Abstract
Intrinsic signals and external cues from the environment drive cell fate decisions. In budding yeast, the decision to enter meiosis is controlled by nutrient and mating-type signals that regulate expression of the master transcription factor for meiotic entry, IME1. How nutrient signals control IME1 expression remains poorly understood. Here, we show that IME1 transcription is regulated by multiple sequence-specific transcription factors (TFs) that mediate association of Tup1-Cyc8 co-repressor to its promoter. We find that at least eight TFs bind the IME1 promoter when nutrients are ample. Remarkably, association of these TFs is highly regulated by different nutrient cues. Mutant cells lacking three TFs (Sok2/Phd1/Yap6) displayed reduced Tup1-Cyc8 association, increased IME1 expression, and earlier onset of meiosis. Our data demonstrate that the promoter of a master regulator is primed for rapid activation while repression by multiple TFs mediating Tup1-Cyc8 recruitment dictates the fate decision to enter meiosis.
Similar content being viewed by others
Introduction
The choice of whether to differentiate into another cell type is directed by multiple cell intrinsic and extrinsic environmental factors. These cues signal to master regulatory genes, which in turn control the initiation of cell differentiation programmes. As a result, multiple signals are transformed into a binary decision: whether to undergo cell differentiation or not.
Budding yeast cells undergo a differentiation programme called gametogenesis or sporulation during which a diploid cell gives rise to four haploid spores. Yeast gametogenesis is characterised by one round of DNA replication and recombination, two consecutive chromatin segregation events called meiosis followed by spore formation1. As a result, an ascus with four haploid spores is produced. In yeast, the decision to enter meiosis is controlled by a master regulatory transcription factor (TF) named inducer of meiosis 1, IME12,3. In the absence of IME1, cells cannot enter meiosis and produce gametes. Thus, understanding how IME1 is regulated is key to understanding how the decision to enter meiosis is made.
Multiple transcriptional control mechanisms regulate IME1 expression. The IME1 gene has an unusually large promoter for the yeast genome (over 2.4 kb) that integrates multiple signals4. Nutrient and mating type signals ensure that IME1 is only expressed in the appropriate nutrient environment and in the correct cell type. Only cells harbouring opposite mating-type loci (MATa and MATα) can induce IME15,6. In cells with a single mating type (MATa or MATα), the TF Rme1 is expressed and induces transcription of the long noncoding RNA (lncRNA) IRT1, which in turn transcribes through the IME1 promoter and represses IME1 expression7. In MATa/α diploid cells, a second lncRNA upstream of IRT1 named IRT2 interferes with IRT1 transcription, thus forming a positive feedback loop by which Ime1 promotes its own expression8.
In order to induce IME1 transcription, diploid cells must be starved for glucose and nitrogen, and cells need to be respiring4,9. The glucose and nitrogen signals integrate at the IME1 promoter. Distinct sequence element mediates IME1 repression by glucose signalling, while other parts of the promoter respond to nitrogen availability10. Notably, the TF Sok2 controls IME1 promoter activity via the glucose responding element11. Multiple other TFs contribute to regulation of IME1 transcription12,13,14. Moreover, over 50 TFs have a conserved consensus site in the IME1 promoter and about 30 TFs may directly or indirectly control IME1 transcription12.
The nutrient control of IME1 expression is mediated by multiple signalling pathways, including PKA, TOR complex 1 (TORC1), AMP-activated protein kinase (AMPK) and mitogen-activated protein kinase (MAPK)15,16,17. Inhibiting two signalling pathways, PKA and TORC1, is sufficient to induce IME1 expression in cells exposed to a nutrient rich environment where IME1 expression is normally repressed16. Thus, PKA and TORC1 signalling is essential for controlling IME1 expression and hence the decision to enter meiosis (Fig. 1a). Previously, we showed that Tup1 represses the IME1 promoter under nutrient rich conditions16. Tup1 is part of the Tup1–Cyc8 co-repressor complex, which is involved in repression of more than 300 gene promoters in yeast18,19,20. During starvation, when PKA and TORC1 activity is reduced, Tup1 dissociates from the IME1 promoter and IME1 transcription is concomitantly induced.16. How Tup1–Cyc8 association with the IME1 promoter is regulated may be key to how IME1 promoter activity is controlled.
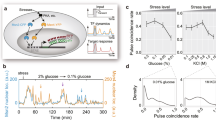
a Schematic representation of nutrient control of the IME1 promoter. b Effects of truncations in the IME1 promoter on meiosis. Diploid cells with one copy of IME1 deleted (control, FW4128) and harbouring promoter truncations at the WT IME1 copy (pIME1(−1600-2315Δ), FW3946; pIME1(−1400-2315Δ), FW3947; pIME1(−1200-2315Δ), FW3948; pIME1(−1000-2315Δ), FW3949; pIME1(−800-2315Δ), FW3950; pIME1(−600-2315Δ), FW3951) were induced to enter meiosis. Samples were taken at the indicated time points, fixed and DAPI masses were counted (n = 200 cells per sample) to determine meiosis (MI + MII). c Cyc8 binding to IME1 promoter determined by chromatin immunoprecipitation (ChIP). Cyc8 bound DNA fragments were isolated and quantified by qPCR using eight different primer pairs from cells expressing V5 epitope-tagged Cyc8 (FW6381). The signals were normalised over HMR. Mean of n = 2 is shown. d Similar as c except that the binding of Cyc8 (Cyc8-V5, FW6381) and Pog1 (Pog1-V5, FW968) is shown alongside untagged wild-type (control, FW1511) cells. The region around 1000 bp upstream of the IME1 AUG was analysed. Mean of n = 3 (control is n = 2) and SEM are shown. e Tup1 and Cyc8 depletion detected by western blot. Diploid cells harbouring Tup1 or Cyc8 fused to auxin induced degron (AID) (TUP1-AID, FW5057; CYC8-AID, FW6371) were treated with IAA or DMSO. As a control, Hxk1 levels were determined. *Tup1-AID cleavage product with no detectable function. A representative AID depletion experiment is shown (n > 3). f Similar as e except that IME1 mRNA expression was determined by RT-qPCR. Mean of n = 2 is shown. g Tup1 protein levels (TUP1-AID, FW5057) detected by western blotting as described in (e). *Tup1-AID cleavage product with no detectable function. A representative AID depletion experiment is shown (n > 3). h Distribution of IME1 transcript levels in single cells as described in g determined by single molecule RNA fluorescence in situ hybridisation (smFISH). Cells were hybridised with IME1 (AF594) and ACT1 (Cy5) probes. Cells positive for ACT1 were used for the analyses. Data of n ≥ 50 cells and mean (black line) are displayed. Unpaired parametric two-tailed Welch’s t test with 95% confidence was used. Non-significant (ns) and p values (** = ≤0.01, *** = ≤0.001) are indicated. i Same as h with data binned by expression levels.
Here, we report how the Tup1–Cyc8 co-repressor complex regulates IME1 transcription. In short, we found that regulated repression by multiple sequence specific TFs mediating the association of Tup1–Cyc8 with the IME1 promoter is the means by which IME1 transcription is controlled. Our data indicate that nutrient cues regulate the association of Tup1–Cyc8 interacting TFs with the IME1 promoter, which is key to regulating IME1 expression. Our work provides a framework for understanding how nutrient signals integrate at a cell fate promoter and control a critical developmental decision in yeast.
Results
Tup1–Cyc8 prevents activation of the IME1 promoter
Previously, we reported that Tup1 associates between 800 and 1400 base pairs (bp) upstream of the IME1 translation start site16. If the region of the IME1 promoter where Tup1 binds is also important for IME1 activation, then deleting that part of the promoter should affect the onset of meiosis. We generated six truncation mutants with a 200 bp interval in the IME1 promoter and examined the ability of these mutants to undergo meiosis (Fig. 1b). The largest truncation mutant that underwent meiosis with comparable kinetics as wild-type cells harboured 1400 bp of the IME1 promoter (pIME1(−1400-2315Δ)) indicating that this region harbours the regulatory elements required for complete activation of the IME1 promoter (Fig. 1b). In addition, we found that meiosis in pIME1(−800-2315Δ) was completely impaired, whereas pIME1(−1200-2315Δ) had a much milder effect on meiosis. The result suggests that a region between −800 and −1200 bp harbours regulatory elements essential for IME1 promoter function. Analysis of additional mutants revealed that the region between −800 and −850 bp contains regulatory elements important for IME1 activation (Supplementary Fig. 1). In conclusion, the region essential for Tup1 binding to the IME1 promoter is also required for transcription of IME1.
Tup1 forms a complex with Cyc819,20. The Tup1–Cyc8 co-repressor complex is conserved and plays various roles in regulating gene transcription21. Like Tup1, Cyc8 has also been implicated in regulation of IME1 expression22. To investigate how Cyc8 regulates IME1 expression, we determined Cyc8 binding with the IME1 promoter under nutrient rich conditions. We found that Cyc8 peaked in the same region as Tup1 in the IME1 promoter (Figs. 1c and 1d). These data indicate that Tup1–Cyc8 regulates the IME1 promoter.
Various models for Tup1–Cyc8 mediated repression of target gene promoters have been described21,23,24. It has been proposed that Tup1–Cyc8 primarily regulates promoters by masking TFs from recruiting co-activators25. If Tup1–Cyc8 represses the IME1 promoter by shielding co-activators, then transcriptional activators should be present at the promoter under repressive conditions. To test this, we measured the association of a known transcriptional activator of IME1, Pog17. We found that Pog1 is indeed enriched at the IME1 promoter under repressive conditions (Fig. 1d). To further examine whether transcriptional activators are readily present at the IME1 promoter, we measured IME1 expression after depletion of Tup1 or Cyc8. We reasoned that if Tup1–Cyc8 represses the IME1 promoter by restraining activating TFs, then depletion of Tup1 or Cyc8 should concomitantly allow activators present to induce IME1 transcription. We used the auxin inducible degron (AID) system (TUP1-AID and CYC8-AID), and treated cells with indole-3-acetic acid (IAA) to achieve rapid protein depletion in cells26. Rapid and sustained depletion of Tup1 and Cyc8 was achieved within 30 min after IAA treatment (Fig. 1e). Strikingly, IME1 transcript levels strongly increased concurrently, and were comparable to or even higher than those in wild-type cells entering meiosis when IME1 expression is typically at its peak (Fig. 1f). These data show that Tup1–Cyc8 represses the IME1 promoter under nutrient rich conditions and suggest that the default state of the IME1 promoter is active.
Although Pog1 is already bound at the IME1 promoter in repressive conditions, it is possible that other transcriptional activators associate with the IME1 promoter after Tup1–Cyc8 dissociates. This may result in a delay between Tup1–Cyc8 depletion and activation of IME1 transcription. We therefore decided to monitor IME1 expression by single molecule RNA fluorescence in situ hybridisation (smFISH)27. We found that as soon as Tup1 was depleted, IME1 transcripts were detected in single cells (Fig. 1g–i, Supplementary Fig. 2a). An increase in IME1 transcripts was detected as early as 10 min after IAA treatment when Tup1 was partially depleted (Fig. 1g). After 15 min, 5.3 IME1 transcripts were detected per cell on average, and 12% of the cells (TUP1-AID + IAA) had more than 10 IME1 transcripts compared to 2% in control cells (Fig. 1i). After 30 min, more than 55% of cells expressed more than 10 IME1 transcripts (Fig. 1i). The AID-tag fused to Tup1 had some effect on IME1 expression in the absence of IAA as IME1 transcript levels were increased by five-fold in TUP1-AID compared to wild-type cells (Fig. 1h). Our analysis indicates that there is little temporal delay between Tup1–Cyc8 depletion and IME1 transcript accumulation suggesting that transcriptional activators are readily available for activating the IME1 promoter.
Multiple Tup1–Cyc8 interacting TFs bind to the IME1 promoter
The Tup1–Cyc8 complex interacts with DNA sequence specific TFs to form co-repressor complexes at promoters28,29,30,31,32,33,34. These TFs facilitate Tup1–Cyc8 association with promoters and mediate repression of target genes. To investigate which TFs recruit Tup1–Cyc8 to the IME1 promoter and how they control IME1 transcription, we assembled a list of TFs previously reported to interact with Tup1 or Cyc8. In addition, we examined the region of the IME1 promoter (−600 to −1200 bp) where Tup1–Cyc8 binds and scanned for sequence motifs among TFs known to interact with Tup1–Cyc8 (Fig. 2a, Supplementary Fig. 3). We identified 13 candidate TFs that were known or implicated to interact with Tup1–Cyc8 and have binding sites in the IME1 promoter (Fig. 2a, Supplementary Fig. 3). We also included the TF Sok2 in our analyses because it has been proposed to interact with Tup1–Cyc8 and Sok2 is known to directly repress IME1 transcription11,28. After the curation of the list of TFs, we measured their binding under nutrient rich conditions. Eight TFs displayed enrichment (three-fold or more over background) at the IME1 promoter (Fig. 2b). As expected, a known regulator of the IME1 promoter, Sok2, was strongly enriched11. Phd1 (a paralogue of Sok2) and Yap6 also displayed strong enrichment (Fig. 2b). In addition, the TF Sut1, which is known to interact with Cyc8, was enriched31. The TFs Mot3, Sko1, Nrg1 and Nrg2 displayed a milder enrichment (between three-fold and six-fold). For the TFs that displayed enrichment, we also assessed their binding to other parts of the IME1 promoter (Fig. 2c). The binding of these TFs peaked in the same region of the IME1 promoter as Tup1–Cyc8. Thus, at least eight TFs that have been implicated to interact with Tup1–Cyc8 associate with the IME1 promoter.
a Table of TFs that have been implicated to interact with Tup1–Cyc8 and have motif sequences within the IME1 promoter. BS = binding site. b Multiple TFs associate with the IME1 promoter in the same region where Tup1–Cyc8 binds. TFs listed in a were tagged with V5 epitope tag. Diploid cells harbouring V5-tagged transcription factor (YAP6-V5, FW3833; SOK2-V5, FW5638; PHD1-V5, FW4466; SUT1-V5, FW6974; MOT3-V5, FW4383; SKO1-V5, FW4389; NRG1-V5, FW4393; NRG2-V5, FW4396; SKN7-V5, FW4399; CIN5-V5, FW7072; SFL1-V5, FW7070; MIG1-V5, FW4665; RGT1-V5, FW4386) and control cells (untagged, FW1511) were grown till exponential growth. The region around 1000 bp upstream of IME1 AUG was analysed for binding by ChIP. The signals were normalised over HMR. Red bars represent a signal of three-fold over HMR or more. Mean and SEM of n = 4 (Yap6-V5 and Sut1-V5), n = 3 (Sok2-V5 and Phd1-V5) and n = 2 for other TFs plus untagged control are shown. c Similar as b, except that TFs that showed at least three-fold enrichment in b were analysed for binding throughout the whole IME1 promoter. Eight primer pairs were used for the analyses. The mean of n = 2 is shown.
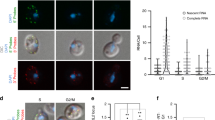
Next, we examined whether these TFs are responsible for recruiting Tup1 to the IME1 promoter. We reasoned that candidate TFs should associate independently of Tup1–Cyc8 to the IME1 promoter, while the binding of Tup1–Cyc8 should depend on the TFs and thus the presence of their binding motifs (Fig. 3a). First, we depleted Tup1 (TUP1-AID + IAA) and measured binding for a subset of the bound TFs (Fig. 3b, Supplementary Fig. 4a). Except for Sko1, the binding of the TFs to the IME1 promoter was not affected by Tup1 depletion (Fig. 3b). Thus, multiple TFs known to interact with Tup1–Cyc8 associate with the IME1 promoter independently of Tup1–Cyc8. Second, we examined whether the candidate TFs contribute to Tup1–Cyc8 recruitment. We mutated putative binding sites of the TFs that showed binding to the IME1 promoter. To do so, we generated a construct containing the full-length promoter controlling the expression of a sfGFP fused to the IME1 gene (pIME1-WT). Subsequently, we mutated 103 nucleotides distributed across a region of 400 bp in the IME1 promoter where most TF binding sites were present (pIME1-bs∆) (Fig. 3c, Supplementary Fig. 4b, c). We integrated the constructs into the TRP1 locus in cells harbouring a deletion of the endogenous IME1 gene and promoter sequence. Tup1 association with the IME1 promoter was nearly completely lost in pIME1-bs∆ cells (Fig. 3c). As expected, control cells (pIME1-WT) displayed strong Tup1 binding. Finally, we assessed how IME1 expression is affected in pIME1-bs∆ cells. Surprisingly, IME1 expression in pIME1-bs∆ cells was reduced, suggesting the regulatory elements essential for Tup1–Cyc8 recruitment are also important for IME1 activation (Fig. 3d). In conclusion, DNA sequence motifs of TFs bound to the IME1 promoter are required for Tup1–Cyc8 association with the IME1 promoter.
a Schematic displaying how TFs and Tup1–Cyc8 could interact at the IME1 promoter. TF = transcription factor. b Diploid cells harbouring TUP1-AID and V5-tagged TFs (YAP6-V5, FW4214; SOK2-V5, FW4218; PHD1-V5, FW5056; MOT3-V5, FW4229; NRG1-V5, FW4230; NRG2-V5, FW5055; SKO1-V5, FW4224) were grown to exponential phase. As a control, TUP1-AID (FW5057) cells were also included, which also harbour a V5 tag. Cells were either treated with IAA or DMSO, and the binding of each transcription factor was determined by ChIP and normalised over HMR. Mean of n = 2 is shown. c Binding of Tup1 is affected in cells lacking transcription factor binding motifs in the IME1 promoter. Diploid cells with a chromosomal deletion of the IME1 locus, and an integrated plasmid that contained the full IME1 gene fused with sfGFP at the amino terminus and the wild-type promoter (pIME1-WT, FW5370) or the same construct with all the candidate motif sequences mutated (pIME1-bsΔ, FW5372) were used for the analysis. These cells also expressed TUP1-V5. The binding of Tup1 was determined by ChIP. Mean and SEM of n = 3 are shown. d Expression of Ime1 during entry into meiosis in pIME1-WT and pIME1-bsΔ cells. Strains described in c were grown till saturation in rich medium (YPD), grown for an additional 16–18 h in pre-sporulation medium (BYTA), and subsequently shifted to sporulation medium (SPO). The levels of Ime1 expression were determined by imaging and quantifying the fluorescent signals generated by sfGFP-Ime1 in single cells (n = 50 cells per sample). The mean of signals detected and error bars representing the 95% confidence interval are displayed. Unpaired parametric two-tailed Welch’s t test with 95% confidence was used and p values (** = ≤0.01, *** = ≤0.001) are indicated.
Tup1–Cyc8 does not directly control the chromatin state
The Tup1–Cyc8 complex represses gene promoters, at least in part, by stabilising nucleosomes and establishing repressive chromatin35,36,37. Indeed, the region where Tup1–Cyc8 binds in the IME1 promoter is devoid of nucleosomes in the absence of Tup116,35. One possibility is that the repressive effects of Tup1–Cyc8 on the IME1 promoter are indirect. Instead of Tup1–Cyc8 directly promoting nucleosome occupancy or stability, nucleosomes could be evicted by transcriptional activators together with chromatin remodellers in the absence of Tup1–Cyc8. Such an activator–repressor model has been proposed for Tup1–Cyc8 and functionally demonstrated for other loci25. Since pIME1-bs∆ cells displayed low binding of Tup1 and no de-repression of IME1 expression (Figs. 3c and 4a), we can discriminate between direct and indirect effects of Tup1–Cyc8 in regulating chromatin state. We found that in pIME1-bs∆ cells, relative histone H3 occupancy was slightly increased in the region between 400 and 1000 bp upstream of IME1 AUG and mostly unaltered in the rest of the IME1 promoter when compared to pIME1-WT cells grown in rich medium (Fig. 4b, Supplementary Fig. 5a). Conversely, under activating conditions, histone H3 was strongly reduced in pIME1-WT cells but to a significantly lesser degree in pIME1-bs∆ cells (Supplementary Fig. 5a, bottom panel).
a IME1 expression in pIME1-WT (FW5370) and pIME1-bsΔ cells (FW5372) grown to exponential growth in rich nutrient conditions (YPD) detected by smFISH. The mean number of transcripts per cell for each time point (black line) is displayed. At least 200 cells were used for the analysis. Unpaired parametric two-tailed Welch’s t test with 95% confidence was used. Non-significant (ns) p value is indicated. b Histone H3 occupancy across the IME1 promoter and the IME1 ORF in pIME1-WT and pIME1-bsΔ cells grown as described in (a). The signals were normalised over the HMR locus. The mean and SEM of n = 3 are shown. * corresponds to a p value of ≤0.05 compared to pIME1-WT control on a two-way ANOVA followed by a Fisher’s LSD test with 95% confidence. c Schematic of Tup1–Cyc8 interacting with histone deacetylases (HDACs) (left). IME1 expression in diploid cells harbouring rpd3Δ, hda1Δ, hos1Δ and hos2Δ single deletions (FW8102, FW8426, FW8430 and FW8103) or rpd3Δhda1Δ, rpd3Δhos1Δ and rpd3Δhos2Δ double deletions (FW8457, FW8428 and FW8171) in single cells as determined by smFISH (right). The mean number of transcripts per cell (black line) is displayed. At least 100 cells were used for the analysis. Unpaired parametric two-tailed Welch’s t test with 95% confidence was used to compare IME1 expression in mutant cells with wild-type control cells. p values (ns = non-significant, * = ≤0.05, *** = ≤0.001) are indicated.
Tup1–Cyc8 also directly interacts with class I and II histone deacetylases (HDACs), which in turn confer repression through deacetylation of nucleosomes38,39,40. For example, repression of the FLO1 promoter is achieved by Tup1–Cyc8 mediated recruitment of Hda1 and Rpd341. In hda1Δ rpd3Δ cells strong de-repression of the FLO1 gene can be observed. To examine whether histone deacetylases mediate Tup1–Cyc8 repression at the IME1 promoter, we generated single and double mutants of known Tup1–Cyc8 interacting histone deacetylases (Rpd3, Hda1, Hos1 and Hos2) and measured IME1 expression levels by smFISH (Fig. 4c, Supplementary Fig. 5b). Deletion of individual HDACs (rpd3Δ, hda1Δ, hos1Δ and hos2Δ) did not increase expression of IME1. About 10% of rpd3Δhda1Δ cells expressed four or more IME1 transcripts, which is a marginal increase when compared to Tup1 depleted cells (Supplementary Fig. 5b, Fig. 1i). Two other double mutants (rpd3Δhos1Δ and rpd3Δhos2Δ) displayed no detectable increase in IME1 expression but showed a decrease in IME1 levels similar to that observed in the rpd3Δ mutant (Fig. 4c). Our data suggest HDACs that are known to interact with Tup1–Cyc8 play only a marginal role in repressing the IME1 promoter. It is worth noting that our analysis does not exclude the possibility that there is further redundancy between HDACs in regulating the IME1 promoter. Taken together, our analyses suggest a marginal role for Tup1–Cyc8 in regulating the IME1 promoter through chromatin directly.
Multiple TFs are required for Tup1–Cyc8 mediated repression
Our analyses of the IME1 promoter suggest that multiple TFs and binding sites are essential for Tup1–Cyc8 recruitment. Next, we examined how the different TFs control IME1 expression and mediate Tup1–Cyc8 recruitment. First, we assessed how the paralogues Sok2 and Phd1 control IME1 expression. sok2Δ cells displayed a marginal yet significant increase in IME1 expression (average transcripts per cell: 0.6 for sok2Δ versus 0.3 for control) (Fig. 5a, b). In the sok2Δphd1Δ double mutant, IME1 expression was further increased (average transcripts per cell: 2.2) and about 5% of cells displayed more than 10 transcripts per cell suggesting that Sok2 and Phd1 play redundant roles in tightly repressing the IME1 promoter (Fig. 5a, b). IME1 repression was not affected in single or double mutant cells containing yap6Δ, but the sok2Δphd1Δyap6Δ triple deletion mutant showed the largest increase in IME1 expression (average transcripts per cell: 2.8 for sok2Δphd1Δyap6Δ versus 2.2 for sok2Δphd1Δ) (Fig. 5a). About 8% of sok2Δphd1Δyap6Δ cells expressed more than 10 IME1 transcripts per cell (Fig. 5b), which was still much lower than that in cells depleted for Tup1 (Fig. 1i), suggesting that additional TFs contribute to IME1 repression.
a IME1 expression in single cells harbouring: sok2Δ, phd1Δ and yap6Δ single deletions (FW3979, FW3991, FW3603); sok2Δphd1Δ, yap6Δphd1Δ and sok2Δyap6Δ double deletions (FW4710, FW4406, FW4239); and sok2Δphd1Δyap6Δ triple deletions (FW4010). Cells were grown to exponential phase in nutrient rich conditions (YPD), fixed and hybridised with IME1 (AF594) and ACT1 (Cy5) probes. Only cells positive for ACT1 were used for the analyses. The mean number of transcripts per cell (black line) is displayed. Between 190 and 310 cells were used for the analysis. Unpaired parametric two-tailed Welch’s t test with 95% confidence was used to compare IME1 expression in mutant cells against wild-type control cells. Non-significant (ns) and p values (* = ≤0.05, *** = ≤0.001) are indicated. b Same data as in a, except that the single cell data for IME1 were binned according to expression levels. c Sok2, Phd1, Yap6 and Tup1 binding in cells harbouring the pIME1-WT (FW8081, FW8083, FW8079, FW5370), pIME1-bs∆ (FW8087, FW8089, FW8085, FW5372) and pIME1-spy (FW8093, FW8095, FW8091, FW7733) constructs as determined by ChIP. Mean of n = 2 are displayed, except for Tup1-V5 (n = 3) for which the error bars represent SEM values. d IME1 expression in single cells as determined by smFISH in cells harbouring pIME1-WT, pIME1-bsΔ or pIME1-spy. These cells either expressed wild-type SOK2, PHD1 and YAP6 (FW5370, FW5372, FW7733) or harboured the sok2Δphd1Δyap6Δ triple deletions (FW7650, FW8420, FW8177). Unpaired parametric two-tailed Welch’s t test with 95% confidence was used. p values (** = ≤0.01, *** = ≤0.001) are indicated. e Same data as in d, except that the single cell data were binned according to IME1 expression levels.
Our data demonstrate that Sok2, Phd1 and Yap6 associate with the IME1 promoter and contribute to IME1 repression in nutrient rich conditions. Yet, IME1 expression was reduced in cells with DNA sequence motifs mutated (pIME1-bsΔ). One explanation is that the mutated binding sites in pIME1-bsΔ facilitate Tup1–Cyc8 recruitment as well as binding of transcriptional activators. Another possibility is that TFs important for Tup1–Cyc8 recruitment are also required for IME1 activation. To discriminate between the two possibilities, we generated a construct that contained binding sites for Sok2, Phd1 and Yap6 (pIME1-spy), while the other TF binding sites remained mutated (Fig. 5c, Supplementary Fig. 5c). By combining pIME1-spy with sok2Δphd1Δyap6Δ, we determined whether Sok2, Phd1 and Yap6 are important for IME1 activation or repression. Tup1 binding was restored in cells harbouring pIME1-spy (Fig. 5c). Furthermore, Yap6, Sok2 and Phd1 were enriched at the IME1 promoter in pIME1-spy cells but their binding was reduced compared to the wild-type promoter—suggesting that additional binding sites are present or cooperative interactions with remaining TFs exist (Fig. 5c). Next, we measured IME1 expression in sok2Δphd1Δyap6Δ mutant cells harbouring pIME1-spy (Fig. 5d). We found that IME1 expression was significantly de-repressed—about 17% of cells harbouring pIME1-spy and sok2Δphd1Δyap6Δ expressed more than 10 IME1 transcripts per cell (Fig. 5d, e). As expected, the sok2Δphd1Δyap6Δ triple deletion only had a mild effect on IME1 levels in cells expressing pIME1-WT or pIME1-bs∆. We conclude that Sok2, Phd1 and Yap6 are important for repression of the IME1 promoter and play little role in IME1 activation.
The Tup1–Cyc8 complex dissociates from the IME1 promoter in cells exposed to nutrient starvation16. We hypothesised that TFs interacting with Tup1–Cyc8 at the IME1 promoter control Tup1–Cyc8 dissociation during IME1 activation. To examine this, we measured the binding of the TFs during activation of the IME1 promoter. In order to induce IME1 expression and meiotic entry, we typically grow cells in rich medium conditions containing glucose until saturation, then shift to pre-sporulation medium containing acetate to ensure that cells are not subjected to repressive glucose signalling to the IME1 promoter4,9. Subsequently, cells are starved in sporulation (SPO) medium (0.3% acetate), which induces IME1 transcription and meiotic entry. Both Pog1 and Tup1 were enriched at 0 h in SPO prior to IME1 induction. As expected, during meiotic entry (4 h in SPO) Tup1 dissociated from the IME1 promoter completely while Pog1 binding was maintained albeit to a reduced level (Fig. 6a, right panel). In addition, we found that all eight TFs were enriched at the IME1 promoter prior to induction of IME1 (Fig. 6a, left panel). Upon entry into meiosis (4 h in SPO), five TFs showed near background binding (less than three-fold) to the IME1 promoter, while three TFs (Yap6, Phd1 and Nrg1) displayed marginal enrichment (less than five-fold over background) (Fig. 6a, right panel). We further performed a time course experiment with three TFs (Yap6/Sok2/Phd1) and Tup1. We found that IME1 bulk expression levels peaked at 3 h in SPO (Supplementary Fig. 6a). Tup1 and Sok2 dissociated from the IME1 promoter in the early time points (1 and 2 h in SPO), while the majority of Tup1 and Sok2 was evicted at 4 h in SPO (Fig. 6b). Yap6 and Phd1 displayed reduced binding at the IME1 promoter at 3 h in SPO and was thus slower than Sok2 and Tup1. We propose that the gradual dissociation of multiple TFs evicts Tup1–Cyc8 from the IME1 promoter.
a Binding of TFs at the IME1 promoter prior to and during entry into meiosis. Diploid cells harbouring V5 tagged TFs (YAP6-V5, FW3833; PHD1-V5, FW4466; NRG1-V5, FW4393; SOK2-V5, FW5638; MOT3-V5, FW4383; SKO1-V5, FW4389; NRG2-V5, FW4396; SUT1-V5, FW6974; TUP1-V5, FW3456; POG1-V5, FW968) and control cells (untagged, FW1511) were used for the analyses. Samples for ChIP were taken at 0 and 4 h in SPO. Mean and SEM values of n = 3 is displayed, except for control (n = 2). b Similar as in a, except that multiple time points were taken to analyse the timing of dissociation of Tup1-V5, Yap6-V5, Sok2-V5 and Phd1-V5 from the IME1 promoter. ChIP signals were normalised over HMR, and the 0 h time point was set to 100%. The mean and SEM values of n = 3 (Tup1–V5), and the mean of n = 2 (Yap6-V5, Sok2-V5 and Phd1-V5) are displayed. c Nuclear signal of Yap6-mNG (FW7473), Sok2-mNG (FW7475), Phd1-mNG (FW7477), Tup1-mNG (FW7644) and Cyc8-mNG (FW7642) prior to (0 h in SPO) and during entry into meiosis (4 h in SPO). Each transcription factor was fused to mNeonGreen (mNG). These cells also expressed mCherry fused to SV40 nuclear localisation signal (NLS) (mCherry-NLS). As a control, the signals of cells harbouring no mNG-tag (FW5199) are displayed. The black bar indicates the mean signal, and each point indicates a single cell measurement (n = 50 for each sample). The error bars represent the 95% confidence interval.
One possible mechanism by which Tup1–Cyc8 and TFs dissociate from the IME1 promoter is by re-localisation to the cytoplasm. Indeed, nutrient signalling via PKA and TORC1 can impact the localisation of several TFs42,43. We fused mNeonGreen to Sok2, Phd1, Yap6, Tup1 and Cyc8 (Supplementary Fig. 6b). As expected, Sok2, Phd1 and Yap6 were concentrated in the nucleus. Neither protein abundance in the nucleus nor the nuclear-to-cytoplasmic ratios were altered in cells prior to (0 h in SPO) and during entry into meiosis (4 h in SPO) (Fig. 6c, Supplementary Fig. 6c). Taken together, activation of the IME1 promoter correlates with dissociation of Tup1–Cyc8 and Tup1–Cyc8 recruiting TFs from the IME1 promoter, but likely not via a mechanism involving re-localisation of TFs to the cytoplasm.
Sok2, Phd1 and Yap6 control the onset of meiosis
Our observations indicate that Sok2, Phd1 and Yap6 are important TFs for IME1 repression. Next, we investigated how the three TFs control Tup1–Cyc8 recruitment in different nutrient conditions. We found that Tup1 binding to the IME1 promoter was not decreased by sok2∆, phd1∆ and yap6∆ single/double/triple deletions in rich medium containing glucose, suggesting that other TFs contribute to IME1 repression via Tup1–Cyc8 when glucose is used by cells as the carbon source (Fig. 7a). In contrast, prior to meiotic entry (0 h in SPO) Tup1 binding was diminished in sok2Δ and sok2Δphd1Δ cells, but not in yap6Δ and phd1Δ cells (Fig. 7a). Strikingly, Tup1 association with the IME1 promoter was severely reduced (less than three-fold over background) in sok2Δyap6Δ and sok2Δphd1Δyap6Δ cells at 0 h in SPO. IME1 expression was inversely correlated with Tup1–Cyc8 recruitment to the IME1 promoter since IME1 was significantly de-repressed in sok2Δyap6Δ, sok2Δphd1Δ or sok2Δphd1Δyap6Δ cells at 0 h in SPO (Fig. 7b). Finally, we examined how Sok2, Phd1 and Yap6 mediated Tup1–Cyc8 recruitment contributes to the onset of meiotic entry. Cells harbouring sok2Δ or sok2Δphd1Δ underwent meiosis much faster than wild-type cells (Fig. 7c). There was little effect on the onset of meiosis in the yap6Δ or yap6Δphd1Δ mutants. In sok2Δyap6Δ and sok2Δphd1Δyap6Δ cells the kinetics of meiosis was slightly faster than sok2Δphd1Δ cells (Fig. 7c). Approximately 50% of cells underwent meiotic divisions within 2 h in SPO for sok2Δphd1Δyap6Δ cells compared to 3 h for sok2Δphd1Δ cells. We also analysed how the onset of meiosis is affected in sok2Δphd1Δyap6Δnrg1Δ cells (Supplementary Fig. 7a). We found no difference in the onset of meiosis between sok2Δphd1Δyap6Δnrg1Δ and sok2Δphd1Δyap6Δ cells, suggesting that Nrg1 is not involved in IME1 activation (Fig. 6a, right panel). We conclude that Sok2, Phd1 and Yap6 direct Tup1–Cyc8 association with the IME1 promoter to ensure timely expression of IME1 in cells grown in acetate-containing medium. Our data further suggest that the IME1 promoter is regulated by multiple Tup1–Cyc8 co-repressor complexes.
a Tup1 binding at the IME1 promoter in nutrient rich conditions (YPD) and prior to entry into meiosis (0 h in SPO) in wild-type cells (FW3456) or cells harbouring sok2Δ, phd1Δ and yap6Δ single deletions (FW3979, FW3991, FW3603); sok2Δphd1Δ, yap6Δphd1Δ and sok2Δyap6Δ double deletions (FW4710, FW4406, FW4239); and sok2Δphd1Δyap6Δ triple deletions (FW4010). An untagged control (FW1511) was also included in the analyses. The mean and SEM values of n = 3 is displayed, except for sok2Δ, phd1Δ and the untagged control for which the mean of n = 2 is displayed. b IME1 expression in deletion mutants described in (a). The signals were normalised over the ACT1 gene signals. Mean and SEM values of n = 3 are displayed. Two-way ANOVA analysis was carried out using the uncorrected Fisher’s LSD method with 95% confidence to compare IME1 expression in mutant cells against wild-type control cells at SPO 0 h. Non-significant (ns) and p values (** = ≤0.01, *** = ≤0.001, **** = ≤0.0001) are indicated. c Meiosis in mutant strains described in (a). Samples were taken at the indicated time points, fixed and DAPI masses were counted (n = 200–300 cells per sample) to determine the percentage of cells that underwent meiosis (MI + MII). Cells harbouring two, three or four DAPI masses were classified as meiosis.
Binding of TFs is highly regulated by nutrient cues
Why do so many TFs (at least eight) associate with the IME1 promoter? One possibility is that the TFs facilitate Tup1–Cyc8 recruitment under different nutrient conditions. With this logic, repression of the IME1 promoter can be maintained under various nutrient conditions and will only be fully activated when all the nutrient signalling requirements are met. In agreement with this model, IME1 expression was only marginally increased in sok2Δphd1Δyap6Δ cells grown in the presence of ample nutrients with glucose as the carbon source (YPD), and was significantly increased by nearly ten-fold in sok2Δphd1Δyap6Δ cells grown in acetate-containing medium (Figs. 5a, b and 7b). Furthermore, in YPD saturation when glucose is depleted from the growth medium, we observed that some TFs displayed altered association with the IME1 promoter. For example, Sok2 binding was increased, while Yap6 and Cyc8 association with the IME1 promoter was decreased (Supplementary Fig. 7b). To examine how the different TFs respond to nutrient signalling at the IME1 promoter more systematically, we measured their association under different nutrient conditions (Fig. 8a). We grew cells until the pre-sporulation stage, and subsequently shifted cells to sporulation medium (SPO) (1), SPO plus 2% glucose (2), YP (3) or YP plus 2% glucose (YPD) (4). First, we measured Tup1 association with the IME1 promoter. We found that in SPO plus glucose, Tup1 binding to the IME1 promoter was partially restored (Fig. 8b). The association of Tup1 with the IME1 promoter was further increased in YP and was the highest in YPD growth medium. Pog1, the transcriptional activator of IME1, was enriched in all four nutrient conditions, but at higher levels in YP and YPD (Fig. 8b). Interestingly, TFs important for Tup1–Cyc8 recruitment to the IME1 promoter responded to nutrient signals in distinct ways (Fig. 8c). For example, Yap6, Sok2, Sko1 and Nrg1 associated with the IME1 promoter in response to the nutrient cues present in YP, but not to glucose signals alone. Phd1 binding partially recovered in the presence of glucose and showed the strongest enrichment in cells exposed to YP and YPD. Conversely, glucose signalling, but not YP, maintained association of Mot3 and Nrg2 with the IME1 promoter (Fig. 8c). Finally, Sut1 association with the IME1 promoter was restored in YPD only.
a Schematic of the experimental set up. Following growth in YPD and pre-sporulation medium, cells were shifted to SPO, SPO plus 2% glucose, YP or YP plus 2% glucose for 4 h. b Binding of Tup1 and Pog1 at the IME1 promoter under distinct nutrient conditions described in (a), as measured by ChIP. Diploid cells harbouring V5 tagged Tup1 (FW3456) or Pog1 (FW968) were used for the analyses. Normalised mean signals and SEM of n = 3 are displayed, except for Pog1-V5 in SPO plus 2% glucose, YP and YPD conditions for which the mean of n = 2 is shown. c Similar to b, except that V5 tagged Yap6, Sok2, Phd1, Nrg1, Mot3, Nrg2, Sko1 and Sut1 were used for the analyses (FW3833, FW5638, FW4466, FW4393, FW4383, FW4396, FW4389 and FW6974). Mean signals and SEM of n = 3 are displayed, except for Sut1-V5 for SPO plus 2% glucose, YP and YPD conditions for which the mean of n = 2 is shown. d Similar to b, except that Tup1 binding to the IME1 promoter was determined in the sok2Δphd1Δyap6Δ cells (FW4010). Mean signals for n = 2 are displayed, except for 0 h in SPO for which the mean and SEM of n = 3 are shown. e Model for how nutrient signalling controls the IME1 promoter. Multiple Tup1–Cyc8 recruiting TFs associate in nutrient rich conditions with the IME1 promoter. During partial starvation some TFs dissociate from the IME1 promoter, but Tup1–Cyc8 remains bound. Upon entry into meiosis all TFs and Tup1–Cyc8 dissociate, and consequently IME1 transcription is induced.
Given that Sok2, Phd1 and Yap6 were strongly enriched in cells exposed to YP medium (Fig. 8c), we hypothesised that Tup1–Cyc8 association with the IME1 promoter is affected in sok2Δphd1Δyap6Δ cells in YP, but not in SPO containing glucose. We therefore examined how Tup1–Cyc8 association with the IME1 promoter was affected in sok2Δphd1Δyap6Δ cells under different nutrient conditions. Indeed, in sok2Δphd1Δyap6Δ cells, Tup1 binding was detected in SPO plus glucose, but not in YP medium (Fig. 8d). These data indicate that Sok2, Phd1 and Yap6 are important for mediating Tup1–Cyc8 association in YP, while other TFs are required for glucose signalling to the IME1 promoter (Fig. 8e). In summary, our analyses revealed that the association of one set of TFs (i.e., Mot3 and Nrg2) with the IME1 promoter is induced by glucose signalling, while another set of TFs (i.e., Yap6, Sok2, Phd1, Sko1 and Nrg1) was recruited to the IME1 promoter primarily in response to the nutrient cues in YP. Thus, only when all the required nutrient signalling pathways are repressed, all TFs interacting with Tup1–Cyc8 dissociate from the IME1 promoter allowing activation of IME1 transcription. These data show that TFs important for Tup1–Cyc8 recruitment to the IME1 promoter respond to different environmental cues to ensure Tup1–Cyc8 mediated repression under various nutrient conditions.
Discussion
We report that the Tup1–Cyc8 complex together with multiple sequence-specific TFs constitute the essential components that control repression of the IME1 promoter. The decision to enter meiosis and produce gametes is remarkably simple in yeast: environmental signals regulate the association and dissociation of TFs that recruit Tup1–Cyc8 to the IME1 promoter. We propose that regulated repression of IME1 by multiple TFs and Tup1–Cyc8 ensures tight control of the decision to enter meiosis in yeast.
Our data show that repression of IME1 transcription, and not activation, is highly regulated. Depletion of either Tup1 or Cyc8 completely de-repressed IME1 expression (Fig. 1). We detected little delay between depletion of Tup1 and de-repression of IME1 expression in the presence of ample nutrients (Fig. 1g–i). From these two observations, we can infer two important features of the IME1 promoter. First, the transcriptional activators are bound to the IME1 promoter or readily available prior to activation of IME1 transcription. Second, key transcriptional activators of the IME1 promoter can be active under nutrient rich conditions when Tup1–Cyc8 is unbound. We found that the activator Pog1 is bound to the IME1 promoter prior to activation. A pog1∆ mutant only has a mild effect on IME1 expression indicating that there must be other transcriptional activators controlling IME1 transcription7. Several other transcriptional activators have been implicated in regulating IME1 transcription12.
How does Tup1–Cyc8 control the IME1 promoter? The Tup1–Cyc8 complex regulates transcription of a subset of promoters in yeast18,44. Several mechanisms have been described for Tup1–Cyc8 mediated gene repression21,23,24. Our data are largely consistent with a model in which Tup1–Cyc8 masks or shields activating TFs from recruiting co-activators at promoters25,32. We showed that multiple (at least eight) TFs that are known to interact with Tup1–Cyc8 associate with the IME1 promoter (Fig. 2b). Our data suggest that these TFs are important for facilitating Tup1–Cyc8 binding but play little role in IME1 transcriptional activation. First, almost all TFs involved in Tup1–Cyc8 recruitment dissociated from the IME1 promoter upon activation of IME1 transcription (Fig. 6a, right panel). Second, deleting multiple TFs led to activation, but not repression of IME1 transcription (Fig. 7c, Supplementary Fig. 7a). However, we cannot exclude that the Tup1–Cyc8 recruiting TFs can function as transcriptional activators in some conditions. Indeed, Yap6 and Sok2 have both been implicated as activators of transcription at some promoters45,46. In the context of the IME1 promoter, each TF likely has a designated function in either repression or activation of IME1 transcription. We propose that multiple TFs are required to recruit the Tup1–Cyc8 co-repressor to the IME1 promoter. The Tup1–Cyc8 co-repressor complexes, in turn, mask transcriptional activators (which are different from the Tup1–Cyc8 recruiting TFs) and prevent co-activator recruitment.
Under most environmental conditions, the IME1 promoter must be repressed to prevent cells from inappropriately entering meiosis and forming gametes only unless when cells are starved. We propose that multiple TFs ensure IME1 repression under various environmental conditions. First, we found that distinct sets of TFs associate with the IME1 promoter in different nutrient environments (Fig. 8). Second, deleting three TFs (Sok2, Phd1 and Yap6) led to very mild IME1 expression in rich medium containing glucose (Fig. 5a, b), but IME1 was almost fully expressed in cells grown in an acetate-containing medium (Fig. 7b). Thus, additional TFs must facilitate Tup1–Cyc8 association with the IME1 promoter in rich medium containing glucose.
We previously showed that inhibiting PKA and TORC1 is sufficient to drive entry into meiosis16. Understanding how PKA and TORC1 regulate TFs controlling Tup1–Cyc8 recruitment to the IME1 promoter may be key to understanding how the decision to enter meiosis is regulated. However, the mechanism remains to be deciphered. Inhibiting PKA and TORC1 activity can trigger the re-localisation of TFs, alter protein–protein interactions and protein turnover, and more32,47. With this view, we observed no depletion of TFs from the nucleus during activation of the IME1 promoter (Fig. 6c, Supplementary Fig. 6c). Nutrient signalling may also regulate Tup1–Cyc8 itself through post-translational modifications48,49.
Our work in yeast shows similarities to how multicellular organisms undertake developmental decisions. In Drosophila, plants, and mammals, transcriptional repressors of the Groucho family (structurally related to Tup1) are important for regulating various developmental processes such as body patterning and determination of organ identity50,51,52. Like Tup1–Cyc8, the association of Groucho repressor with promoters relies on sequence specific TFs, and Groucho repressor integrates multiple signals to control gene expression and cell fate outcomes. Regulation of the IME1 promoter also demonstrates features of enhancer-directed transcriptional control of cell-fate master regulators in mammalian cells53,54,55. Like at the IME1 promoter, an array of TFs associates and controls the activity of enhancers. In addition, developmentally controlled enhancers are typically regulated by multiple upstream signalling pathways and are often primed for activation56. Our findings in yeast may provide insights to better understand how signal integration controls master regulatory genes and developmental decisions in all eukaryotic cells.
Methods
Yeast strains and plasmids
The Saccharomyces cerevisiae SK1 genetic background was employed for all experiments in this study. Experiments were carried out with diploid cells and the list of yeast strains described in this study can be found in Supplementary Data 1. Gene deletions, IME1 promoter truncations, and protein fusions were achieved by the single step PCR-based gene modification protocol described in ref. 57. Auxin-based depletion of Tup1 and Cyc8 was achieved by fusing Tup1 and Cyc8 with the auxin-induced degron (AID) tag containing 3×V5 epitope and the Arabidopsis thaliana IAA7 protein26. The Oryza sativa TIR1 ligase (osTIR1) was also expressed under the TEF1 promoter from a plasmid integrated at the HIS3 locus (courtesy of Leon Chan) in the TUP1-AID and CYC8-AID strains. Protein-mNeonGreen fusions were constructed by tagging the proteins with mNeonGreen tagging cassettes (courtesy of Andreas Doncic) described in ref. 58. Cells expressing protein-mNeonGreen fusions also harboured a nuclear localisation signal peptide derived from simian virus 40 tagged with two copies of mCherry (2xmCherry-SV40NLS)58.
Single-copy integration plasmids containing IME1 N-terminally fused with sfGFP and full length IME1 promoter were derived from pNH60459. The pIME1-sfGFP-IME1 fragment (~4.6 kb) was amplified from yeast cells expressing sfGFP-Ime1 and was cloned into pNH604 plasmid in the NotI and BamHI sites by restriction digestion. The resulting plasmid (pIME1-WT) consists of pIME1-sfGFP-IME1 followed by C. glabrata TRP1 and the whole cassette is flanked by the 5′ and 3′ UTRs of S. cerevisiae TRP1. To mutate TF binding sites, DNA fragments of 500 bp in length corresponding to 701–1100 bp upstream of the IME1 start codon with binding site mutations were synthesised (gBlocks Gene Fragments, Integrated DNA Technologies) and cloned into the pIME1-WT plasmid by Gibson assembly using the NEBuilder HiFi DNA Assembly Master Mix (New England BioLabs). The pIME1-bs∆ plasmid carried 103 mutated nucleotides (nt) between 701 and 1100 bp upstream of the IME1 start codon to disrupt Yap6, Sok2, Phd1, Mot3, Sko1, Nrg1 and Nrg2 binding sites (Supplementary Fig. 4c). The pIME1-spy plasmid was designed based on the promoter sequence in pIME1-bs∆ by restoring the Yap6, Sok2 and Phd1 sites (33 nt) between 701–1100 bp upstream of the IME1 start codon while other TF sites remained mutated (Supplementary Fig. 5c). Plasmid sequences were verified by Sanger sequencing. Plasmids (pIME1-WT, pIME1-bs∆ and pIME1-spy) were linearised with PmeI and integrated by transformation into the TRP1 locus.
Growth conditions
Yeast cells were grown in YPD medium (1% yeast extract, 2% peptone, 2% glucose) supplemented with 96 µg/mL tryptophan, 24 µg/mL uracil and 12 µg/mL adenine, grown at 30 °C and liquid cultures were agitated at 300 r.p.m. To obtain exponentially growing cells (YPD (E)) and cells grown to saturation (YPD (S)), cells were grown in YPD to saturation overnight, diluted to OD600 = 0.2 and subsequently YPD (E) cells were harvested after two to three doublings. YPD (S) cells were grown for 20–24 h in YPD. To induce entry into meiosis, cells were grown overnight in YPD, shifted to pre-sporulation medium BYTA (1% yeast extract, 2% tryptone, 1% potassium acetate, 50 mM potassium phthalate) at OD600 = 0.4 for 16–18 h, and subsequently transferred to sporulation medium SPO (0.3% potassium acetate, 0.02% raffinose, pH 7.0) at OD600 = 1.8.
To study the responses of the TFs in distinct nutrient conditions in Fig. 8b–d, cells were grown in YPD and pre-sporulation medium following the standard sporulation induction protocol. Subsequently, cells were shifted to four different types of media including sporulation medium (SPO), glucose-only medium (SPO + 2% glucose), YP medium without glucose (YP + 0.05% glucose) and YPD medium (YP + 2% glucose). Yeast cells were harvested for ChIP analyses at the point of shift (0 h SPO) and after four hours in the different nutrient conditions.
To study the effect of Tup1 and Cyc8 depletion on IME1 expression and TF binding (Fig. 1e–i, Fig. 3b, Supplementary Figs. 2b and 4a), 500 µM of indole-3-acetic acid (IAA) (Aldrich) was added to exponentially growing cells to induce degradation of Tup1-AID and Cyc8-AID proteins. As control, same volume of dimethyl sulphoxide (DMSO) was added to yeast cells. Cells were harvested at the indicated time points for ChIP, RT-qPCR, smFISH and western blot analyses.
Chromatin immunoprecipitation (ChIP)
Harvested cells were crosslinked with formaldehyde for 20 min at room temperature and reaction was quenched by the addition of 100 mM glycine. Cells were washed with FA lysis buffer (50 mM HEPES pH 7.5, 150 mM sodium chloride, 1 mM EDTA pH 7.6, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% sodium dodecyl sulphate (SDS)), snap frozen and stored at −80 °C. Cell lysis was performed in cold FA lysis buffer with cOmplete Mini Protease Inhibitor Cocktail (Roche). Samples were homogenised with zirconia beads (BioSpec) using Mini-Beadbeater-96 (BioSpec). The chromatin fraction was subjected to shearing by sonication on Bioruptor Plus (Diagenode) using 9 cycles of 30 s on, 30 s off or until the majority of fragments were under 850 bp. V5 epitope-tagged proteins were immunoprecipitated with anti-V5 agarose beads (Sigma-Aldrich) at room temperature for 2 h with rotation. Histone H3 was immunoprecipitated (Fig. 4b, Supplementary Fig. 5a) with Dynabeads Protein A (Invitrogen) coupled with anti-H3 antibodies (ab1791, Abcam) using 2 µg of antibodies per sample. Subsequently, the beads were washed with FA lysis buffer, FA lysis buffer with 260 mM sodium chloride and a lithium chloride/detergent buffer (10 mM Tris pH 8, 250 mM lithium chloride, 0.5% NP-40, 0.5% sodium deoxycholate, 1 mM EDTA). Samples were reverse crosslinked in TE buffer with 1% SDS at 65 °C, 500 r.p.m. overnight and treated with 80 µg/mL proteinase K (Thermo Scientific) at 37 °C for 2 h. Purified DNA fragments were quantified by quantitative PCR using EXPRESS SYBR GreenER SuperMix (Thermo Fisher Scientific) or PowerUp SYBR Green Master Mix (Thermo Fisher Scientific) on Applied Biosystems 7500 Fast Real-Time PCR System (Thermo Fisher Scientific). ChIP signals were typically corrected by an input, and subsequently were normalised over the silent mating type cassette HMR. Primer sequences are listed in Supplementary Table 1. The HMR locus is known to be transcriptionally inactive but binding of Tup1 has been detected near silent mating loci. To examine whether HMR is an appropriate background control for Tup1 ChIP, we calculated Tup1–V5 ChIP signals normalised over input for the IME1 promoter and HMR, which showed that Tup1 was not enriched at the HMR locus (Supplementary Fig. 8).
RNA isolation and reverse transcription
Total RNA was extracted from harvested cells using the hot phenol method. Briefly, TES buffer (10 mM Tris-HCl pH 7.5, 10 mM EDTA, 0.5% SDS) and acid-phenol:chloroform (Ambion) were added to samples and incubated at 65 °C. RNA was subsequently purified using the NucleoSpin RNA kit (Macherey-Nagel) according to the manufacturer’s instructions. rDNase was added to remove residual genomic DNA during the purification procedure. For reverse transcription, ProtoScript II First Strand cDNA Synthesis Kit (New England BioLabs) was used and 500 ng of total RNA was provided as template in each reaction. qPCR reactions were prepared using EXPRESS SYBR GreenER SuperMix (Thermo Fisher Scientific) or PowerUp SYBR Green Master Mix (Thermo Fisher Scientific) and IME1 level was quantified on Applied Biosystems 7500 Fast Real-Time PCR System (Thermo Fisher Scientific). Signals were normalised over ACT1. Primer sequences are listed in Supplementary Table 1.
Western blotting
Proteins were extracted from cells fixed with 5% trichloroacetic acid by lysing cells in protein breakage buffer (50 mM Tris at pH 7.5, 1 mM EDTA, 27.5 mM DTT) with 0.5 mm glass beads (BioSpec) on the Mini-Beadbeater-96 (BioSpec). Samples were denatured in SDS loading buffer (62.5 mM Tris (pH 6.8), 2% β-mercaptoethanol, 10% glycerol, 3% SDS and 0.017% Bromophenol Blue) and separated by SDS-PAGE in Tris-glycine buffer (25 mM Tris base, 192 mM glycine, 0.1% SDS). V5-epitope tagged proteins were detected using anti-V5 primary antibodies (Invitrogen, 1:2000, mouse) and IRDye 800CW (LI-COR, 1:15000) or HRP-conjugated (GE Healthcare, 1:8000) secondary antibodies. For equal loading Hxk1 protein levels were determined using anti-hexokinase primary antibodies (Stratech Scientific, 1:2000, rabbit), and IRDye 680RD (LI-COR, 1:15000) or HRP-conjugated (GE Healthcare, 1:8000) secondary antibodies. Images were acquired on the Odyssey CLx imaging system (LI-COR) or by ECL Prime (GE Healthcare) using Amersham Imager 600 (GE Healthcare). Uncropped versions of the western blots presented in Fig. 1e, g, Supplementary Fig. 4a are provided in the Source Data file.
Nuclei/DAPI counting
Samples were fixed in 80% ethanol and stained with 1 µg/mL 4′,6-diamidino-2-phenylindole (DAPI) in PBS buffer. Cells with two, three or four DAPI masses were considered meiosis, while cells harbouring one DAPI mass were counted as NO meiosis. At least 200 cells were analysed for each sample.
Microscopy
For single molecule RNA fluorescence in situ hybridisation (smFISH), cells were fixed with formaldehyde and washed with Buffer B (1.2 M sorbitol, 0.1 M potassium phosphate dibasic, pH 7.5). To spheroplast the cells, samples were treated with 40 µg/mL Zymolyase-100T (MP Biomedicals) and 57.2 mM β-mercaptoethanol in Buffer B at 30 °C. Spheroplasted cells were washed and smFISH probes recognising IME1 (AF594) and ACT1 (Cy5)16 were hybridised overnight in hybridisation buffer (10% dextran, 2 mM vanadyl-ribonucleoside complex (New England BioLabs), 0.02% RNAse-free BSA, 1 mg/mL E. coli tRNA, 2×SSC and 10% formamide) at 30 °C. Cells were washed with wash buffer (2×SSC, 10% formamide), stained with 1 µg/mL DAPI in wash buffer and re-suspended in 2×SSC. Subsequently, cells were spun down and re-suspended prior to imaging in anti-fade GLOX with 1% catalase (Sigma) and 1% glucose oxidase (Sigma)27. Images were acquired using the Eclipse Ti–E inverted microscope system (Nikon) using the 100× oil objective with the ORCA-FLASH 4.0 camera (Hamamatsu) and NIS-elements software (Nikon). Cells were imaged at every 0.3 µm along the z-axis using the built-in z-axis drive and a total of 25 images were taken for each z-stack. Signals from all the planes were merged into a 2D image by applying maximum intensity z-projection in ImageJ 1.52a60. Only cells with ACT1 signals were considered for IME1 quantification using the StarSearch software (http://rajlab.seas.upenn.edu/StarSearch/launch.html).
For the quantification of sfGFP expressed in pIME1-WT and pIME1-bs∆ presented in Fig. 3d, cells were induced to sporulate using the standard protocol and samples were taken from the sporulation culture at indicated time points. Harvested cells were fixed with formaldehyde and re-suspended prior to imaging in a buffer containing 16.6 mM potassium phosphate monobasic, 83.4 mM potassium phosphate dibasic and 1.2 M sorbitol. Imaging was carried out on the Eclipse Ti–E inverted microscope system (Nikon) using the 100× oil objective with the ORCA-FLASH 4.0 camera (Hamamatsu) and NIS-elements software (Nikon). sfGFP signals in each cell were quantified using the ImageJ 1.52a software60.
For determining the localisation of mNeonGreen-tagged TFs described in Fig. 6c. and Supplementary Fig. 6c, cells were induced to sporulate with the standard protocol. Subsequently, cells were imaged at 0 h in SPO and 4 h in SPO. Images were acquired using the same imaging system and set up described for quantification of sfGFP expressed in pIME1-WT and pIME1-bs∆ cells. Signals from whole cell and cell nucleus were quantified in the ImageJ 1.52a software and with use of the nuclear marker (2xmCherry-SV40NLS)60. Signal from the cytosol was inferred from the difference between whole cell signal and nuclear signal.
IME1 promoter motif analysis
TF binding sites presented in Fig. 2a, Supplementary Figs. 3 and 4b were predicted by scanning the IME1 promoter with the curated TF motifs in the YeTFaSCo database (version 1.02)61. Binding sites were predicted to have at least 75% of the maximum possible score, with the exception of the Sut1 binding site which was predicted with a 70% threshold. The Sko1 binding site was identified manually by scanning the promoter sequence for the consensus motif TGACG as described in ref. 61.
Statistical analyses
Data statistics and statistical analyses indicated in the figure legends were computed using GraphPad Prism version 8.2.0 for Windows, GraphPad Software, San Diego, CA, USA, www.graphpad.com. Data from the smFISH and imaging experiments presented in Figs. 1h, 3d, 4a, c and 5a, d were analysed using unpaired parametric two-tailed Welch’s t test with 95% confidence. The H3 ChIP data presented in Fig. 4b and Supplementary Fig. 5a and the IME1 expression data presented in Fig. 7b were analysed by two-way ANOVA using the uncorrected Fisher’s LSD method with 95% confidence. p Values are indicated in the figures, where ns stands for non-significant, * = ≤0.05, ** = ≤0.01, *** = ≤0.001, **** = ≤0.0001.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Supplementary Figs. 1–8 and Supplementary Table 1 are available in the supplementary information. A reporting summary for this article is available as a Supplementary Information file. The source data underlying Figs. 1b–I, 2b, c, 3b–d, 4a–c, 5a–e, 6a–c, 7a–c and 8b–d and Supplementary Figs. 1, 2b, 4a, 5a, b, 6a, c, 7a, b and 8 are provided as a Source Data file. All data are available from the corresponding author upon reasonable request.
References
Neiman, A. M. Sporulation in the budding yeast Saccharomyces cerevisiae. Genetics 189, 737–765 (2011).
Kassir, Y., Granot, D. & Simchen, G. IME1, a positive regulator gene of meiosis in S. cerevisiae. Cell 52, 853–862 (1988).
Nachman, I., Regev, A. & Ramanathan, S. Dissecting timing variability in yeast meiosis. Cell 131, 544–556 (2007).
van Werven, F. J. & Amon, A. Regulation of entry into gametogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 3521–3531 (2011).
Covitz, P. A. & Mitchell, A. P. Repression by the yeast meiotic inhibitor RME1. Genes Dev. 7, 1598–1608 (1993).
Mitchell, A. P. & Herskowitz, I. Activation of meiosis and sporulation by repression of the RME1 product in yeast. Nature 319, 738–742 (1986).
van Werven, F. J. et al. Transcription of two long noncoding RNAs mediates mating-type control of gametogenesis in budding yeast. Cell 150, 1170–1181 (2012).
Moretto, F., Wood, N. E., Kelly, G., Doncic, A. & van Werven, F. J. A regulatory circuit of two lncRNAs and a master regulator directs cell fate in yeast. Nat. Commun. 9, 780 (2018).
Jambhekar, A. & Amon, A. Control of meiosis by respiration. Curr. Biol. 18, 969–975 (2008).
Sagee, S. et al. Multiple and distinct activation and repression sequences mediate the regulated transcription of IME1, a transcriptional activator of meiosis-specific genes in Saccharomyces cerevisiae. Mol. Cell Biol. 18, 1985–1995 (1998).
Shenhar, G. & Kassir, Y. A positive regulator of mitosis, Sok2, functions as a negative regulator of meiosis in Saccharomyces cerevisiae. Mol. Cell Biol. 21, 1603–1612 (2001).
Kahana, S. et al. Functional dissection of IME1 transcription using quantitative promoter-reporter screening. Genetics 186, 829–841 (2010).
Zhao, H. et al. A role for the respiratory chain in regulating meiosis initiation in Saccharomyces cerevisiae. Genetics 208, 1181–1194 (2018).
Kunoh, T., Kaneko, Y. & Harashima, S. YHP1 encodes a new homeoprotein that binds to the IME1 promoter in Saccharomyces cerevisiae. Yeast 16, 439–449 (2000).
Kahana-Edwin, S., Stark, M. & Kassir, Y. Multiple MAPK cascades regulate the transcription of IME1, the master transcriptional activator of meiosis in Saccharomyces cerevisiae. PLoS ONE 8, e78920 (2013).
Weidberg, H., Moretto, F., Spedale, G., Amon, A. & van Werven, F. J. Nutrient control of yeast gametogenesis is mediated by TORC1, PKA and energy availability. PLoS Genet. 12, e1006075 (2016).
Honigberg, S. M. & Lee, R. H. Snf1 kinase connects nutritional pathways controlling meiosis in Saccharomyces cerevisiae. Mol. Cell Biol. 18, 4548–4555 (1998).
DeRisi, J. L., Iyer, V. R. & Brown, P. O. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science 278, 680–686 (1997).
Keleher, C. A., Redd, M. J., Schultz, J., Carlson, M. & Johnson, A. D. Ssn6-Tup1 is a general repressor of transcription in yeast. Cell 68, 709–719 (1992).
Tzamarias, D. & Struhl, K. Functional dissection of the yeast Cyc8-Tup1 transcriptional co-repressor complex. Nature 369, 758–761 (1994).
Smith, R. L. & Johnson, A. D. Turning genes off by Ssn6-Tup1: a conserved system of transcriptional repression in eukaryotes. Trends Biochem. Sci. 25, 325–330 (2000).
Mizuno, T. et al. The Tup1-Ssn6 general repressor is involved in repression of IME1 encoding a transcriptional activator of meiosis in Saccharomyces cerevisiae. Curr. Genet. 33, 239–247 (1998).
Parnell, E. J. & Stillman, D. J. Shields up: the Tup1-Cyc8 repressor complex blocks coactivator recruitment. Genes Dev. 25, 2429–2435 (2011).
Malave, T. M. & Dent, S. Y. Transcriptional repression by Tup1-Ssn6. Biochem. Cell Biol. 84, 437–443 (2006).
Wong, K. H. & Struhl, K. The Cyc8-Tup1 complex inhibits transcription primarily by masking the activation domain of the recruiting protein. Genes Dev. 25, 2525–2539 (2011).
Nishimura, K., Fukagawa, T., Takisawa, H., Kakimoto, T. & Kanemaki, M. An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat. Methods 6, 917–922 (2009).
Raj, A., van den Bogaard, P., Rifkin, S. A., van Oudenaarden, A. & Tyagi, S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat. Methods 5, 877–879 (2008).
Hanlon, S. E., Rizzo, J. M., Tatomer, D. C., Lieb, J. D. & Buck, M. J. The stress response factors Yap6, Cin5, Phd1, and Skn7 direct targeting of the conserved co-repressor Tup1-Ssn6 in S. cerevisiae. PLoS ONE 6, e19060 (2011).
Park, S. H., Koh, S. S., Chun, J. H., Hwang, H. J. & Kang, H. S. Nrg1 is a transcriptional repressor for glucose repression of STA1 gene expression in Saccharomyces cerevisiae. Mol. Cell Biol. 19, 2044–2050 (1999).
Proft, M. & Serrano, R. Repressors and upstream repressing sequences of the stress-regulated ENA1 gene in Saccharomyces cerevisiae: bZIP protein Sko1p confers HOG-dependent osmotic regulation. Mol. Cell Biol. 19, 537–546 (1999).
Regnacq, M., Alimardani, P., El Moudni, B. & Berges, T. SUT1p interaction with Cyc8p(Ssn6p) relieves hypoxic genes from Cyc8p-Tup1p repression in Saccharomyces cerevisiae. Mol. Microbiol. 40, 1085–1096 (2001).
Treitel, M. A. & Carlson, M. Repression by SSN6-TUP1 is directed by MIG1, a repressor/activator protein. Proc. Natl Acad. Sci. USA 92, 3132–3136 (1995).
Ozcan, S. & Johnston, M. Three different regulatory mechanisms enable yeast hexose transporter (HXT) genes to be induced by different levels of glucose. Mol. Cell Biol. 15, 1564–1572 (1995).
Berkey, C. D., Vyas, V. K. & Carlson, M. Nrg1 and nrg2 transcriptional repressors are differently regulated in response to carbon source. Eukaryot. Cell 3, 311–317 (2004).
Rizzo, J. M., Mieczkowski, P. A. & Buck, M. J. Tup1 stabilizes promoter nucleosome positioning and occupancy at transcriptionally plastic genes. Nucleic Acids Res. 39, 8803–8819 (2011).
Davie, J. K., Trumbly, R. J. & Dent, S. Y. Histone-dependent association of Tup1-Ssn6 with repressed genes in vivo. Mol. Cell Biol. 22, 693–703 (2002).
Edmondson, D. G., Smith, M. M. & Roth, S. Y. Repression domain of the yeast global repressor Tup1 interacts directly with histones H3 and H4. Genes Dev. 10, 1247–1259 (1996).
Watson, A. D. et al. Ssn6-Tup1 interacts with class I histone deacetylases required for repression. Genes Dev. 14, 2737–2744 (2000).
Davie, J. K., Edmondson, D. G., Coco, C. B. & Dent, S. Y. Tup1-Ssn6 interacts with multiple class I histone deacetylases in vivo. J. Biol. Chem. 278, 50158–50162 (2003).
Wu, J., Suka, N., Carlson, M. & Grunstein, M. TUP1 utilizes histone H3/H2B-specific HDA1 deacetylase to repress gene activity in yeast. Mol. Cell 7, 117–126 (2001).
Fleming, A. B., Beggs, S., Church, M., Tsukihashi, Y. & Pennings, S. The yeast Cyc8-Tup1 complex cooperates with Hda1p and Rpd3p histone deacetylases to robustly repress transcription of the subtelomeric FLO1 gene. Biochim. Biophys. Acta 1839, 1242–1255 (2014).
Loewith, R. & Hall, M. N. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics 189, 1177–1201 (2011).
Conrad, M. et al. Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 38, 254–299 (2014).
Green, S. R. & Johnson, A. D. Promoter-dependent roles for the Srb10 cyclin-dependent kinase and the Hda1 deacetylase in Tup1-mediated repression in Saccharomyces cerevisiae. Mol. Biol. Cell 15, 4191–4202 (2004).
Wasserstrom, L., Dunkler, A., Walther, A. & Wendland, J. The APSES protein Sok2 is a positive regulator of sporulation in Ashbya gossypii. Mol. Microbiol. 106, 949–960 (2017).
Rodrigues-Pousada, C. et al. Yeast AP-1 like TFs (Yap) and stress response: a current overview. Microb. Cell 6, 267–285 (2019).
Proft, M. & Struhl, K. Hog1 kinase converts the Sko1-Cyc8-Tup1 repressor complex into an activator that recruits SAGA and SWI/SNF in response to osmotic stress. Mol. Cell 9, 1307–1317 (2002).
Ng, C. H. et al. Sumoylation controls the timing of Tup1-mediated transcriptional deactivation. Nat. Commun. 6, 6610 (2015).
Oeser, M. L. et al. Dynamic sumoylation of a conserved transcription corepressor prevents persistent inclusion formation during hyperosmotic stress. PLoS Genet. 12, e1005809 (2016).
Buscarlet, M. & Stifani, S. The ‘Marx’ of Groucho on development and disease. Trends Cell Biol. 17, 353–361 (2007).
Liu, Z. & Karmarkar, V. Groucho/Tup1 family co-repressors in plant development. Trends Plant Sci. 13, 137–144 (2008).
Turki-Judeh, W. & Courey, A. J. Groucho: a corepressor with instructive roles in development. Curr. Top. Dev. Biol. 98, 65–96 (2012).
Jin, F., Li, Y., Ren, B. & Natarajan, R. Enhancers: multi-dimensional signal integrators. Transcription 2, 226–230 (2011).
Pott, S. & Lieb, J. D. What are super-enhancers? Nat. Genet. 47, 8–12 (2015).
Rickels, R. & Shilatifard, A. Enhancer logic and mechanics in development and disease. Trends Cell Biol. 28, 608–630 (2018).
Spitz, F. & Furlong, E. E. TFs: from enhancer binding to developmental control. Nat. Rev. Genet. 13, 613–626 (2012).
Longtine, M. S. et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 (1998).
Arguello-Miranda, O., Liu, Y., Wood, N. E., Kositangool, P. & Doncic, A. Integration of multiple metabolic signals determines cell fate prior to commitment. Mol. Cell 71, 733–744 e711 (2018).
Youk, H. & Lim, W. A. Secreting and sensing the same molecule allows cells to achieve versatile social behaviors. Science 343, 1242782 (2014).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
de Boer, C. G. & Hughes, T. R. YeTFaSCo: a database of evaluated yeast transcription factor sequence specificities. Nucleic Acids Res. 40, D169–D179 (2012).
Acknowledgements
We are grateful to Andrew Wu and Fabien Moretto for their critical reading of the paper, and to Andreas Doncic for providing the mNeongreen tagging cassette. This work was supported by the Francis Crick Institute (FC001203), which receives its core funding from Cancer Research UK (FC001203), the UK Medical Research Council (FC001203) and the Wellcome Trust (FC001203).
Author information
Authors and Affiliations
Contributions
Conceptualisation: F.W. and J.T., Methodology: F.W. and J.T., Investigation: J.T., Resources: F.W. and J.T., Writing and Revisions: F.W. and J.T., Supervision: F.W., Funding acquisition: F.W.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source Data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tam, J., van Werven, F.J. Regulated repression governs the cell fate promoter controlling yeast meiosis. Nat Commun 11, 2271 (2020). https://doi.org/10.1038/s41467-020-16107-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-020-16107-w
This article is cited by
-
Sublinear scaling of the cellular proteome with ploidy
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.