Abstract
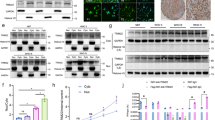
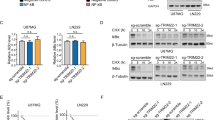
The transcription factor GLI2 has an important role in the transduction of Hedgehog signaling and thereby regulates tumorigenesis in a wide variety of human tumors. However, the mechanisms controlling GLI2 protein expression and stabilization are incompletely understood. In this study, we show that the mitogen-activated protein kinase MEK1 modulates GLI2 both at the mRNA and protein level. Constitutively activated MEK1 prolonged the half-life of GLI2 and increased its nuclear translocation, accompanied by attenuated ubiquitination of GLI2 protein. RSK2, a protein kinase lying downstream of MEK–ERK cascade, mimicked the effect of MEK on GLI2 stabilization. MEK1 and RSK2 failed to augment the half-life of GLI2 lacking GSK-3β phosphorylation sites, indicating that MEK–RSK stabilizes GLI2 by controlling targeting GSK-3β-mediated phosphorylation and ubiquitination of GLI2. The significance of MEK–RSK stabilization was demonstrated in experiments showing that activation of MEK–RSK paralleled higher protein level of GLI2 in several multiple myelomas (MM) cells relative to normal B cells. Moreover, combined treatment with RSK and GLI inhibitors led to an enhanced apoptosis of MM cells. Thus, our results indicate that MEK–RSK cascade positively regulates GLI2 stabilization and represses its degradation via inhibiting GSK-3β-dependent phosphorylation and ubiquitination of GLI2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Jiang J, Hui CC . Hedgehog signaling in development and cancer. Dev Cell 2008; 15: 801–812.
Cai J, Deng L . [Regulations of hedgehog signaling pathway on mesenchymal stem cells]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2010; 24: 993–996.
Fujii K, Miyashita T . [Hedgehog signaling pathway and human disorders]. No To Hattatsu 2009; 41: 247–252.
Onishi H, Katano M . Hedgehog signaling pathway as a therapeutic target in various types of cancer. Cancer Sci 2011; 102: 1756–1760.
Varjosalo M, Taipale J . Hedgehog: functions and mechanisms. Genes Dev 2008; 22: 2454–2472.
Duman-Scheel M, Weng L, Xin S, Du W . Hedgehog regulates cell growth and proliferation by inducing Cyclin D and Cyclin E. Nature 2002; 417: 299–304.
Aza-Blanc P, Lin HY, Ruiz i Altaba A, Kornberg TB . Expression of the vertebrate gli proteins in drosophila reveals a distribution of activator and repressor activities. Development 2000; 127: 4293–4301.
Dai P, Akimaru H, Tanaka Y, Maekawa T, Nakafuku M, Ishii S . Sonic hedgehog-induced activation of the gli1 promoter is mediated by GLI3. J Biol Chem 1999; 274: 8143–8152.
Bhatia N, Thiyagarajan S, Elcheva I, Saleem M, Dlugosz A, Mukhtar H et al. Gli2 is targeted for ubiquitination and degradation by beta-TrCP ubiquitin ligase. J Biol Chem 2006; 281: 19320–19326.
Pan Y, Wang C, Wang B . Phosphorylation of Gli2 by protein kinase A is required for Gli2 processing and degradation and the sonic hedgehog-regulated mouse development. Dev Biol 2009; 326: 177–189.
Marini KD, Payne BJ, Watkins DN, Martelotto LG . Mechanisms of hedgehog signalling in cancer. Growth Factors 2011; 29: 221–234.
Teichert AE, Elalieh H, Elias PM, Welsh J, Bikle DD . Overexpression of hedgehog signaling is associated with epidermal tumor formation in vitamin D receptor-null mice. J Invest Dermatol 2011; 131: 2289–2297.
Alexaki VI, Javelaud D, Van Kempen LC, Mohammad KS, Dennler S, Luciani F et al. GLI2-mediated melanoma invasion and metastasis. J Natl Cancer Inst 2010; 102: 1148–1159.
Thomas GM, Rumbaugh GR, Harrar DB, Huganir RL . Ribosomal S6 kinase 2 interacts with and phosphorylates PDZ domain-containing proteins and regulates AMPA receptor transmission. Proc Natl Acad Sci USA 2005; 102: 15006–15011.
Tam M, Lin P, Hu P, Lennon PA . Examining hedgehog pathway genes GLI3, SHH, and PTCH1 and the p53 target GLIPR1/GLIPR1L1/GLIPR1L2 gene cluster using fluorescence in situ hybridization uncovers GLIPR1/GLIPR1L1/GLIPR1L2 deletion in 9% of patients with multiple myeloma. J Assoc Genet Technol 2010; 36: 111–114.
Javelaud D, Alexaki VI, Dennler S, Mohammad KS, Guise TA, Mauviel A . TGF-beta/SMAD/GLI2 signaling axis in cancer progression and metastasis. Cancer Res 2011; 71: 5606–5610.
Kump E, Ji J, Wernli M, Hausermann P, Erb P . Gli2 upregulates cFlip and renders basal cell carcinoma cells resistant to death ligand-mediated apoptosis. Oncogene 2008; 27: 3856–3864.
Thiyagarajan S, Bhatia N, Reagan-Shaw S, Cozma D, Thomas-Tikhonenko A, Ahmad N et al. Role of GLI2 transcription factor in growth and tumorigenicity of prostate cells. Cancer Res 2007; 67: 10642–10646.
Grachtchouk M, Mo R, Yu S, Zhang X, Sasaki H, Hui CC et al. Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat Genet 2000; 24: 216–217.
Kim Y, Yoon JW, Xiao X, Dean NM, Monia BP, Marcusson EG . Selective down-regulation of glioma-associated oncogene 2 inhibits the proliferation of hepatocellular carcinoma cells. Cancer Res 2007; 67: 3583–3593.
Kim J, Kato M, Beachy PA . Gli2 trafficking links hedgehog-dependent activation of smoothened in the primary cilium to transcriptional activation in the nucleus. Proc Natl Acad Sci USA 2009; 106: 21666–21671.
Pan Y, Bai CB, Joyner AL, Wang B . Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol 2006; 26: 3365–3377.
Jiao X, Wood LD, Lindman M, Jones S, Buckhaults P, Polyak K et al. Somatic mutations in the notch, NF-KB, PIK3CA, and hedgehog pathways in human breast cancers. Genes Chromosomes Cancer 2012; 51: 480–489.
Keshet Y, Seger R . The MAP kinase signaling cascades: a system of hundreds of components regulates a diverse array of physiological functions. Methods Mol Biol 2010; 661: 3–38.
Lauth M, Toftgard R . Non-canonical activation of GLI transcription factors: implications for targeted anti-cancer therapy. Cell Cycle 2007; 6: 2458–2463.
Lewis MT, Visbal AP . The hedgehog signaling network, mammary stem cells, and breast cancer: connections and controversies. Ernst Schering Found Symp Proc 2006. 181–217.
Reinhold MI, Kapadia RM, Liao Z, Naski MC . The Wnt-inducible transcription factor Twist1 inhibits chondrogenesis. J Biol Chem 2006; 281: 1381–1388.
Liu Z, Li T, Liu Y, Jia Z, Li Y, Zhang C et al. WNT signaling promotes Nkx2.5 expression and early cardiomyogenesis via downregulation of Hdac1. Biochim Biophys Acta 2009; 1793: 300–311.
Acknowledgements
This work was supported by National Institutes of Health R01 (AR053100) (M C Naski), the National Natural Sciences Foundation of China (31101057), and the Sciences Foundation of Zhejiang Normal University (ZC304009172). We thank Dr Natalia Riobo from Thomas Jefferson University; Dr John Blenis from Department of Cell Biology of Harvard Medical School; Dr Richard L Huganir from the Department of Neuroscience of Howard Hughes Medical Institute; Dr Fritz Aberger, University of Salzburg; and Dr Baolin Wang, Associate Professor of Cell and Developmental Biology Weill Cornell Medical College, for their generous contributions of the plasmids used in this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, Z., Li, T., Reinhold, M. et al. MEK1–RSK2 contributes to hedgehog signaling by stabilizing GLI2 transcription factor and inhibiting ubiquitination. Oncogene 33, 65–73 (2014). https://doi.org/10.1038/onc.2012.544
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.544
Keywords
This article is cited by
-
High GLI-1 Expression is a Reliable Indicator of Bad Prognosis in Newly Diagnosed Acute Leukemia Patients
Indian Journal of Hematology and Blood Transfusion (2023)
-
GLI3: a mediator of genetic diseases, development and cancer
Cell Communication and Signaling (2020)
-
Proteasome inhibitor induced SIRT1 deacetylates GLI2 to enhance hedgehog signaling activity and drug resistance in multiple myeloma
Oncogene (2020)
-
Hedgehog/GLI1 activation leads to leukemic transformation of myelodysplastic syndrome in vivo and GLI1 inhibition results in antitumor activity
Oncogene (2019)
-
The Effect of SHH-Gli Signaling Pathway on the Synovial Fibroblast Proliferation in Rheumatoid Arthritis
Inflammation (2016)