with Nanodrop One (Thermo Fisher), and labelling was verified by SDS–PAGE and fluorescence imaging.
In vivo infection
hC5aR1KI or C57BL6/N (WT, Charles River) mice, aged between 6 and 12 weeks and matched for weight and sex, were injected intraperitoneally with 5 × 107 c.f.u. Mice were killed 24-h post-infection and the relevant compartments were harvested as described elsewhere16. Briefly, peripheral blood, peritoneal lavage fluid and homogenized organs were serially diluted and plated on Tryptic Soy Agar for incubation at 37 °C overnight, followed by c.f.u. counting. For the skin infection model, mice were shaved on their back and hair removal cream was applied for 1 min before being extensively washed. Three days later, mice were injected subcutaneously on both sides of their back with 2 × 106 c.f.u. in 100 μl PBS. At day 5 post-inoculation, mice were euthanized and the skin lesion and the underneath tissue were collected in 1 ml PBS. Tissue was grinded using the Precellys homogenizer (Bertin Instruments). The homogenate was diluted and plated on blood agar plates (BioMérieux) using the easySpiral Dilute (Interscience). The c.f.u. were automatically counted in a blinded manner using the Scan300 counter with a manual correction to remove non-haemolytic c.f.u. Sample sizes were determined following previous16,18 and preliminary experiments showing sufficient power.
Calcium mobilization assays
Murine neutrophils or immortalized murine hC5aR1KI bone-marrow-derived macrophages were loaded with Fluo-4-AM (Molecular Probes/Thermo Fisher) for 30 min at 37 °C in the dark following the manufacturer’s instructions. Cells were then washed twice in Hanks’ Balanced Salt Solution (HBSS) supplemented with 2.5 mM probenicid, 0.1% (w/v) BSA and HEPES 25 mM. Cells were incubated for 5 min at 37 °C before FACS analysis (Accuri C6). Cells were analysed for 20 s to obtain the baseline fluorescence before the addition of murine C5a (Prospec) or human C5a (Prospec or Peprotech), after which acquisition was further continued.
U937 cells were loaded with 2 mM Fluo-3AM (Molecular Probes/Thermo Fisher) in RPMI/HSA for 20 min at room temperature under constant agitation, washed with buffer and suspended to 106 cells per ml in RPMI/HSA. Each sample of cells was first measured for approximately 10 s to determine the basal fluorescence level. Next, a titrated range of C5a (Sigma) was added and rapidly placed back in the sample holder to continue the measurement. Cells were analysed by flow cytometry, gated on forward and side scatter to exclude dead cells and debris.
Cell permeability assays
All human and murine primary cells were pre-stained before toxin treatment. Human peripheral blood mononuclear cells were stained for CD14 expression (clone M5E2, BD Biosciences). Bone-marrow-derived murine cells were stained in the presence of a Fcγ-receptor block (TruStain fcX, BioLegend) with Ly6G (clone 1A8, BD) and Ly6C (clone AL-21, BD) antibodies for 20 min at 4 °C. Cells were washed once in PBS 3% FCS and exposed to recombinant proteins in 100 μl of PBS with 3% FCS at room temperature. Cells were subsequently analysed by flow cytometry using propidium iodide at 10 μg ml−1. For competition experiments, human neutrophils were pre-incubated with 10 μg ml−1 mouse anti-hCD45 (clone 4B2, obtained from the ATCC) or isotype control for 15 min at room temperature. Thirty minutes after subsequent addition of the toxin, cells were analysed by flow cytometry using intracellular staining by 1 μg ml−1 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes/Thermo Fisher).
U937 cells were exposed to canonical or non-canonical recombinant proteins and measured for 30 min at 37 °C in a monochromator-based microplate reader (FLUOstar Omega, BMG Labtech) using 2.5 μg ml−1 DAPI. As PVL and HlgCB are two-component toxins, equimolar concentrations of polyhistidine-tagged LukS-PV, LukF-PV, HlgC and HlgB were used. Pore formation was defined as a collective positive DAPI signal and the area under the curve was calculated for comparison.
Binding assays
Binding of proteins was measured by incubating cells with directly labelled proteins for 30 min at room temperature. After washing, binding was detected by flow cytometry. For competition experiments, human neutrophils were pre-incubated with 10 μg ml−1 of different mouse anti-hCD45 monoclonal antibodies (clone 4B2, obtained from the ATCC; clone 2D1, BD Biosciences; clone F10-89-4, Bio-Rad) or isotype control for 15 min at room temperature.
Lysis assays
U937 cells were exposed to PVL for 1 h followed by a short spin down. Supernatants were collected and the presence of lactate dehydrogenase was detected using the Cytotox-96 Non-Radioactive Cytotoxicity Assay (Promega), following the manufacturer’s instruction. Optical density was measured at 490 nm on a microplate reader (Bio-Rad). Results were normalized to the manufacturer’s lysis solution (9% v/v Triton X-100).
Determination of receptor expression levels
Receptor expression levels were determined as described elsewhere16. For quantification, single-cell suspensions were stained with mouse anti-hC5aR1 (clone S5/1, AbD Serotec), mouse anti-hCD45 (clone 2D1, BD Biosciences) or isotype controls, followed by FITC-conjugated goat anti-mouse antibody (Dako). Antibody binding was quantified by calibration to the defined antibody-binding capacity unites, using QIFIkit (Dako). For validation of receptor expression after complementation, cells were incubated with phycoerythrin (PE)-labelled mouse anti-hC5aR1 (clone S5/1, AbD Serotec), allophycocyanin (APC)-labelled mouse anti-hCD45 (clone 2D1, BD Biosciences) or Cy-Chrome-labelled rat anti-mouse CD45 (clone 30-F11, BD Biosciences). Samples were subsequently measured using flow cytometry.
SPR analyses
The recombinant human and mouse CD45 isoform R0 protein (R&D Systems) was purchased and immobilized onto a Series S CM5 chip using a Biacore S200 system (GE Healthcare) using methods previously described13. Whole U937 cells expressing the human or murine CD45 isoform R0 (U937-hC5aR1-SpCas9 cells transduced with a sgRNA targeting PTPRC (hC5aR1+ CD45−) and subsequently transduced with a plasmid containing either the hCD45 isoform R0 (phCD45-R0) or the murine CD45 isoform R0 (pmCD45-R0)), were fixed using 4% formaldehyde and washed three times with PBS and resuspended at 107 cells per ml. Cells were immobilized onto Series S C1 sensor chips using the C1 wizard methodology on the Biacore T200 control system as previously described52. Cells were flowed at 5 µl per min for 900 s to load the chip to saturation. Whole U937 cells not expressing CD45 (U937-hC5aR1-SpCas9 cells transduced with a sgRNA targeting PTPRC (hC5aR1+ CD45−)) were loaded using the same methodology onto flow cell one to allow for double reference subtraction. For both the whole-cell and the recombinant protein assays, LukF-PV was flowed over the immobilized CD45 in two concentration ranges (1.6 nM to 1.0 µM and 16.0 nM to 10.0 µM). Data for mouse CD45 were obtained from only the higher concentration series.
Statistical analyses
Calculations of the area under the curves, calculations of the EC50 using linear regression analyses and all statistical analyses were performed using Prism 7.0 (GraphPad Software). Flow cytometric analyses were performed with FlowJo (Tree Star Software). Significance was calculated using analysis of variance (ANOVA) and Student’s t-test with post-test correction for multiple comparison, where appropriate. Exact P values are provided in Supplementary Table 3.
Ethics statement
Human leukocytes were isolated after informed consent was obtained from all subjects in accordance with the Declaration of Helsinki. In the Netherlands, approval was obtained from the medical ethics committee of the UMC Utrecht, the Netherlands (protocol METC 07-125/C). In France, blood was obtained from healthy donors from the Etablissement Français du Sang Auvergne Rhône Alpes, France, under the convention EFS 16-2066. Ethical approval was obtained from the Comité de Protection des Personnes Sud Méditerranée I. All experiments involving animals were reviewed and approved by the animal ethics committees of Lyon, France (CECCAPP, protocol number ENS2012_033, ENS2013_033, ENS2014_035 and ENS2017_022).
Reporting Summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and the supplementary information, or from the corresponding authors upon request. Relevant accession codes are provided within the specific Methods sections.
Change history
03 September 2018
In the version of this Article originally published, the name of author Robert Jan Lebbink was coded wrongly, resulting in it being incorrect when exported to citation databases. This has now been corrected, though no visible changes will be apparent.
References
Thwaites, G. E. et al. Clinical management of Staphylococcus aureus bacteraemia. Lancet Infect. Dis. 11, 208–222 (2011).
Deleo, F. R., Otto, M., Kreiswirth, B. N. & Chambers, H. F. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375, 1557–1568 (2010).
Fowler, V. G. et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 309, 1368–1378 (2013).
Spaan, A. N., Surewaard, B. G., Nijland, R. & van Strijp, J. A. Neutrophils versus Staphylococcus aureus: a biological tug of war. Annu. Rev. Microbiol. 67, 629–650 (2013).
Alonzo, F. III. & Torres, V. J. The bicomponent pore-forming leucocidins of Staphylococcus aureus. Microbiol. Mol. Biol. Rev. 78, 199–230 (2014).
Vandenesch, F., Lina, G. & Henry, T. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: a redundant arsenal of membrane-damaging virulence factors? Front. Cell Infect. Microbiol. 2, 12 (2012).
Spaan, A. N., van Strijp, J. A. G. & Torres, V. J. Leukocidins: staphylococcal bi-component pore-forming toxins find their receptors. Nat. Rev. Microbiol. 15, 435–447 2017).
Peraro, M. D. & van der Goot, F. G. Pore-forming toxins: ancient, but never really out of fashion. Nat. Rev. Microbiol. 14, 77–92 (2016).
Ferreras, M. et al. The interaction of Staphylococcus aureus bi-component γ-hemolysins and leucocidins with cells and lipid membranes. Biochim. Biophys. Acta 1414, 108–126 (1998).
Dalla Serra, M. et al. Staphylococcus aureus bicomponent γ-hemolysins, HlgA, HlgB, and HlgC, can form mixed pores containing all components. J. Chem. Inf. Model. 45, 1539–1545 (2005).
Konig, B., Prevost, G. & Konig, W. Composition of staphylococcal bi-component toxins determines pathophysiological reactions. J. Med. Microbiol. 46, 479–485 (1997).
Yoong, P. & Torres, V. J. Counter inhibition between leukotoxins attenuates Staphylococcus aureus virulence. Nat. Commun. 6, 8125 (2015).
DuMont, A. L. et al. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc. Natl Acad. Sci. USA 110, 10794–10799 (2013).
Spaan, A. N. et al. The staphylococcal toxin Panton–Valentine leukocidin targets human C5a receptors. Cell Host Microbe 13, 584–594 2013).
Reyes-Robles, T. et al. Staphylococcus aureus leukotoxin ED targets the chemokine receptors CXCR1 and CXCR2 to kill leukocytes and promote infection. Cell Host Microbe 14, 453–459 (2013).
Spaan, A. N. et al. The staphylococcal toxins γ-haemolysin AB and CB differentially target phagocytes by employing specific chemokine receptors. Nat. Commun. 5, 5438 (2014).
Koop, G. et al. Identification of LukPQ, a novel, equid-adapted leukocidin of Staphylococcus aureus. Sci. Rep. 7, 40660 (2017).
Spaan, A. N. et al. Staphylococcus aureus targets the Duffy antigen receptor for chemokines (DARC) to lyse erythrocytes. Cell Host Microbe 18, 363–370 2015).
Vrieling, M. et al. Bovine Staphylococcus aureus secretes the leukocidin LukMF’ to kill migrating neutrophils through CCR1. mBio 6, e00335 (2015).
Alonzo, F. III. et al. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature 493, 51–55 (2013).
Spaan, A. N. et al. Differential interaction of the staphylococcal toxins Panton–Valentine leukocidin and γ-hemolysin CB with human C5a receptors. J. Immunol. 195, 1034–1043 (2015).
Tawk, M. Y. et al. Internalization of staphylococcal leukotoxins that bind and divert the C5a receptor is required for intracellular Ca2+ mobilization by human neutrophils. Cell. Microbiol. 17, 1241–1257 (2015).
Labandeira-Rey, M. et al. Staphylococcus aureus Panton–Valentine leukocidin causes necrotizing pneumonia. Science 315, 1130–1133 (2007).
Bubeck Wardenburg, J., Bae, T., Otto, M., Deleo, F. R. & Schneewind, O. Poring over pores: α-hemolysin and Panton–Valentine leukocidin in Staphylococcus aureus pneumonia. Nat. Med. 13, 1405–1406 (2007).
Diep, B. A. et al. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton–Valentine leukocidin-induced lung inflammation and injury. Proc. Natl Acad. Sci. USA 107, 5587–5592 (2010).
Cremieux, A. C. et al. Panton–Valentine leukocidin enhances the severity of community-associated methicillin-resistant Staphylococcus aureus rabbit osteomyelitis. PLoS ONE 4, e7204 (2009).
Diep, B. A. et al. Contribution of Panton–Valentine leukocidin in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. PLoS ONE 3, e3198 (2008).
Lipinska, U. et al. Panton–Valentine leukocidin does play a role in the early stage of Staphylococcus aureus skin infections: a rabbit model. PLoS ONE 6, e22864 (2011).
Kobayashi, S. D. et al. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J. Infect. Dis. 204, 937–941 (2011).
Shallcross, L. J., Fragaszy, E., Johnson, A. M., & Hayward, A. C. The role of the Panton-Valentine leucocidin toxin in staphylococcal disease: a systematic review and meta-analysis. Lancet Infect. Dis. 13, 43–54 (2013).
Tseng, C. W. et al. Increased susceptibility of humanized NSG mice to Panton–Valentine leukocidin and Staphylococcus aureus skin infection. PLoS Pathog. 11, e1005292 (2015).
Prince, A., Wang, H., Kitur, K. & Parker, D. Humanized mice exhibit increased susceptibility to Staphylococcus aureus pneumonia. J. Infect. Dis. 215, 1386–1395 (2017).
Monk, P. N., Scola, A. M., Madala, P. & Fairlie, D. P. Function, structure and therapeutic potential of complement C5a receptors. Br. J. Pharmacol. 152, 429–448 (2007).
Otter, J. A. & French, G. L. Molecular epidemiology of community-associated meticillin-resistant Staphylococcus aureus in Europe. Lancet Infect. Dis. 10, 227–239 (2010).
Hermiston, M. L., Xu, Z. & Weiss, A. CD45: a critical regulator of signaling thresholds in immune cells. Annu. Rev. Immunol. 21, 107–137 (2003).
Lowy, F. D. Staphylococcus aureus infections. N. Engl. J. Med. 339, 520–532 1998).
Gillet, Y. et al. Association between Staphylococcus aureus strains carrying gene for Panton–Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359, 753–759 (2002).
Alcais, A., Abel, L. & Casanova, J. L. Human genetics of infectious diseases: between proof of principle and paradigm. J. Clin. Invest. 119, 2506–2514 (2009).
Casanova, J. L. Severe infectious diseases of childhood as monogenic inborn errors of immunity. Proc. Natl Acad. Sci. USA 112, E7128–E7137 (2015).
Kung, C. et al. Mutations in the tyrosine phosphatase CD45 gene in a child with severe combined immunodeficiency disease. Nat. Med. 6, 343–345 (2000).
Tchilian, E. Z. et al. A deletion in the gene encoding the CD45 antigen in a patient with SCID. J. Immunol. 166, 1308–1313 (2001).
Tchilian, E. Z. et al. The exon A (C77G) mutation is a common cause of abnormal CD45 splicing in humans. J. Immunol. 166, 6144–6148 (2001).
Lee, H. et al. Human C5aR knock-in mice facilitate the production and assessment of anti-inflammatory monoclonal antibodies. Nat. Biotechnol. 24, 1279–1284(2006).
Birling, M. C., Dierich, A., Jacquot, S., Herault, Y. & Pavlovic, G. Highly-efficient, fluorescent, locus directed cre and FlpO deleter mice on a pure C57BL/6N genetic background. Genesis 50, 482–489 (2012).
van de Weijer, M. L. et al. A high-coverage shRNA screen identifies TMEM129 as an E3 ligase involved in ER-associated protein degradation. Nat. Commun. 5, 3832 (2014).
van Diemen, F. R. et al. CRISPR/Cas9-mediated genome editing of herpesviruses limits productive and latent infections. PLoS Pathog. 12, e1005701 (2016).
Blasi, E. et al. Selective immortalization of murine macrophages from fresh bone marrow by a raf/myc recombinant murine retrovirus. Nature 318, 667–670 (1985).
Perret, M. et al. Cross-talk between S. aureus leukocidins-intoxicated macrophages and lung epithelial cells triggers chemokine secretion in an inflammasome-dependent manner. Cell. Microbiol. 14, 1019–1036 (2012).
Garnier, F. et al. Pneumonia and new methicillin-resistant Staphylococcus aureus clone. Emerg. Infect. Dis. 12, 498–500 (2006).
Gauduchon, V., Werner, S., Prevost, G., Monteil, H. & Colin, D. A. Flow cytometric determination of Panton–Valentine leucocidin S component binding. Infect. Immun. 69, 2390–2395 (2001).
Meyer, F., Girardot, R., Piemont, Y., Prevost, G. & Colin, D. A. Analysis of the specificity of Panton–Valentine leucocidin and gamma-hemolysin F component binding. Infect. Immun. 77, 266–273 (2009).
Mubaiwa, T. D. et al. The glycointeractome of serogroup B Neisseria meningitidis strain MC58. Sci. Rep. 7, 5693 (2017).
Acknowledgements
We thank L. Scheepmaker and P. C. Aerts (University Medical Center Utrecht, Utrecht, The Netherlands) for technical support, C. Badiou (CIRI Inserm U111, Lyon, France) and G. Prevost (Strasbourg University, Strasbourg, France) for providing toxins and Y. Benito (CIRI Inserm U111) for providing S. aureus strains; PBES (J. F. Henry), lentivectors production facility (C. Costa) and flow cytometry platforms of SFR Biosciences Gerland—Lyon Sud. This work is supported by grants from the Agence Nationale de la Recherche (ANR-12-BSV3-0003 to F.V. and T.H.), the Finovi foundation (to T.H.), the Australian National Health and Medical Research Council (1071659 and 1138466 to M.P.J. and 1108124 to M.P.J. and C.J.D.) and the Dutch Cancer Society (UU 2012-5667 to R.J.L.). This work was performed within the framework of LABEX ECOFECT (ANR-11−LABX-0048) of Université de Lyon and ANR ‘Investissements d’Avenir’ (ANR-11-IDEX-0007).
Author information
Authors and Affiliations
Contributions
A.T.T., M.V.G., B.W.B., F.V., T.H. and A.N.S. conceptualized the study. A.T.T., M.V.G., R.J.L., P.-J.A.H., K.P.M.V.K., C.J.D., M.P.J., T.H. and A.N.S. designed the methodology. A.T.T., M.V.G., P.A., A.M., J.P.J., C.J.C.D.H., E.B., C.J.D., T.H. and A.N.S. conducted the investigation. E.K., C.J.C.D.H., M.B., C.J.D., M.P.J. and M.T.M. provided resources. G.L., F.V., J.A.G.V.S., P.-J.A.H. and T.H. provided funding. A.T.T., M.V.G., T.H. and A.N.S. wrote the paper. R.J.L., P.-J.A.H., T.H. and A.N.S. provided supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–6.
Supplementary Table 1
Screening results for resistance to PVL toxicity.
Supplementary Table 2
Selected sgRNAs for a genome-wide library.
Supplementary Table 3
Exact P values.
Rights and permissions
About this article
Cite this article
Tromp, A.T., Van Gent, M., Abrial, P. et al. Human CD45 is an F-component-specific receptor for the staphylococcal toxin Panton–Valentine leukocidin. Nat Microbiol 3, 708–717 (2018). https://doi.org/10.1038/s41564-018-0159-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-018-0159-x
This article is cited by
-
Staphylococcus aureus host interactions and adaptation
Nature Reviews Microbiology (2023)
-
Neutralization of the Staphylococcus aureus Panton-Valentine leukocidin by African and Caucasian sera
BMC Microbiology (2022)
-
Cross-species RNA-seq for deciphering host–microbe interactions
Nature Reviews Genetics (2021)
-
Genetic variation of staphylococcal LukAB toxin determines receptor tropism
Nature Microbiology (2021)
-
A common approach to toxin specificity
Nature Microbiology (2018)