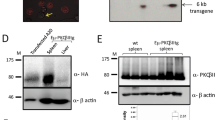
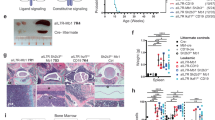
Abstract
The non-receptor protein tyrosine kinase Syk (spleen tyrosine kinase) is an important mediator of signal transduction in B cells. By acting downstream of the B-cell antigen receptor, Syk promotes signaling pathways involved in proliferation, differentiation and survival of B cells. To study the oncogenic potential of Syk, we generated a mouse model for the inducible expression of the leukemia-derived TEL-Syk fusion protein exhibiting constitutive kinase activity. To achieve B-cell-specific expression of TEL-Syk in adult mice, we used a tamoxifen-inducible Cre mouse line. This study shows that inducible expression of TEL-Syk in B cells leads to transient proliferation and subsequent plasma cell differentiation. However, it does not lead to B-cell transformation. Instead, Syk activation induces the tumor suppressor B-lymphocyte-induced maturation protein-1 (Blimp-1), which interferes with the expression of the antiapoptotic protein Bcl-2. Combined induction of TEL-Syk with transgenic expression of Bcl-2 results in a severe phenotype and plasma cell expansion. Our results suggest that deregulated Syk activity by itself is not sufficient for the transformation of B cells, as downstream effectors, such as Blimp-1, limit the survival and expansion of the activated B cell.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Beitz LO, Fruman DA, Kurosaki T, Cantley LC, Scharenberg AM . SYK is upstream of phosphoinositide 3-kinase in B cell receptor signaling. J Biol Chem 1999; 274: 32662–32666.
Marshall AJ, Niiro H, Yun TJ, Clark EA . Regulation of B-cell activation and differentiation by the phosphatidylinositol 3-kinase and phospholipase Cgamma pathway. Immunol Rev 2000; 176: 30–46.
Werner M, Hobeika E, Jumaa H . Role of PI3K in the generation and survival of B cells. Immunol Rev 2010; 237: 55–71.
Kallies A, Nutt SL . Terminal differentiation of lymphocytes depends on Blimp-1. Curr Opin Immunol 2007; 19: 156–162.
Martins G, Calame K . Regulation and functions of Blimp-1 in T and B lymphocytes. Annu Rev Immunol 2008; 26: 133–169.
Shapiro-Shelef M, Lin KI, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, Calame K . Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity 2003; 19: 607–620.
Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 2002; 17: 51–62.
Calado DP, Zhang B, Srinivasan L, Sasaki Y, Seagal J, Unitt C et al. Constitutive canonical NF-kappaB activation cooperates with disruption of BLIMP1 in the pathogenesis of activated B cell-like diffuse large cell lymphoma. Cancer Cell 2010; 18: 580–589.
Mandelbaum J, Bhagat G, Tang H, Mo T, Brahmachary M, Shen Q et al. BLIMP1 is a tumor suppressor gene frequently disrupted in activated B cell-like diffuse large B cell lymphoma. Cancer Cell 2010; 18: 568–579.
Kanie T, Abe A, Matsuda T, Kuno Y, Towatari M, Yamamoto T et al. TEL-Syk fusion constitutively activates PI3-K/Akt, MAPK and JAK2-independent STAT5 signal pathways. Leukemia 2004; 18: 548–555.
Wossning T, Herzog S, Kohler F, Meixlsperger S, Kulathu Y, Mittler G et al. Deregulated Syk inhibits differentiation and induces growth factor-independent proliferation of pre-B cells. J Exp Med 2006; 203: 2829–2840.
Gobessi S, Laurenti L, Longo PG, Carsetti L, Berno V, Sica S et al. Inhibition of constitutive and BCR-induced Syk activation downregulates Mcl-1 and induces apoptosis in chronic lymphocytic leukemia B cells. Leukemia 2009; 23: 686–697.
Leseux L, Hamdi SM, Al Saati T, Capilla F, Recher C, Laurent G et al. Syk-dependent mTOR activation in follicular lymphoma cells. Blood 2006; 108: 4156–4162.
Rinaldi A, Kwee I, Taborelli M, Largo C, Uccella S, Martin V et al. Genomic and expression profiling identifies the B-cell associated tyrosine kinase Syk as a possible therapeutic target in mantle cell lymphoma. Br J Haematol 2006; 132: 303–316.
Young RM, Hardy IR, Clarke RL, Lundy N, Pine P, Turner BC et al. Mouse models of non-Hodgkin lymphoma reveal Syk as an important therapeutic target. Blood 2009; 113: 2508–2516.
Chen L, Monti S, Juszczynski P, Daley J, Chen W, Witzig TE et al. SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood 2008; 111: 2230–2237.
Riccaboni M, Bianchi I, Petrillo P . Spleen tyrosine kinases: biology, therapeutic targets and drugs. Drug Discov Today 2010; 15: 517–530.
Kuno Y, Abe A, Emi N, Iida M, Yokozawa T, Towatari M et al. Constitutive kinase activation of the TEL-Syk fusion gene in myelodysplastic syndrome with t(9;12)(q22;p12). Blood 2001; 97: 1050–1055.
Kulathu Y, Hobeika E, Turchinovich G, Reth M . The kinase Syk as an adaptor controlling sustained calcium signalling and B-cell development. EMBO J 2008; 27: 1333–1344.
Soriano P . Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 1999; 21: 70–71.
Feil R, Wagner J, Metzger D, Chambon P . Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun 1997; 237: 752–757.
Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 2001; 1: 4.
Shaffer AL, Shapiro-Shelef M, Iwakoshi NN, Lee AH, Qian SB, Zhao H et al. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 2004; 21: 81–93.
Saijo K, Schmedt C, Su IH, Karasuyama H, Lowell CA, Reth M et al. Essential role of Src-family protein tyrosine kinases in NF-kappaB activation during B cell development. Nat Immunol 2003; 4: 274–279.
Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K . Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell 2004; 117: 787–800.
Cretney E, Xin A, Shi W, Minnich M, Masson F, Miasari M et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol 2011; 12: 304–311.
Strasser A, Whittingham S, Vaux DL, Bath ML, Adams JM, Cory S et al. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci USA 1991; 88: 8661–8665.
Visan I, Goller M, Berberich I, Kneitz C, Tony HP . Pax-5 is a key regulator of the B cell-restricted expression of the CD23a isoform. Eur J Immunol 2003; 33: 1163–1173.
Holmes ML, Pridans C, Nutt SL . The regulation of the B-cell gene expression programme by Pax5. Immunol Cell Biol 2008; 86: 47–53.
Grossmann M, O'Reilly LA, Gugasyan R, Strasser A, Adams JM, Gerondakis S . The anti-apoptotic activities of Rel and RelA required during B-cell maturation involve the regulation of Bcl-2 expression. EMBO J 2000; 19: 6351–6360.
Merino D, Khaw SL, Glaser SP, Anderson DJ, Belmont LD, Wong C et al. Bcl-2, Bcl-x(L), and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood 2012; 119: 5807–5816.
Carrington EM, Vikstrom IB, Light A, Sutherland RM, Londrigan SL, Mason KD et al. BH3 mimetics antagonizing restricted prosurvival Bcl-2 proteins represent another class of selective immune modulatory drugs. Proc Natl Acad Sci USA 2010; 107: 10967–10971.
Peperzak V, Vikstrom I, Walker J, Glaser SP, LePage M, Coquery CM et al. Mcl-1 is essential for the survival of plasma cells. Nat Immunol 2013; 14: 290–297.
De Vos J, Hose D, Reme T, Tarte K, Moreaux J, Mahtouk K et al. Microarray-based understanding of normal and malignant plasma cells. Immunol Rev 2006; 210: 86–104.
Knodel M, Kuss AW, Lindemann D, Berberich I, Schimpl A . Reversal of Blimp-1-mediated apoptosis by A1, a member of the Bcl-2 family. Eur J Immunol 1999; 29: 2988–2998.
Kallies A, Hawkins ED, Belz GT, Metcalf D, Hommel M, Corcoran LM et al. Transcriptional repressor Blimp-1 is essential for T cell homeostasis and self-tolerance. Nat Immunol 2006; 7: 466–474.
Homig-Holzel C, Hojer C, Rastelli J, Casola S, Strobl LJ, Muller W et al. Constitutive CD40 signaling in B cells selectively activates the noncanonical NF-kappaB pathway and promotes lymphomagenesis. J Exp Med 2008; 205: 1317–1329.
Herzog S, Hug E, Meixlsperger S, Paik JH, DePinho RA, Reth M et al. SLP-65 regulates immunoglobulin light chain gene recombination through the PI(3)K-PKB-Foxo pathway. Nat Immunol 2008; 9: 623–631.
Adachi T, Schamel WW, Kim KM, Watanabe T, Becker B, Nielsen PJ et al. The specificity of association of the IgD molecule with the accessory proteins BAP31/BAP29 lies in the IgD transmembrane sequence. EMBO J 1996; 15: 1534–1541.
Acknowledgements
We thank A Nil for critical discussion and help with mouse analyses. We thank M Bach, C Eschbach, I Fiedler, C Sainz Rueda for technical assistance; U Zimber-Strobl for providing Rosa26-targeting construct; Benoit Kanzler and the transgenic mouse unit for ES cell injection; A Wuerch and S Hobitz for cell sorting; F Koehler and M Bach for proofreading the manuscript; S Srinivas for the Rosa26-YFP mice, A Strasser for the Bcl-2 tg mice, A Tarakhovsky for the Sykfl/fl mice and K Rajewsky for the CD21-Cre mice. This work was supported by the Deutsche Krebshilfe (project no. 108935), the Deutsche Forschungsgemeinschaft (SFB620) and the Excellence Initiative of the German Federal and State Governments (EXC294).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Author contributions
E Hug generated the Rosa26-TS mouse strain, designed and performed the experiments, analyzed the data and wrote the manuscript; E Hobeika and MR provided mb1-CreERT2 mice; E Hobeika carried out the tamoxifen treatment of mice, provided materials and suggestions for experimental design; and HJ supervised the work, designed experiments and wrote the manuscript together with E Hug.
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Hug, E., Hobeika, E., Reth, M. et al. Inducible expression of hyperactive Syk in B cells activates Blimp-1-dependent terminal differentiation. Oncogene 33, 3730–3741 (2014). https://doi.org/10.1038/onc.2013.326
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.326
Keywords
This article is cited by
-
Targeted PI3K/AKT-hyperactivation induces cell death in chronic lymphocytic leukemia
Nature Communications (2021)
-
Metabolic gatekeepers to safeguard against autoimmunity and oncogenic B cell transformation
Nature Reviews Immunology (2019)
-
Autoimmunity checkpoints as therapeutic targets in B cell malignancies
Nature Reviews Cancer (2018)
-
Secreted IgM deficiency leads to increased BCR signaling that results in abnormal splenic B cell development
Scientific Reports (2017)
-
Autonomous membrane IgE signaling prevents IgE-memory formation
Nature Immunology (2016)