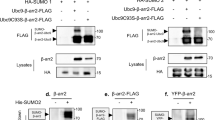
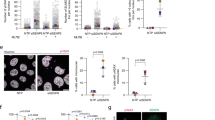
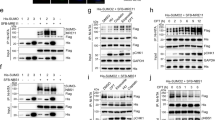
Abstract
The retinoblastoma tumor suppressor protein (RB) plays a critical role in cell proliferation and differentiation and its inactivation is a frequent underlying factor in tumorigenesis. While the regulation of RB function by phosphorylation is well studied, proteasome-mediated RB protein degradation is emerging as an important regulatory mechanism. Although our understanding of RB turnover is currently limited, there is evidence that the nuclear lamina filament protein Lamin A/C protects RB from proteasomal degradation. Here we show that SUMO1 conjugation of RB and Lamin A/C is modulated by the SUMO protease SENP1 and that sumoylation of both proteins is required for their interaction. Importantly, this SUMO1-dependent complex protects both RB and Lamin A/C from proteasomal turnover.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ezhevsky SA, Ho A, Becker-Hapak M, Davis PK, Dowdy SF . Differential regulation of retinoblastoma tumor suppressor protein by G(1) cyclin-dependent kinase complexes in vivo. Mol Cell Biol 2001; 21: 4773–4784.
Harbour JW, Dean DC . The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev 2000; 14: 2393–2409.
Harbour JW, Dean DC . Rb function in cell-cycle regulation and apoptosis. Nat Cell Biol 2000; 2: E65–E67.
Narasimha AM, Kaulich M, Shapiro GS, Choi YJ, Sicinski P, Dowdy SF . Cyclin D activates the Rb tumor suppressor by mono-phosphorylation. Elife 2014, e-pub ahead of print 4 June 2014 doi:10.7554/eLife.02872.
Macdonald JI, Dick FA . Posttranslational modifications of the retinoblastoma tumor suppressor protein as determinants of function. Genes Cancer 2012; 3: 619–633.
Ying H, Xiao ZX . Targeting retinoblastoma protein for degradation by proteasomes. Cell Cycle 2006; 5: 506–508.
Dechat T, Adam SA, Taimen P, Shimi T, Goldman RD . Nuclear lamins. Cold Spring Harb Perspect Biol 2010; 2: a000547.
Dittmer TA, Misteli T . The lamin protein family. Genome Biol 2011; 12: 222.
Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L et al. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev 2008; 22: 832–853.
Dechat T, Gesson K, Foisner R . Lamina-independent lamins in the nuclear interior serve important functions. Cold Spring Harb Symp Quant Biol 2010; 75: 533–543.
Bonne G, Di Barletta MR, Varnous S, Becane HM, Hammouda EH, Merlini L et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet 1999; 21: 285–288.
Fatkin D, MacRae C, Sasaki T, Wolff MR, Porcu M, Frenneaux M et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med 1999; 341: 1715–1724.
Muchir A, Bonne G, van der Kooi AJ, van Meegen M, Baas F, Bolhuis PA et al. Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B). Hum Mol Genet 2000; 9: 1453–1459.
Cao H, Hegele RA . LMNA is mutated in Hutchinson-Gilford progeria (MIM 176670) but not in Wiedemann-Rautenstrauch progeroid syndrome (MIM 264090). J Hum Genet 2003; 48: 271–274.
De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I et al. Lamin a truncation in Hutchinson-Gilford progeria. Science 2003; 300: 2055.
Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 2003; 423: 293–298.
Butin-Israeli V, Adam SA, Goldman AE, Goldman RD . Nuclear lamin functions and disease. Trends Genet 2012; 28: 464–471.
Maraldi NM, Capanni C, Cenni V, Fini M, Lattanzi G . Laminopathies and lamin-associated signaling pathways. J Cell Biochem 2011; 112: 979–992.
Schreiber KH, Kennedy BK . When lamins go bad: nuclear structure and disease. Cell 2013; 152: 1365–1375.
Kennedy BK, Pennypacker JK . RB and lamins in cell cycle regulation and aging. Adv Exp Med Biol 2014; 773: 127–142.
Markiewicz E, Dechat T, Foisner R, Quinlan RA, Hutchison CJ . Lamin A/C binding protein LAP2alpha is required for nuclear anchorage of retinoblastoma protein. Mol Biol Cell 2002; 13: 4401–4413.
Pekovic V, Harborth J, Broers JL, Ramaekers FC, van Engelen B, Lammens M et al. Nucleoplasmic LAP2alpha-lamin A complexes are required to maintain a proliferative state in human fibroblasts. J Cell Biol 2007; 176: 163–172.
Van Berlo JH, Voncken JW, Kubben N, Broers JL, Duisters R, van Leeuwen RE et al. A-type lamins are essential for TGF-beta1 induced PP2A to dephosphorylate transcription factors. Hum Mol Genet 2005; 14: 2839–2849.
Johnson BR, Nitta RT, Frock RL, Mounkes L, Barbie DA, Stewart CL et al. A-type lamins regulate retinoblastoma protein function by promoting subnuclear localization and preventing proteasomal degradation. Proc Natl Acad Sci USA 2004; 101: 9677–9682.
Marji J, O'Donoghue SI, McClintock D, Satagopam VP, Schneider R, Ratner D et al. Defective lamin A-Rb signaling in Hutchinson-Gilford Progeria Syndrome and reversal by farnesyltransferase inhibition. PLoS One 2010; 5: e11132.
Nitta RT, Jameson SA, Kudlow BA, Conlan LA, Kennedy BK . Stabilization of the retinoblastoma protein by A-type nuclear lamins is required for INK4A-mediated cell cycle arrest. Mol Cell Biol 2006; 26: 5360–5372.
Gareau JR, Lima CD . The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition. Nat Rev Mol Cell Biol 2010; 11: 861–871.
Wilkinson KA, Henley JM . Mechanisms, regulation and consequences of protein SUMOylation. Biochem J 2010; 428: 133–145.
Mukhopadhyay D, Dasso M . Modification in reverse: the SUMO proteases. Trends Biochem Sci 2007; 32: 286–295.
Xu Z, Chan HY, Lam WL, Lam KH, Lam LS, Ng TB et al. SUMO proteases: redox regulation and biological consequences. Antioxid Redox Signal 2009; 11: 1453–1484.
Ledl A, Schmidt D, Muller S . Viral oncoproteins E1A and E7 and cellular LxCxE proteins repress SUMO modification of the retinoblastoma tumor suppressor. Oncogene 2005; 24: 3810–3818.
Simon DN, Domaradzki T, Hofmann WA, Wilson KL . Lamin A tail modification by SUMO1 is disrupted by familial partial lipodystrophy-causing mutations. Mol Biol Cell 2013; 24: 342–350.
Zhang YQ, Sarge KD . Sumoylation regulates lamin A function and is lost in lamin A mutants associated with familial cardiomyopathies. J Cell Biol 2008; 182: 35–39.
Sharma P, Yamada S, Lualdi M, Dasso M, Kuehn MR . Senp1 is essential for desumoylating Sumo1-modified proteins but dispensable for Sumo2 and Sumo3 deconjugation in the mouse embryo. Cell Rep 2013; 3: 1640–1650.
Yamaguchi T, Sharma P, Athanasiou M, Kumar A, Yamada S, Kuehn MR . Mutation of SENP1/SuPr-2 reveals an essential role for desumoylation in mouse development. Mol Cell Biol 2005; 25: 5171–5182.
Sharma P, Murillas R, Zhang H, Kuehn MR . N4BP1 is a newly identified nucleolar protein that undergoes SUMO-regulated polyubiquitylation and proteasomal turnover at promyelocytic leukemia nuclear bodies. J Cell Sci 2010; 123: 1227–1234.
Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA . Effects of an Rb mutation in the mouse. Nature 1992; 359: 295–300.
Jiao W, Datta J, Lin HM, Dundr M, Rane SG . Nucleocytoplasmic shuttling of the retinoblastoma tumor suppressor protein via Cdk phosphorylation-dependent nuclear export. J Biol Chem 2006; 281: 38098–38108.
Evdokimov E, Sharma P, Lockett SJ, Lualdi M, Kuehn MR . Loss of SUMO1 in mice affects RanGAP1 localization and formation of PML nuclear bodies, but is not lethal as it can be compensated by SUMO2 or SUMO3. J Cell Sci 2008; 121: 4106–4113.
Ulrich HD . Mutual interactions between the SUMO and ubiquitin systems: a plea of no contest. Trends Cell Biol 2005; 15: 525–532.
Wang J, Sampath A, Raychaudhuri P, Bagchi S . Both Rb and E7 are regulated by the ubiquitin proteasome pathway in HPV-containing cervical tumor cells. Oncogene 2001; 20: 4740–4749.
Hay RT . SUMO: a history of modification. Mol Cell 2005; 18: 1–12.
Dick FA, Rubin SM . Molecular mechanisms underlying RB protein function. Nat Rev Mol Cell Biol 2013; 14: 297–306.
Bawa-Khalfe T, Cheng J, Lin SH, Ittmann MM, Yeh ET . SENP1 induces prostatic intraepithelial neoplasia through multiple mechanisms. J Biol Chem 2010; 285: 25859–25866.
Burdelski C, Menan D, Tsourlakis MC, Kluth M, Hube-Magg C, Melling N et al. The prognostic value of SUMO1/Sentrin specific peptidase 1 (SENP1) in prostate cancer is limited to ERG-fusion positive tumors lacking PTEN deletion. BMC Cancer 2015; 15: 538.
Huang W, He T, Chai C, Yang Y, Zheng Y, Zhou P et al. Triptolide inhibits the proliferation of prostate cancer cells and down-regulates SUMO-specific protease 1 expression. PLoS One 2012; 7: e37693.
Li T, Huang S, Dong M, Gui Y, Wu D . Prognostic impact of SUMO-specific protease 1 (SENP1) in prostate cancer patients undergoing radical prostatectomy. Urol Oncol 2013; 31: 1539–1545.
Ma C, Wu B, Huang X, Yuan Z, Nong K, Dong B et al. SUMO-specific protease 1 regulates pancreatic cancer cell proliferation and invasion by targeting MMP-9. Tumour Biol 2014; 35: 12729–12735.
Mu J, Zuo Y, Yang W, Chen Z, Liu Z, Tu J et al. Over-expression of small ubiquitin-like modifier proteases 1 predicts chemo-sensitivity and poor survival in non-small cell lung cancer. Chin Med J (Engl) 2014; 127: 4060–4065.
Wang C, Tao W, Ni S, Chen Q, Zhao Z, Ma L et al. Tumor-suppressive microRNA-145 induces growth arrest by targeting SENP1 in human prostate cancer cells. Cancer Sci 2015; 106: 375–382.
Wang Q, Xia N, Li T, Xu Y, Zou Y, Zuo Y et al. SUMO-specific protease 1 promotes prostate cancer progression and metastasis. Oncogene 2013; 32: 2493–2498.
Xu Y, Li J, Zuo Y, Deng J, Wang LS, Chen GQ . SUMO-specific protease 1 regulates the in vitro and in vivo growth of colon cancer cells with the upregulated expression of CDK inhibitors. Cancer Lett 2011; 309: 78–84.
Yan XM, Xu ZQ, Zhou Y, Wang J, Pan J, Yuan LQ et al. SENP1 regulates cell migration and invasion in neuroblastoma. Biotechnol Appl Biochem 2015, e-pub ahead of print 27 March 2015 doi:10.1002/bab.1375.
Ahmed SA, Gogal RM Jr, Walsh JE . A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J Immunol Methods 1994; 170: 211–224.
Acknowledgements
We thank Stefano Campaner (IIT, Milan) for discussions, and Alessandra Mazzoni (AIRC, Milan), Shyam Sharan (NCI, Frederick), Sushil Rane (NIDDK, Bethesda) and Tom Misteli (NCI, Bethesda) for comments on the manuscript. This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Sharma, P., Kuehn, M. SENP1-modulated sumoylation regulates retinoblastoma protein (RB) and Lamin A/C interaction and stabilization. Oncogene 35, 6429–6438 (2016). https://doi.org/10.1038/onc.2016.177
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.177
This article is cited by
-
Post-translational modifications on the retinoblastoma protein
Journal of Biomedical Science (2022)
-
Nuclear accumulation of UBC9 contributes to SUMOylation of lamin A/C and nucleophagy in response to DNA damage
Journal of Experimental & Clinical Cancer Research (2019)