Abstract
Control of mRNA levels, a fundamental aspect in the regulation of gene expression, is achieved through a balance between mRNA synthesis and decay. E26-related gene (Erg) proteins are canonical transcription factors whose previously described functions are confined to the control of mRNA synthesis. Here, we report that ERG also regulates gene expression by affecting mRNA stability and identify the molecular mechanisms underlying this function in human cells. ERG is recruited to mRNAs via interaction with the RNA-binding protein RBPMS, and it promotes mRNA decay by binding CNOT2, a component of the CCR4–NOT deadenylation complex. Transcriptome-wide mRNA stability analysis revealed that ERG controls the degradation of a subset of mRNAs highly connected to Aurora signaling, whose decay during S phase is necessary for mitotic progression. Our data indicate that control of gene expression by mammalian transcription factors may follow a more complex scheme than previously anticipated, integrating mRNA synthesis and degradation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Braun, K.A. & Young, E.T. Coupling mRNA synthesis and decay. Mol. Cell. Biol. 34, 4078–4087 (2014).
Darnell, J.E. Jr. Reflections on the history of pre-mRNA processing and highlights of current knowledge: a unified picture. RNA 19, 443–460 (2013).
Wahle, E. & Winkler, G.S. RNA decay machines: deadenylation by the Ccr4-not and Pan2-Pan3 complexes. Biochim. Biophys. Acta 1829, 561–570 (2013).
Keene, J.D. RNA regulons: coordination of post-transcriptional events. Nat. Rev. Genet. 8, 533–543 (2007).
Chen, C.Y. & Shyu, A.B. Deadenylation and P-bodies. Adv. Exp. Med. Biol. 768, 183–195 (2013).
Marguerat, S., Lawler, K., Brazma, A. & Bähler, J. Contributions of transcription and mRNA decay to gene expression dynamics of fission yeast in response to oxidative stress. RNA Biol. 11, 702–714 (2014).
Shalem, O., Groisman, B., Choder, M., Dahan, O. & Pilpel, Y. Transcriptome kinetics is governed by a genome-wide coupling of mRNA production and degradation: a role for RNA Pol II. PLoS Genet. 7, e1002273 (2011).
Sun, M. et al. Global analysis of eukaryotic mRNA degradation reveals Xrn1-dependent buffering of transcript levels. Mol. Cell 52, 52–62 (2013).
Sun, M. et al. Comparative dynamic transcriptome analysis (cDTA) reveals mutual feedback between mRNA synthesis and degradation. Genome Res. 22, 1350–1359 (2012).
Collart, M.A. & Timmers, H.T. The eukaryotic Ccr4-not complex: a regulatory platform integrating mRNA metabolism with cellular signaling pathways? Prog. Nucleic Acid Res. Mol. Biol. 77, 289–322 (2004).
Miller, J.E. & Reese, J.C. Ccr4-Not complex: the control freak of eukaryotic cells. Crit. Rev. Biochem. Mol. Biol. 47, 315–333 (2012).
Bregman, A. et al. Promoter elements regulate cytoplasmic mRNA decay. Cell 147, 1473–1483 (2011).
Trcek, T., Larson, D.R., Moldón, A., Query, C.C. & Singer, R.H. Single-molecule mRNA decay measurements reveal promoter- regulated mRNA stability in yeast. Cell 147, 1484–1497 (2011).
Haimovich, G., Choder, M., Singer, R.H. & Trcek, T. The fate of the messenger is pre-determined: a new model for regulation of gene expression. Biochim. Biophys. Acta 1829, 643–653 (2013).
Sharrocks, A.D. The ETS-domain transcription factor family. Nat. Rev. Mol. Cell Biol. 2, 827–837 (2001).
Hollenhorst, P.C., Shah, A.A., Hopkins, C. & Graves, B.J. Genome-wide analyses reveal properties of redundant and specific promoter occupancy within the ETS gene family. Genes Dev. 21, 1882–1894 (2007).
Hollenhorst, P.C., McIntosh, L.P. & Graves, B.J. Genomic and biochemical insights into the specificity of ETS transcription factors. Annu. Rev. Biochem. 80, 437–471 (2011).
Uhlen, M. et al. Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol. 28, 1248–1250 (2010).
Eulalio, A., Behm-Ansmant, I. & Izaurralde, E. P bodies: at the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 8, 9–22 (2007).
Castello, A. et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell 149, 1393–1406 (2012).
Singh, G., Ricci, E.P. & Moore, M.J. RIPiT-Seq: a high-throughput approach for footprinting RNA:protein complexes. Methods 65, 320–332 (2014).
Norris, J.D., Fan, D., Sherk, A. & McDonnell, D.P. A negative coregulator for the human ER. Mol. Endocrinol. 16, 459–468 (2002).
Decker, C.J. & Parker, R. P-bodies and stress granules: possible roles in the control of translation and mRNA degradation. Cold Spring Harb. Perspect. Biol. 4, a012286 (2012).
Tourrière, H. et al. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J. Cell Biol. 160, 823–831 (2003).
Kedersha, N.L., Gupta, M., Li, W., Miller, I. & Anderson, P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J. Cell Biol. 147, 1431–1442 (1999).
Barreau, C., Watrin, T., Beverley Osborne, H. & Paillard, L. Protein expression is increased by a class III AU-rich element and tethered CUG-BP1. Biochem. Biophys. Res. Commun. 347, 723–730 (2006).
Hoesel, B. et al. Sequence-function correlations and dynamics of ERG isoforms: ERG8 is the black sheep of the family. Biochim. Biophys. Acta 1863, 205–218 (2016).
Liu, J. et al. A role for the P-body component GW182 in microRNA function. Nat. Cell Biol. 7, 1261–1266 (2005).
Dreze, M. et al. High-quality binary interactome mapping. Methods Enzymol. 470, 281–315 (2010).
Ito, K. et al. CNOT2 depletion disrupts and inhibits the CCR4-NOT deadenylase complex and induces apoptotic cell death. Genes Cells 16, 368–379 (2011).
Winkler, G.S., Mulder, K.W., Bardwell, V.J., Kalkhoven, E. & Timmers, H.T. Human Ccr4-Not complex is a ligand-dependent repressor of nuclear receptor-mediated transcription. EMBO J. 25, 3089–3099 (2006).
Boland, A. et al. Structure and assembly of the NOT module of the human CCR4–NOT complex. Nat. Struct. Mol. Biol. 20, 1289–1297 (2013).
Barckmann, B. & Simonelig, M. Control of maternal mRNA stability in germ cells and early embryos. Biochim. Biophys. Acta 1829, 714–724 (2013).
Kuroyanagi, H. Fox-1 family of RNA-binding proteins. Cell. Mol. Life Sci. 66, 3895–3907 (2009).
Song, H.W. et al. Hermes RNA-binding protein targets RNAs-encoding proteins involved in meiotic maturation, early cleavage, and germline development. Differentiation 75, 519–528 (2007).
Farazi, T.A. et al. Identification of the RNA recognition element of the RBPMS family of RNA-binding proteins and their transcriptome-wide mRNA targets. RNA 20, 1090–1102 (2014).
Sun, S., Zhang, Z., Fregoso, O. & Krainer, A.R. Mechanisms of activation and repression by the alternative splicing factors RBFOX1/2. RNA 18, 274–283 (2012).
Pines, J. & Hunter, T. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J. Cell Biol. 115, 1–17 (1991).
Ross, J. A hypothesis to explain why translation inhibitors stabilize mRNAs in mammalian cells: mRNA stability and mitosis. BioEssays 19, 527–529 (1997).
Nikonova, A.S., Astsaturov, I., Serebriiskii, I.G., Dunbrack, R.L. Jr. & Golemis, E.A. Aurora A kinase (AURKA) in normal and pathological cell division. Cell. Mol. Life Sci. 70, 661–687 (2013).
Ruchaud, S., Carmena, M. & Earnshaw, W.C. Chromosomal passengers: conducting cell division. Nat. Rev. Mol. Cell Biol. 8, 798–812 (2007).
Goler-Baron, V. et al. Transcription in the nucleus and mRNA decay in the cytoplasm are coupled processes. Genes Dev. 22, 2022–2027 (2008).
Lotan, R. et al. The RNA polymerase II subunit Rpb4p mediates decay of a specific class of mRNAs. Genes Dev. 19, 3004–3016 (2005).
Lotan, R., Goler-Baron, V., Duek, L., Haimovich, G. & Choder, M. The Rpb7p subunit of yeast RNA polymerase II plays roles in the two major cytoplasmic mRNA decay mechanisms. J. Cell Biol. 178, 1133–1143 (2007).
Yu, J. et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell 17, 443–454 (2010).
Wilson, N.K. et al. Combinatorial transcriptional control in blood stem/progenitor cells: genome-wide analysis of ten major transcriptional regulators. Cell Stem Cell 7, 532–544 (2010).
Rolland, T. et al. A proteome-scale map of the human interactome network. Cell 159, 1212–1226 (2014).
Carmena, M., Ruchaud, S. & Earnshaw, W.C. Making the Auroras glow: regulation of Aurora A and B kinase function by interacting proteins. Curr. Opin. Cell Biol. 21, 796–805 (2009).
Gill, T., Cai, T., Aulds, J., Wierzbicki, S. & Schmitt, M.E. RNase MRP cleaves the CLB2 mRNA to promote cell cycle progression: novel method of mRNA degradation. Mol. Cell. Biol. 24, 945–953 (2004).
Alvarez, B., Blanco, M.A. & Moreno, S. The fission yeast APC activator Ste9 is regulated by mRNA decay. Cell Cycle 5, 865–868 (2006).
Pai, A.A. et al. The contribution of RNA decay quantitative trait loci to inter-individual variation in steady-state gene expression levels. PLoS Genet. 8, e1003000 (2012).
Rabani, M. et al. Metabolic labeling of RNA uncovers principles of RNA production and degradation dynamics in mammalian cells. Nat. Biotechnol. 29, 436–442 (2011).
Lykke-Andersen, J., Shu, M.D. & Steitz, J.A. Human Upf proteins target an mRNA for nonsense-mediated decay when bound downstream of a termination codon. Cell 103, 1121–1131 (2000).
Souquere, S. et al. Unravelling the ultrastructure of stress granules and associated P-bodies in human cells. J. Cell Sci. 122, 3619–3626 (2009).
Raj, A., van den Bogaard, P., Rifkin, S.A., van Oudenaarden, A. & Tyagi, S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat. Methods 5, 877–879 (2008).
James, P., Halladay, J. & Craig, E.A. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144, 1425–1436 (1996).
Walhout, A.J. & Vidal, M. High-throughput yeast two-hybrid assays for large-scale protein interaction mapping. Methods 24, 297–306 (2001).
Rual, J.F. et al. Towards a proteome-scale map of the human protein-protein interaction network. Nature 437, 1173–1178 (2005).
Keene, J.D., Komisarow, J.M. & Friedersdorf, M.B. RIP-Chip: the isolation and identification of mRNAs, microRNAs and protein components of ribonucleoprotein complexes from cell extracts. Nat. Protoc. 1, 302–307 (2006).
Acknowledgements
We thank the Vidal laboratory at the Center for Cancer Systems Biology at the Dana-Farber Cancer Institute (CCSB, DFCI) for supplying reagents, advice and technical support for the HT-Y2H screen. We thank the Tuschl laboratory (Howard Hughes Medical Institute, Rockefeller University) for sharing reagents. We are grateful to E. Izaurralde (European Molecular Biology Laboratory) for kindly providing HA-tagged CNOT2, CNOT3, CNOT6 and CNOT. We would like to thank the laboratories of the Molecular Biology in Diseases Unit (GIGA-R, ULg) for helpful discussions and C. Vindry from the Kruys laboratory for technical assistance. We thank the GIGA interactomics, imaging and flow cytometry and genotranscriptomics platforms for their technical support. This work was supported by grants from the University of Liège, the Fonds Léon Frédéricq, the Belgian Foundation against Cancer (Special Equipment Fund), the Belgian National Fund for Scientific Research (FNRS), Télévie, the Interuniversity Attraction Poles Program–Belgian Science Policy (IUAP-BELSPO PVI/28 and PVII/13 to F.D. and M. Bollen) and the National Human Genome research Institute (grant R01HG001715 to M.V. and D.E.H.). X.R., J-C.T. and N.S. are supported as an FNRS Research Fellow, Research Associate and Postdoctoral Fellow, respectively. C.D. and K.G. are supported as Télévie-FNRS PhD students.
Author information
Authors and Affiliations
Contributions
F.D. and X.R. conceived the project and designed the experiments. X.R. performed most experiments and processed and analyzed the data. C.D., J.B., E.M., M.C., P.D., M.L., R.S., K.G., R.K., I.S. and V.K. assisted with experiments. D.E.H., M.V., N.S. and J.-C.T. helped perform and analyze the HT-Y2H screen. S.B. performed bioinformatic analysis for the transcriptome-wide decay experiments and contributed to the corresponding manuscript sections. M. Bollen, M. Beullens and B.L. contributed to analysis of ERG in mitotic progression and Aurora kinase regulation and participated in writing the corresponding manuscript sections. T.A.F. assisted in analyzing RBPMS CLIP data. F.D. and X.R. wrote the manuscript, which was edited by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
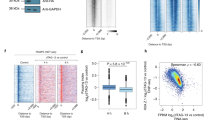
Supplementary Figure 1 ERG localizes in PBs and promotes decay of tethered mRNAs.
Related to Figure 1.
(a) Immunofluorescence of ERG (green), DPC1A (PBs marker, red), EDC4 (PBs marker, white) and Hoechst (nuclear stain, blue) in HeLa cells. Scale bars, 10 µm.
(b) Immunofluorescence of YFP-tagged Erg members FLI1 and FEV (green), RFP-tagged DCP1A (PB marker, in red) and Hoechst (nuclear stain, blue) in HeLa cells. Scale bars, 10 µm.
(c) Immunofluorescence of ERG (green), DPC1A (PBs marker, red), TIA1 (upper panel) or TIAR (lower panel) (SGs markers, white) and Hoechst (nuclear stain, blue) in arsenite-treated HeLa cells. Scale bars, 10 µm.
(d,e) RT-qPCR analysis of ERG mRNA levels (d) and western blot against ERG and control GAPDH (e). Samples are HeLa cells transfected with control (siCTL) or anti-ERG siRNA (siERG). (d) Results are means ± s.d. (n = 4 independent experiments). ***P < 0.001 compared to siCTL condition by two-tailed unpaired Student’s t test.
(f) Luciferase assays in HeLa, U2OS and MRC5 cells transfected with MS2-CP or ERG-MS2-CP together with R-Luc-8MS2 or R-Luc-0MS2 reporters. Results are means of normalized R-Luc activities (white bars) and relative mRNA levels (grey bars) ± s.d. (see Online Methods) (n = 3 independent experiments). *P < 0.05; **P < 0.01; ***P < 0.001 compared to MS2-CP conditions by two-tailed unpaired Student’s t test.
(g,h) RT-qPCR analysis of the stability of the R-Luc-8MS2 mRNA reporter (g) and luciferase assays (h). Samples are RNA (g) or lysates (h) from HeLa cells ActD-treated (g) or not (h) and transfected with MS2-CP (blue, diamond), ERG-MS2-CP (red, squares), or ERG-W334R-MS2-CP (green, triangles) together with R-Luc-8MS2 (or R-Luc-0MS2 (h)) reporters. Results are means ± s.d. (n = 3 independent experiments). *P < 0.05; **P < 0.01; NS, not significant compared to MS2-CP condition by two-tailed unpaired Student’s t test.
(i) Luciferase assays of HeLa cells transfected as in Fig. 1g. Results are means of normalized R-Luc activities ± s.d. (see Online Methods) (n = 3 independent experiments). **P < 0.01; NS, not significant compared to MS2-CP condition by two-tailed unpaired Student’s t test.
(j) Anti-FLAG, -GW182, and -GAPDH western blots. Samples are lysates from HeLa cells transfected as in Fig. 1g.
Supplementary Figure 2 ERG interacts with the CNOT2 component of the CCR4–NOT deadenylation complex and induces mRNA decay.
Related to Figure 2.
(a) Upper panel: Domain structure of CNOT2 and description of CNOT2 deletion mutants. Lower panel: Immunoprecipitation of FLAG-tagged ERG and anti-FLAG and -Myc western blots. Samples are lysates from HEK-293 cells transfected with either Myc-tagged CNOT2 deletion mutants alone or with FLAG-ERG.
(b) Immunofluorescence of ERG (green), CNOT2 (red), G3BP (SGs marker, white), and Hoechst (nuclear stain, blue) in arsenite-treated HeLa cells. Scale bars, 10 µm.
(c) Immunoprecipitation of FLAG-tagged ERG and anti-FLAG and –HA western blot. Samples are lysates from HEK-293 cells transfected with either of HA-tagged subunits of the CCR4-NOT complex (CNOT2, CNOT3, CNOT6, CNOT7) alone or with FLAG-ERG.
(d) Luciferase assays of HeLa cells transfected as in Fig. 2f. Results are means of normalized R-Luc activities ± s.d. (see Online Methods) (n = 3 independent experiments). *P < 0.05; **P < 0.01; NS, not significant compared to MS2-CP condition by two-tailed unpaired Student’s t test.
(e) Luciferase assays of HeLa cells transfected as in Fig. 2g. Results are means of normalized R-Luc activities ± s.d. (see Online Methods) (n = 3 independent experiments). *P < 0.05; **P < 0.01; NS, not significant compared to MS2-CP or siCTL condition by two-tailed unpaired Student’s t test.
(f) Anti-FLAG, -CNOT2, and -GAPDH western blots. Samples are lysates from HeLa cells transfected as in Fig. 2g.
Supplementary Figure 3 ERG is recruited on mRNA through its interaction with RBPMS.
Related to Figure 3.
(a) Immunoprecipitation of ERG and anti-ERG and -RBPMS western blots. Samples are control (CTL) or anti-ERG immunoprecipitates from HeLa cells.
(b) Immunoprecipitation of FLAG-tagged ERG deletion mutants and anti-FLAG and -HA western blots. Samples are lysates from HEK-293 cells transfected with HA-RBPMS alone or with either of the FLAG-tagged Erg members.
(c) Immunoprecipitation of FLAG-tagged ERG and anti-FLAG or -HA western blots. Samples are nontreated (NT) or RNase A-treated lysates from HEK-293 cells transfected with HA-RBPMS alone or with FLAG-ERG.
(d) Upper panel: Domain structure of RBPMS and description of RBPMS deletion mutants. Lower panel: Immunoprecipitation of FLAG-tagged ERG and anti-FLAG and -HA western blots. Samples are lysates from HEK-293 cells transfected with HA-RBPMS alone or with FLAG-ERG.
(e) Immunoprecipitation of RBPMS and western blot against RBPMS, CNOT2 and CNOT3. Samples are control (CTL) or anti-RBPMS immunoprecipitates from HeLa cells.
(f) Immunofluorescence of ERG (green), RBPMS (red), Hoechst (nuclear stain, blue) and CNOT2 (upper panel) or CNOT6 (lower panel) (white). Scale bars, 10 µm. White and red arrows indicate ERG cytoplasmic foci positive for CNOT2 or CNOT6, and positive or negative for RBPMS, respectively.
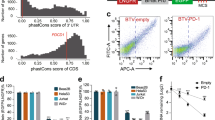
Supplementary Figure 4 ERG promotes decay of mitotic mRNAs.
Related to Figure 4.
(a) List of the 22 mitotic genes (GO:0000279 – M phase) identified in Figure 4a.
(b) RT-qPCR stability analysis of the 22 ERG mitotic targets. Samples are RNA from HeLa cells transfected with control (siCTL: blue, diamonds), anti-ERG (siERG: red, squares), anti-CNOT2 (siCNOT2: green, triangles), or anti-RBPMS siRNA (siRBPMS: purple, circles) (n = 5, 4, 3 and 4 independent experiments respectively) and treated with ActD for 0, 1, 2, or 4 hours. *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant; ND, not determined compared to siCTL condition by two-tailed unpaired Student’s t test.
(c) RT-qPCR analysis of RBPMS mRNA levels (upper panel) and anti-RBPMS and -GAPDH western blots (lower panel). Samples are HeLa cells transfected with control or anti-RBPMS siRNA. Upper panel: Results are means ± s.d. (n = 4 independent experiments). ***P < 0.001 compared to siCTL condition by two-tailed unpaired Student’s t test.
Supplementary Figure 5 ERG depletion promotes mitotic arrest and impairs decay of mitotic mRNAs during the S phase of the cell cycle.
Related to Figure 5.
(a) Quantification of the western blot analyses, one representative of which is shown in Fig. 5b. Samples are lysates from HeLa cells transfected with control (siCTL), anti-ERG (siERG) or anti-CNOT2 siRNA (siCNOT2) and released from a G2-M block. Cyclin signals were normalized to GAPDH, and set to 1 at the time of release (t = 0 h) for each siRNA. Results are means ± s.d. (n = 3 independent experiments). **P < 0.01; NS, not significant for siERG (higher labels) or siCNOT2 (lower labels) conditions compared to siCTL condition by two-tailed unpaired Student’s t test.
(b) Immunofluorescence of Hoechst (chromosomes, blue), ACA (centromeres, white), α-tubulin (mitotic spindle, green), and γ-tubulin (centrosomes, red) in mitotic HeLa cells. Scale bars, 5 µm.
(c) RT-qPCR stability analysis of the 19 validated ERG mitotic targets presented in Fig. 5d. Samples are RNA from HeLa cells transfected with control (plain, triangles) or anti-ERG siRNA (dashed, squares), synchronized in S-phase (blue), late G2-phase (red), or mitosis (green) (n = 4, 3 and 3 independent experiments respectively) and treated with ActD for 0, 2, or 4 hours. Results are means ± s.d. *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant; ND, not determined compared to siCTL condition by two-tailed unpaired Student’s t test.
Supplementary Figure 6 ERG depletion promotes mitotic defects.
Related to Figure 6.
(a-d) Quantification of centrosome defects (a), spindle multipolarity (b), increased length (c) and chromosome congression defects (d) presented in Fig. 6a,b. Results are mean percentages (a,b,d) or length (c) ± s.d. (n = 3 independent experiments, 50-100 (a,b,d) and 5-10 (d) cells in each replicate). *P < 0.05; **P < 0.01; ***P < 0.001 compared to siCTL condition by two-tailed unpaired Student’s t test.
(e) Quantification of mitotic defects. Samples are HeLa cells transfected with control (siCTL), anti-ERG (siERG), anti-CNOT2 (siCNOT2) or anti-RBPMS siRNA (siRBPMS). Results are mean percentages ± s.d. (n = 3 independent experiments). *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant compared to siCTL condition by two-tailed unpaired Student’s t test.
(f) RT-qPCR stability analysis of the 9 Aurora-related ERG mitotic. Samples are RNA from HeLa cells transfected with control (siCTL) or an alternate anti-ERG siRNA (siERG#2), synchronized in S-phase, and treated with ActD for 0, 2, or 4 hours. Results are means ± s.d. (n = 3 independent experiments). Representation is similar to Fig. 4d.
(g) Immunofluorescence of AURKA mRNAs (smFISH, red) and Hoechst (nuclear stain, blue). Samples are HeLa cells transfected with control or anti-ERG siRNA and synchronized in S-phase. Scale bars, 10 µm.
(h) Quantification of the number of AURKA mRNAs in the cytoplasm and nucleus from immunofluorescence images in (g). Results are means ± s.d. (n = 3 independent experiments). *P < 0.05; NS, not significant compared to siCTL condition by two-tailed unpaired Student’s t test.
(i) Quantification of western blot analyses, one representative of which is presented in Fig. 6e. Aurora signals were normalized to GAPDH, and set to 1 in control cells at t = 0 h for AURKA and AURKB, or at t = 14 h for P-Aurora. Results are means ± s.d. (n = 4 independent experiments). *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant compared to siCTL condition by two-tailed unpaired Student’s t test.
(j) Anti-ERG, -phospho-Aurora and -GAPDH western blots. Samples are lysates from HeLa cells transfected with control or anti-ERG siRNAs.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Notes 1–2 (PDF 1999 kb)
Supplementary Data Set 1
Original uncropped western blots (PDF 29563 kb)
Supplementary Table 1
List of ERG-interacting partners identified by HT-Y2H (XLSX 43 kb)
Supplementary Table 2
List of mRNAs whose stability changed after ERG knock-down in HeLa cells (XLSX 72 kb)
Rights and permissions
About this article
Cite this article
Rambout, X., Detiffe, C., Bruyr, J. et al. The transcription factor ERG recruits CCR4–NOT to control mRNA decay and mitotic progression. Nat Struct Mol Biol 23, 663–672 (2016). https://doi.org/10.1038/nsmb.3243
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3243
This article is cited by
-
The X-linked trichothiodystrophy-causing gene RNF113A links the spliceosome to cell survival upon DNA damage
Nature Communications (2020)
-
hnRNPA2B1 inhibits the exosomal export of miR-503 in endothelial cells
Cellular and Molecular Life Sciences (2020)
-
The CCR4-NOT complex contributes to repression of Major Histocompatibility Complex class II transcription
Scientific Reports (2017)