Key Points
-
Mitochondrial homeostasis requires a fine-tuned balance between mitochondrial dynamics and mitochondrial energetics, and ensures the maintenance of properly functioning mitochondria
-
Mitochondria can adapt to different metabolic conditions via the regulation of mechanistic target of rapamycin (mTOR) and AMP-activated protein kinase (AMPK) nutrient sensing pathways, to maintain a healthy population of mitochondria
-
External stimuli can augment mitochondrial processes, such as mitophagy, fission and fusion, and mitochondrial biogenesis to attenuate irregular levels of ATP production
-
The disruption of mitochondrial homeostasis in the early stages of acute kidney injury is an important factor that drives tubular injury and persistent renal dysfunction
-
Hyperglycaemia-induced ATP depletion triggers changes in mitochondrial morphology that lead to the onset of diabetic nephropathy in diabetes mellitus
-
Correcting abnormal electron transport chain function directly, and/or by targeting the pathways that regulate mitochondrial biogenesis, is likely to improve renal outcomes by restoring mitochondrial function and stimulating organ repair
Abstract
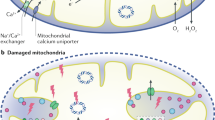
The kidney requires a large number of mitochondria to remove waste from the blood and regulate fluid and electrolyte balance. Mitochondria provide the energy to drive these important functions and can adapt to different metabolic conditions through a number of signalling pathways (for example, mechanistic target of rapamycin (mTOR) and AMP-activated protein kinase (AMPK) pathways) that activate the transcriptional co-activator peroxisome proliferator-activated receptor-γ co-activator 1α (PGC1α), and by balancing mitochondrial dynamics and energetics to maintain mitochondrial homeostasis. Mitochondrial dysfunction leads to a decrease in ATP production, alterations in cellular functions and structure, and the loss of renal function. Persistent mitochondrial dysfunction has a role in the early stages and progression of renal diseases, such as acute kidney injury (AKI) and diabetic nephropathy, as it disrupts mitochondrial homeostasis and thus normal kidney function. Improving mitochondrial homeostasis and function has the potential to restore renal function, and administering compounds that stimulate mitochondrial biogenesis can restore mitochondrial and renal function in mouse models of AKI and diabetes mellitus. Furthermore, inhibiting the fission protein dynamin 1-like protein (DRP1) might ameliorate ischaemic renal injury by blocking mitochondrial fission.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Wang, Z. M. et al. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am. J. Clin. Nutr. 92, 1369–1377 (2010).
Pagliarini, D. J. et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112–123 (2008).
O'Connor, P. M. Renal oxygen delivery: matching delivery to metabolic demand. Clin. Exp. Pharmacol. Physiol. 33, 961–967 (2006).
Soltoff, S. P. ATP and the regulation of renal cell function. Annu. Rev. Physiol. 48, 9–31 (1986).
Holechek, M. J. et al. Glomerular filtration: an overview. Nephrol. Nurs. J. 30, 285–290, quiz 281–282 (2003).
Dimmer, K. S. & Scorrano, L. (De)constructing mitochondria: what for? Physiol. (Bethesda) 21, 233–241 (2006).
Lodish, H. et al. in Molecular Cell Biology (W. H. Freeman and Company, 2000).
Weinberg, J. M. et al. Anaerobic and aerobic pathways for salvage of proximal tubules from hypoxia-induced mitochondrial injury. Am. J. Physiol. Renal Physiol. 279, F927–F943 (2000).
Pollak, M. R., Quaggin, S. E., Hoenig, M. P. & Dworkin, L. D. The glomerulus: the sphere of influence. Clin. J. Am. Soc. Nephrol. 9, 1461–1469 (2014).
Chen, Y., Fry, B. C. & Layton, A. T. Modeling glucose metabolism and lactate production in the kidney. Math. Biosci. 289, 116–129 (2017).
Gerich, J. E. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med. 27, 136–142 (2010).
Thomas, S. R. Inner medullary lactate production and accumulation: a vasa recta model. Am. J. Physiol. Renal Physiol. 279, F468–F481 (2000).
Ross, B. D., Espinal, J. & Silva, P. Glucose metabolism in renal tubular function. Kidney Int. 29, 54–67 (1986).
Scott, C. Misconceptions about aerobic and anaerobic energy expenditure. J. Int. Soc. Sports Nutr. 2, 32 (2005).
Wirthensohn, G. & Guder, W. G. Renal substrate metabolism. Physiol. Rev. 66, 469–497 (1986).
Guder, W. G. & Ross, B. D. Enzyme distribution along the nephron. Kidney Int. 26, 101–111 (1984).
Lewy, P. R., Quintanilla, A., Levin, N. W. & Kessler, R. H. Renal energy metabolism and sodium reabsorption. Annu. Rev. Med. 24, 365–384 (1973).
Simon, N. & Hertig, A. Alteration of fatty acid oxidation in tubular epithelial cells: from acute kidney injury to renal fibrogenesis. Front. Med. (Lausanne) 2, 52 (2015). This review discusses the mechanisms behind fatty acid transport and oxidation in proximal tubules and how therapeutic agents restore β -oxidation in renal diseases.
Iwao, Y. et al. CD36 is one of important receptors promoting renal tubular injury by advanced oxidation protein products. Am. J. Physiol. Renal Physiol. 295, F1871–F1880 (2008).
Sabbahy, M. E. & Vaidya, V. S. Ischemic kidney injury and mechanisms of tissue repair. Wiley Interdiscip. Rev. Syst. Biol. Med. 3, 606–618 (2011).
Forbes, J. M. Mitochondria-power players in kidney function? Trends Endocrinol. Metab. 27, 441–442 (2016).
Bobulescu, I. A. Renal lipid metabolism and lipotoxicity. Curr. Opin. Nephrol. Hypertens. 19, 393–402 (2010).
Proctor, G. et al. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes 55, 2502–2509 (2006).
Arici, M., Chana, R., Lewington, A., Brown, J. & Brunskill, N. J. Stimulation of proximal tubular cell apoptosis by albumin-bound fatty acids mediated by peroxisome proliferator activated receptor-γ. J. Am. Soc. Nephrol. 14, 17–27 (2003).
Ruggiero, C. et al. Albumin-bound fatty acids but not albumin itself alter redox balance in tubular epithelial cells and induce a peroxide-mediated redox-sensitive apoptosis. Am. J. Physiol. Renal Physiol. 306, F896–F906 (2014).
Gutteridge, J. M. C. & Halliwell, B. Invited review free radicals in disease processes: a compilation of cause and consequence. Free Radic. Res. Commun. 19, 141–158 (1993).
Ray, P. D., Huang, B. W. & Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 24, 981–990 (2012).
Holmstrom, K. M. & Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell. Biol. 15, 411–421 (2014).
Ruiz, S. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic. Kidney Int. 83, 1029–1041 (2013).
Weisiger, R. A. & Fridovich, I. Mitochondrial superoxide simutase. Site of synthesis and intramitochondrial localization. J. Biol. Chem. 248, 4793–4796 (1973).
Teruya, R. et al. Expression of oxidative stress and antioxidant defense genes in the kidney of inbred mice after intestinal ischemia and reperfusion. Acta Cir. Bras. 28, 848–855 (2013).
Ribas, V., García-Ruiz, C. & Fernández-Checa, J. C. Glutathione and mitochondria. Front. Pharmacol. 5, 151 (2014).
Lushchak, V. I. Glutathione homeostasis and functions: potential targets for medical interventions. J. Amino Acids 2012, 26 (2012).
Handy, D. E. et al. Glutathione peroxidase-1 regulates mitochondrial function to modulate. J. Biol. Chem. 284, 11913–11921 (2009).
Fedorenko, A., Lishko, P. V. & Kirichok, Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151, 400–413 (2012).
Brand, M. D. & Esteves, T. C. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2, 85–93 (2005).
Brand, M. D. et al. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic. Biol. Med. 37, 755–767 (2004).
Zhou, Y. et al. UCP2 attenuates apoptosis of tubular epithelial cells in renal ischemia/reperfusion injury. Am. J. Physiol. Renal Physiol. http://dx.doi.org/10.1152/ajprenal.00118.2017 (2017). This study suggests a role for UCP2 in restoring tubular function after AKI by reducing tubular cell apoptosis.
Souza, B. M. d. et al. Polymorphisms of the UCP2 gene are associated with glomerular filtration rate in type 2 diabetic patients and with decreased UCP2 gene expression in human kidney. PLoS ONE 10, e0132938 (2015).
Semenza, G. L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 3, 721–732 (2003).
Haase, V. H. Hypoxia-inducible factors in the kidney. Am. J. Physiol. Renal Physiol. 291, F271–F281 (2006).
Semenza, G. L. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem. J. 405, 1–9 (2007).
Chandel, N. S. et al. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl Acad. Sci. USA 95, 11715–11720 (1998).
Chandel, N. S. et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1α during hypoxia: a mechanism of O2 sensing. J. Biol. Chem. 275, 25130–25138 (2000).
Klimova, T. & Chandel, N. S. Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death Differ. 15, 660–666 (2008).
Fantus, D., Rogers, N. M., Grahammer, F., Huber, T. B. & Thomson, A. W. Roles of mTOR complexes in the kidney: implications for renal disease and transplantation. Nat. Rev. Nephrol. 12, 587–609 (2016).
Kim, Y. & Park, C. W. Adenosine monophosphate-activated protein kinase in diabetic nephropathy. Kidney Res. Clin. Pract. 35, 69–77 (2016).
Grahammer, F. et al. mTORC2 critically regulates renal potassium handling. J. Clin. Invest. 126, 1773–1782 (2016).
Gleason, C. E. et al. mTORC2 regulates renal tubule sodium uptake by promoting ENaC activity. J. Clin.Invest. 125, 117–128 (2015).
Cunningham, J. T. et al. mTOR controls mitochondrial oxidative function through a YY1-PGC-1α transcriptional complex. Nature 450, 736–740 (2007).
Grahammer, F. et al. mTORC1 maintains renal tubular homeostasis and is essential in response to ischemic stress. Proc. Natl Acad. Sci. USA 111, E2817–E2826 (2014).
Hardie, D. G. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 25, 1895–1908 (2011).
Mihaylova, M. M. & Shaw, R. J. The AMP-activated protein kinase (AMPK) signaling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 13, 1016–1023 (2011).
Jager, S., Handschin, C., St-Pierre, J. & Spiegelman, B. M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl Acad. Sci. USA 104, 12017–12022 (2007).
Melser, S. et al. Rheb regulates mitophagy induced by mitochondrial energetic status. Cell Metab. 17, 719–730 (2013).
Scarpulla, R. C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta 1813, 1269–1278 (2011).
Scarpulla, R. C., Vega, R. B. & Kelly, D. P. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol. Metab. 23, 459–466 (2012).
Svensson, K., Schnyder, S., Cardel, B. & Handschin, C. Loss of renal tubular PGC-1α exacerbates diet-induced renal steatosis and age-related urinary sodium excretion in mice. PLoS ONE 11, e0158716 (2016). This study shows the importance of PGC1 α in basic renal physiology, further supporting PGC1 α as a therapeutic target for renal diseases.
Rasbach, K. A. & Schnellmann, R. G. PGC-1α over-expression promotes recovery from mitochondrial dysfunction and cell injury. Biochem. Biophys. Res. Commun. 355, 734–739 (2007).
Fan, W. & Evans, R. PPARs and ERRs: molecular mediators of mitochondrial metabolism. Curr. Opin. Cell Biol. 33, 49–54 (2015).
Huang, P., Chandra, V. & Rastinejad, F. Structural overview of the nuclear receptor superfamily: insights into physiology and therapeutics. Annu. Rev. Physiol. 72, 247–272 (2010).
Vega, R. B., Huss, J. M. & Kelly, D. P. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor α in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol. Cell. Biol. 20, 1868–1876 (2000).
Huss, J. M., Kopp, R. P. & Kelly, D. P. Peroxisome proliferator-activated receptor coactivator-1α (PGC-1α) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-α and -γ. Identification of novel leucine-rich interaction motif within PGC-1α. J. Biol. Chem. 277, 40265–40274 (2002).
Whitaker, R. M., Corum, D., Beeson, C. C. & Schnellmann, R. G. Mitochondrial biogenesis as a pharmacological target: A new approach to acute and chronic diseases. Annu. Rev. Pharmacol. Toxicol. 56, 229–249 (2016).
Fernandez-Marcos, P. J. & Auwerx, J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 93, 884S–890S (2011).
Cameron, R. B., Beeson, C. C. & Schnellmann, R. G. Development of therapeutics that induce mitochondrial biogenesis for the treatment of acute and chronic degenerative diseases. J. Med. Chem. 59, 10411–10434 (2016).
Villena, J. A. New insights into PGC-1 coactivators: redefining their role in the regulation of mitochondrial function and beyond. FEBS J. 282, 647–672 (2015).
Finck, B. N. & Kelly, D. P. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J. Clin. Invest. 116, 615–622 (2006).
Palikaras, K. & Tavernarakis, N. Mitochondrial homeostasis: the interplay between mitophagy and mitochondrial biogenesis. Exp. Gerontol. 56, 182–188 (2014).
Lee, H. C. & Wei, Y. H. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int. J. Biochem. Cell Biol. 37, 822–834 (2005).
Ahn, B. H. et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl Acad. Sci. USA 105, 14447–14452 (2008).
Kong, X. et al. Sirtuin 3, a new target of PGC-1α, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS ONE http://dx.doi.org/10.1371/journal.pone.0011707 (2010).
Handschin, C., Rhee, J., Lin, J., Tarr, P. T. & Spiegelman, B. M. An autoregulatory loop controls peroxisome proliferator-activated receptor γ coactivator 1α expression in muscle. Proc. Natl Acad. Sci. USA 100, 7111–7116 (2003).
Nisoli, E. et al. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc. Natl Acad. Sci. USA 101, 16507–16512 (2004).
Nisoli, E. et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310, 314–317 (2005).
Whitaker, R. M., Wills, L. P., Stallons, L. J. & Schnellmann, R. G. cGMP-selective phosphodiesterase inhibitors stimulate mitochondrial biogenesis and promote recovery from acute kidney injury. J. Pharmacol. Exp. Ther. 347, 626–634 (2013).
Lemasters, J. J. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuven. Res. 8, 3–5 (2005).
Alexander, C. et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat. Genet. 26, 211–215 (2000).
Delettre, C. et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat. Genet. 26, 207–210 (2000).
Delettre, C., Lenaers, G., Pelloquin, L., Belenguer, P. & Hamel, C. P. OPA1 (Kjer type) dominant optic atrophy: a novel mitochondrial disease. Mol. Genet. Metab. 75, 97–107 (2002).
Labbe, K., Murley, A. & Nunnari, J. Determinants and functions of mitochondrial behavior. Annu. Rev. Cell Dev. Biol. 30, 357–391 (2014).
Chan, D. C. Fusion and fission: interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 46, 265–287 (2012).
Waterham, H. R. et al. A lethal defect of mitochondrial and peroxisomal fission. N. Engl. J. Med. 356, 1736–1741 (2007).
Rossignol, R. et al. Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Res. 64, 985–993 (2004).
Mishra, P., Carelli, V., Manfredi, G. & Chan, D. C. Proteolytic cleavage of Opa1 stimulates mitochondrial inner membrane fusion and couples fusion to oxidative phosphorylation. Cell Metab. 19, 630–641 (2014).
Lu, B. Mitochondrial dynamics and neurodegeneration. Curr. Neurol. Neurosci. Rep. 9, 212–219 (2009).
Song, M. & Dorn, G. W. II. Mitoconfusion: noncanonical functioning of dynamism factors in static mitochondria of the heart. Cell Metab. 21, 195–205 (2015).
Ziegler, D. V., Wiley, C. D. & Velarde, M. C. Mitochondrial effectors of cellular senescence: beyond the free radical theory of aging. Aging Cell 14, 1–7 (2015).
Yoon, Y. S. et al. Formation of elongated giant mitochondria in DFO-induced cellular senescence: involvement of enhanced fusion process through modulation of Fis1. J. Cell. Physiol. 209, 468–480 (2006).
Romanello, V. & Sandri, M. Mitochondrial quality control and muscle mass maintenance. Front. Physiol. http://dx.doi.org/10.3389/fphys.2015.00422 (2015).
Mourier, A. et al. Mitofusin 2 is required to maintain mitochondrial coenzyme Q levels. J. Cell Biol. 208, 429–442 (2015).
Shutt, T., Geoffrion, M., Milne, R. & McBride, H. M. The intracellular redox state is a core determinant of mitochondrial fusion. EMBO Rep. 13, 909–915 (2012).
Song, Z., Chen, H., Fiket, M., Alexander, C. & Chan, D. C. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J. Cell Biol. 178, 749–755 (2007).
Anand, R. et al. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J. Cell Biol. 204, 919–929 (2014).
Frezza, C. et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126, 177–189 (2006).
Boissan, M. et al. Nucleoside diphosphate kinases fuel dynamin superfamily proteins with GTP for membrane remodeling. Science 344, 1510–1515 (2014).
Mishra, P. & Chan, D. C. Metabolic regulation of mitochondrial dynamics. J. Cell Biol. 212, 379–387 (2016). This review summarizes recent studies and mechanisms that relate metabolism and mitochondrial energetics to mitochondrial dynamics.
Twig, G., Hyde, B. & Shirihai, O. S. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim. Biophys. Acta 1777, 1092–1097 (2008).
Liesa, M. & Shirihai, O. S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 17, 491–506 (2013).
Mears, J. A. et al. Conformational changes in Dnm1 support a contractile mechanism for mitochondrial fission. Nat. Struct. Mol. Biol. 18, 20–26 (2011).
Otera, H., Ishihara, N. & Mihara, K. New insights into the function and regulation of mitochondrial fission. Biochim. Biophys. Acta 1833, 1256–1268 (2013).
Loson, O. C. et al. The mitochondrial fission receptor MiD51 requires ADP as a cofactor. Structure 22, 367–377 (2014).
Richter, V. et al. Structural and functional analysis of MiD51, a dynamin receptor required for mitochondrial fission. J. Cell Biol. 204, 477–486 (2014).
van der Bliek, A. M., Shen, Q. & Kawajiri, S. Mechanisms of mitochondrial fission and fusion. Cold Spring Harb. Perspect. Biol. http://dx.doi.org/10.1101/cshperspect.a011072 (2013).
Chang, C. R. & Blackstone, C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann. NY Acad. Sci. 1201, 34–39 (2010).
Chang, C. R. & Blackstone, C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J. Biol. Chem. 282, 21583–21587 (2007).
Slupe, A. M. et al. A calcineurin docking motif (LXVP) in dynamin-related protein 1 contributes to mitochondrial fragmentation and ischemic neuronal injury. J. Biol. Chem. 288, 12353–12365 (2013).
Cereghetti, G. M. et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc. Natl Acad. Sci. USA 105, 15803–15808 (2008).
Eiyama, A. & Okamoto, K. PINK1/Parkin-mediated mitophagy in mammalian cells. Curr. Opin. Cell Biol. 33, 95–101 (2015).
Greene, A. W. et al. Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep. 13, 378–385 (2012).
Matsuda, N. et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J. Cell Biol. 189, 211–221 (2010).
Narendra, D. P. et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 8, e1000298 (2010).
Okatsu, K. et al. Phosphorylated ubiquitin chain is the genuine Parkin receptor. J. Cell Biol. 209, 111–128 (2015).
Vives-Bauza, C. et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc. Natl Acad. Sci. USA 107, 378–383 (2010).
Tanaka, A. et al. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J. Cell Biol. 191, 1367–1380 (2010).
Youle, R. J. & Narendra, D. P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell. Biol. 12, 9–14 (2011).
Randow, F. & Youle, R. J. Self and nonself: how autophagy targets mitochondria and bacteria. Cell Host Microbe 15, 403–411 (2014).
Sarraf, S. A. et al. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature 496, 372–376 (2013).
Chan, N. C. et al. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum. Mol. Genet. 20, 1726–1737 (2011).
Egan, D. F. et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331, 456–461 (2011).
Groenewoud, M. J. & Zwartkruis, F. J. Rheb and mammalian target of rapamycin in mitochondrial homoeostasis. Open Biol. 3, 130185 (2013).
Toyama, E. Q. et al. Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 351, 275–281 (2016).
Zhang, C.-S. & Lin, S.-C. AMPK promotes autophagy by facilitating mitochondrial fission. Cell Metab. 23, 399–401 (2016). This study suggests a direct role for AMPK in mitophagy by phosphorylating MFF, a mitophagy receptor on the outer mitochondrial membrane, to initiate fission and therefore mitophagy.
Chen, G. et al. A regulatory signaling loop comprising the PGAM5 phosphatase and CK2 controls receptor-mediated mitophagy. Mol. Cell 54, 362–377 (2014).
Liu, L. et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. 14, 177–185 (2012).
Novak, I. et al. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 11, 45–51 (2010).
Zhang, J. & Ney, P. A. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 16, 939–946 (2009).
Thomas, R. L., Kubli, D. A. & Gustafsson, A. B. Bnip3-mediated defects in oxidative phosphorylation promote mitophagy. Autophagy 7, 775–777 (2011).
Hanna, R. A. et al. Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J. Biol. Chem. 287, 19094–19104 (2012).
Kanki, T. Nix, a receptor protein for mitophagy in mammals. Autophagy 6, 433–435 (2010).
Scherz-Shouval, R. & Elazar, Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem. Sci. 36, 30–38 (2011).
Li, Y. et al. Bnip3 mediates the hypoxia-induced inhibition on mammalian target of rapamycin by interacting with Rheb. J. Biol. Chem. 282, 35803–35813 (2007).
Maiuri, M. C. et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 26, 2527–2539 (2007).
Ishihara, M. et al. Sestrin-2 and BNIP3 regulate autophagy and mitophagy in renal tubular cells in acute kidney injury. Am. J. Physiol. Renal Physiol. 305, F495–F509 (2013).
Tang, C., He, L., Liu, J. & Dong, Z. Mitophagy: Basic Mechanism and Potential Role in Kidney Diseases. Kidney Diseases 1, 71–79 (2015).
Che, R., Yuan, Y., Huang, S. & Zhang, A. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am. J. Physiol. - Renal Physiol. 306, F367–F378 (2014).
Yang, Y. et al. Renoprotective approaches and strategies in acute kidney injury. Pharmacol. Ther. 163, 58–73 (2016).
Shusterman, N. et al. Risk factors and outcome of hospital-acquired acute renal failure. Clinical epidemiologic study. Am. J. Med. 83, 65–71 (1987).
Thadhani, R., Pascual, M. & Bonventre, J. V. Acute renal failure. N. Engl. J. Med. 334, 1448–1460 (1996).
Kelly, K. J. & Molitoris, B. A. Acute renal failure in the new millennium: time to consider combination therapy. Semin. Nephrol. 20, 4–19 (2000).
Murugan, R. & Kellum, J. A. Acute kidney injury: what's the prognosis? Nat. Rev. Nephrol. 7, 209–217 (2011).
Waikar, S. S., Liu, K. D. & Chertow, G. M. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin. J. Am. Soc. Nephrol. 3, 844–861 (2008).
Doyle, J. F. & Forni, L. G. Acute kidney injury: short-term and long-term effects. Crit. Care 20, 188 (2016).
Hsu, C. & Liu, K. D. Cardiovascular events after AKI: a new dimension. J. Am. Soc. Nephrol. 25, 425–427 (2014).
Selewski, D. T. & Symons, J. M. Acute kidney injury. Pediatr. Rev. 35, 30–41 (2014).
Paraskevas, K. I. & Mikhailidis, D. P. Contrast-induced acute kidney injury in patients undergoing carotid artery stenting: an underestimated issue. Angiology http://dx.doi.org/10.1177/0003319716668934 (2016).
Schefold, J. C., Filippatos, G., Hasenfuss, G., Anker, S. D. & von Haehling, S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat. Rev. Nephrol. 12, 610–623 (2016).
Basile, D. P., Anderson, M. D. & Sutton, T. A. Pathophysiology of acute kidney injury. Compr. Physiol. 2, 1303–1353 (2012).
Ishimoto, Y. & Inagi, R. Mitochondria: a therapeutic target in acute kidney injury. Nephrol. Dial. Transplant. 31, 1062–1069 (2016).
Emma, F., Montini, G., Parikh, S. M. & Salviati, L. Mitochondrial dysfunction in inherited renal disease and acute kidney injury. Nat. Rev. Nephrol. 12, 267–280 (2016).
Funk, J. A. & Schnellmann, R. G. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am. J. Physiol. Renal Physiol. 302, F853–F864 (2012).
Tran, M. et al. PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J. Clin. Invest. 121, 4003–4014 (2011).
Parikh, S. M. Therapeutic targeting of the mitochondrial dysfunction in septic acute kidney injury. Curr. Opin. Crit. Care 19, 554–559 (2013).
Ruidera, E. et al. Fatty acid metabolism in renal ischemia. Lipids 23, 882–884 (1988).
Johnson, A. C., Stahl, A. & Zager, R. A. Triglyceride accumulation in injured renal tubular cells: alterations in both synthetic and catabolic pathways. Kidney Int. 67, 2196–2209 (2005).
Zager, R. A., Johnson, A. C. & Hanson, S. Y. Renal tubular triglyercide accumulation following endotoxic, toxic, and ischemic injury. Kidney Int. 67, 111–121 (2005).
Portilla, D. Role of fatty acid beta-oxidation and calcium-independent phospholipase A2 in ischemic acute renal failure. Curr. Opin. Nephrol. Hypertens. 8, 473–477 (1999).
Idrovo, J. P., Yang, W. L., Nicastro, J., Coppa, G. F. & Wang, P. Stimulation of carnitine palmitoyltransferase 1 improves renal function and attenuates tissue damage after ischemia/reperfusion. J. Surg. Res. 177, 157–164 (2012).
Smith, J. A., Stallons, L. J. & Schnellmann, R. G. Renal cortical hexokinase and pentose phosphate pathway activation through the EGFR/Akt signaling pathway in endotoxin-induced acute kidney injury. Am. J. Physiol. Renal Physiol. 307, F435–F444 (2014).
Zager, R. A., Johnson, A. C. & Becker, K. Renal cortical pyruvate depletion during AKI. J. Am. Soc. Nephrol. 25, 998–1012 (2014).
Lan, R. et al. Mitochondrial pathology and glycolytic shift during proximal tubule atrophy after ischemic AKI. J. Am. Soc. Nephrol. 27, 3356–3367 (2016).
Venkatachalam, M. A., Weinberg, J. M., Kriz, W. & Bidani, A. K. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J. Am. Soc. Nephrol. 26, 1765–1776 (2015).
Eklund, T., Wahlberg, J., Ungerstedt, U. & Hillered, L. Interstitial lactate, inosine and hypoxanthine in rat kidney during normothermic ischaemia and recirculation. Acta Physiol. Scand. 143, 279–286 (1991).
Zhan, M., Brooks, C., Liu, F., Sun, L. & Dong, Z. Mitochondrial dynamics: regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int. 83, 568–581 (2013).
Brooks, C., Wei, Q., Cho, S. G. & Dong, Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J. Clin. Invest. 119, 1275–1285 (2009).
Cho, S. G., Du, Q., Huang, S. & Dong, Z. Drp1 dephosphorylation in ATP depletion-induced mitochondrial injury and tubular cell apoptosis. Am. J. Physiol. Renal Physiol. 299, F199–F206 (2010).
Jiang, M. et al. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 82, 1271–1283 (2012).
Liu, S. et al. Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy 8, 826–837 (2012).
Kimura, T. et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J. Am. Soc. Nephrol. 22, 902–913 (2011).
Duann, P., Lianos, E. A., Ma, J. & Lin, P. H. Autophagy, innate immunity and tissue repair in acute kidney injury. Int. J. Mol. Sci. 17, 662 (2016).
Wei, Q., Dong, G., Chen, J. K., Ramesh, G. & Dong, Z. Bax and Bak have critical roles in ischemic acute kidney injury in global and proximal tubule-specific knockout mouse models. Kidney Int. 84, 138–148 (2013).
Stallons, L. J., Whitaker, R. M. & Schnellmann, R. G. Suppressed mitochondrial biogenesis in folic acid-induced acute kidney injury and early fibrosis. Toxicol. Lett. 224, 326–332 (2014).
Tran, M. T. et al. PGC1α-dependent NAD biosynthesis links oxidative metabolism to renal protection. Nature 531, 528–532 (2016). This investigation shows the importance of NAD biosynthesis in the recovery phase of AKI and of PGC1 α as an important regulator of NAD biosynthesis, highlighting this pathway as a therapeutic target for AKI.
Jesinkey, S. R. et al. Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. J. Am. Soc. Nephrol. 25, 1157–1162 (2014). This study provides the first proof of principle that stimulation of mitochondrial biogenesis after AKI can restore mitochondrial function and renal function.
Garrett, S. M., Whitaker, R. M., Beeson, C. C. & Schnellmann, R. G. Agonism of the 5-hydroxytryptamine 1F receptor promotes mitochondrial biogenesis and recovery from acute kidney injury. J. Pharmacol. Exp. Ther. 350, 257–264 (2014).
Perico, L., Morigi, M. & Benigni, A. Mitochondrial sirtuin 3 and renal diseases. Nephron 134, 14–19 (2016).
Maahs, D. M. & Rewers, M. Mortality and renal disease in type 1 diabetes mellitus—progress made, more to be done. J. Clin. Endocrinol. Metab. 91, 3757–3759 (2006).
Collins, A. J. et al. US Renal Data System 2011 annual data report. Am. J. Kidney Dis. 59, A7 (2012).
Miranda-Diaz, A. G., Pazarin-Villasenor, L., Yanowsky-Escatell, F. G. & Andrade-Sierra, J. Oxidative stress in diabetic nephropathy with early chronic kidney disease. J. Diabetes Res. 2016, 7047238 (2016).
Flemming, N. B., Gallo, L. A., Ward, M. S. & Forbes, J. M. Tapping into mitochondria to find novel targets for diabetes complications. Curr. Drug Targets 17, 1341–1349 (2016). This review summarizes the role of mitochondrial ROS production in diabetes, which is a controversial contributor to the development and progression of diabetes.
Coughlan, M. T. et al. Mapping time-course mitochondrial adaptations in the kidney in experimental diabetes. Clin. Sci. (Lond.) 130, 711–720 (2016). This study showed that diabetes-induced changes in mitochondrial morphology and energetics occur prior to renal lesions, suggesting that mitochondrial dysfunction is a primary cause of diabetes rather than a contributor.
Higgins, G. C. & Coughlan, M. T. Mitochondrial dysfunction and mitophagy: the beginning and end to diabetic nephropathy? Br. J. Pharmacol. 171, 1917–1942 (2014).
Coughlan, M. T. & Sharma, K. Challenging the dogma of mitochondrial reactive oxygen species overproduction in diabetic kidney disease. Kidney Int. 90, 272–279 (2016).
Giacco, F. & Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 107, 1058–1070 (2010).
Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813–820 (2001).
Lonn, E. et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA 293, 1338–1347 (2005).
Hallan, S. & Sharma, K. The role of mitochondria in diabetic kidney disease. Curr. Diab. Rep. 16, 61 (2016).
Burch, H. B. et al. Metabolic effects of large fructose loads in different parts of the rat nephron. J. Biol. Chem. 255, 8239–8244 (1980).
Lanaspa, M. A. et al. Endogenous fructose production and fructokinase activation mediate renal injury in diabetic nephropathy. J. Am. Soc. Nephrol. 25, 2526–2538 (2014).
Diggle, C. P. et al. Ketohexokinase: expression and localization of the principal fructose-metabolizing enzyme. J. Histochem. Cytochem. 57, 763–774 (2009).
Wai, T. & Langer, T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol. Metab. 27, 105–117 (2016).
Wang, W. et al. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab. 15, 186–200 (2012).
Tang, W. X., Wu, W. H., Zeng, X. X., Bo, H. & Huang, S. M. Early protective effect of mitofusion 2 overexpression in STZ-induced diabetic rat kidney. Endocr 41, 236–247 (2012).
Hickey, F. B. et al. IHG-1 increases mitochondrial fusion and bioenergetic function. Diabetes 63, 4314–4325 (2014).
Hickey, F. B. et al. IHG-1 promotes mitochondrial biogenesis by stabilizing PGC-1α. J. Am. Soc. Nephrol. 22, 1475–1485 (2011).
Guo, K. et al. Protective role of PGC-1α in diabetic nephropathy is associated with the inhibition of ROS through mitochondrial dynamic remodeling. PLoS ONE 10, e0125176 (2015).
Imasawa, T. et al. High glucose repatterns human podocyte energy metabolism during differentiation and diabetic nephropathy. FASEB J. 31, 294–307 (2017).
Qi, W. et al. Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat. Med. 23, 753–762 (2017).
Szeto, H. H. et al. Protection of mitochondria prevents high-fat diet-induced glomerulopathy and proximal tubular injury. Kidney Int. 90, 997–1011 (2016).
Lempiainen, J., Finckenberg, P., Levijoki, J. & Mervaala, E. AMPK activator AICAR ameliorates ischaemia reperfusion injury in the rat kidney. Br. J. Pharmacol. 166, 1905–1915 (2012).
Ruderman, N. B., Carling, D., Prentki, M. & Cacicedo, J. M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Invest. 123, 2764–2772 (2013).
Dugan, L. L. et al. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J. Clin. Invest. 123, 4888–4899 (2013).
Pillai, V. B. et al. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J. Biol. Chem. 285, 3133–3144 (2010).
Palacios, O. M. et al. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1α in skeletal muscle. Aging (Albany NY) 1, 771–783 (2009).
Nogueiras, R. et al. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol. Rev. 92, 1479–1514 (2012)
Morigi, M. et al. Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J. Clin. Invest. 125, 715–726 (2015).
Singh, J. P., Singh, A. P. & Bhatti, R. Explicit role of peroxisome proliferator-activated receptor γ in gallic acid-mediated protection against ischemia-reperfusion-induced acute kidney injury in rats. J. Surg. Res. 187, 631–639 (2014).
Chung, B. H. et al. Protective effect of peroxisome proliferator activated receptor γ agonists on diabetic and non-diabetic renal diseases. Nephrol. (Carlton, Vic.) 10 (Suppl.), S40–S43 (2005).
Sivarajah, A. et al. Agonists of peroxisome-proliferator activated receptor-γ reduce renal ischemia/reperfusion injury. Am. J. Nephrol. 23, 267–276 (2003).
Staels, B. et al. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 98, 2088–2093 (1998).
Wu, Q. Q. et al. Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases expression of protective genes Nrf2, PPARγ, and HO-1. Am. J. Physiol. Renal Physiol. 300, F1180–F1192 (2011).
de Zeeuw, D. et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 369, 2492–2503 (2013).
Park, C. W. et al. PPARα agonist fenofibrate improves diabetic nephropathy in db/db mice. Kidney Int. 69, 1511–1517 (2006).
Stadler, K., Goldberg, I. J. & Susztak, K. The evolving understanding of the contribution of lipid metabolism to diabetic kidney disease. Curr. Diabetes Rep. 15, 40 (2015).
Al-Rasheed, N. M. et al. Fenofibrate attenuates diabetic nephropathy in experimental diabetic rat's model via suppression of augmented TGF-β1/Smad3 signaling pathway. Arch. Physiol. Biochem. 122, 186–194 (2016).
Hong, Y. A. et al. Fenofibrate improves renal lipotoxicity through activation of AMPK-PGC-1α in db/db mice. PLoS ONE 9, e96147 (2014).
Kawanami, D., Matoba, K. & Utsunomiya, K. Dyslipidemia in diabetic nephropathy. Ren. Replace. Ther. 2, 16 (2016).
Szeto, H. H. & Birk, A. V. Serendipity and the discovery of novel compounds that restore mitochondrial plasticity. Clin. Pharmacol. Ther. 96, 672–683 (2014). The Szeto peptides described in this study are novel, as they prevent the peroxidation of cardiolipin and therefore preserve mitochondrial function, demonstrating that they are renoprotective.
Hanske, J. et al. Conformational properties of cardiolipin-bound cytochrome c. Proc. Natl Acad. Sci. USA 109, 125–130 (2012).
Basova, L. V. et al. Cardiolipin switch in mitochondria: shutting off the reduction of cytochrome c and turning on the peroxidase activity. Biochemistry 46, 3423–3434 (2007).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02436447 (2015).
Sedeek, M., Nasrallah, R., Touyz, R. M. & Hebert, R. L. NADPH oxidases, reactive oxygen species, and the kidney: friend and foe. J. Am. Soc. Nephrol. 24, 1512–1518 (2013).
Bai, J. & Cederbaum, A. I. Mitochondrial catalase and oxidative injury. Biol. Signals Recept. 10, 189–199 (2001).
Scarpulla, R. C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 88, 611–638 (2008).
Kaufman, B. A. et al. The mitochondrial transcription factor TFAM coordinates the assembly of multiple DNA molecules into nucleoid-like structures. Mol. Biol. Cell 18, 3225–3236 (2007).
Virbasius, J. V. & Scarpulla, R. C. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc. Natl Acad. Sci. USA 91, 1309–1313 (1994).
Wu, Z. et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115–124 (1999).
Halseth, A. E., Ensor, N. J., White, T. A., Ross, S. A. & Gulve, E. A. Acute and chronic treatment of ob/ob and db/db mice with AICAR decreases blood glucose concentrations. Biochem. Biophys. Res. Commun. 294, 798–805 (2002).
Keech, A. et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 366, 1849–1861 (2005).
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, made substantial contributions to discussions of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Carnitine shuttle
-
Enzymes in the mitochondrial membrane that transport long-chain fatty acids from the cytosol to the mitochondrial matrix by replacing their coA group with carnitine.
- Mitochondrial cristae
-
Folds in the mitochondrial inner membrane that increase the surface area for mitochondrial respiration to take place.
- Streptozotocin
-
A glucosamine-nitrosourea that is used to induce experimental diabetes in animals by specifically targeting and damaging beta cells.
- Dyslipidaemia
-
Abnormalities in lipoprotein metabolism, resulting in elevated or deficient levels of lipids and/or lipoproteins in the body.
Rights and permissions
About this article
Cite this article
Bhargava, P., Schnellmann, R. Mitochondrial energetics in the kidney. Nat Rev Nephrol 13, 629–646 (2017). https://doi.org/10.1038/nrneph.2017.107
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2017.107
This article is cited by
-
Sacubitril/valsartan ameliorates tubulointerstitial fibrosis by restoring mitochondrial homeostasis in diabetic kidney disease
Diabetology & Metabolic Syndrome (2024)
-
Dietary fat supplementation relieves cold temperature-induced energy stress through AMPK-mediated mitochondrial homeostasis in pigs
Journal of Animal Science and Biotechnology (2024)
-
Mapping human tissues with highly multiplexed RNA in situ hybridization
Nature Communications (2024)
-
NDUFS4 regulates cristae remodeling in diabetic kidney disease
Nature Communications (2024)
-
Sirtuins in kidney health and disease
Nature Reviews Nephrology (2024)