Key Points
-
This Review summarizes our current understanding of the mechanisms of tau-mediated neurodegeneration in Alzheimer's disease and related disorders. It is believed that tau-mediated neurodegeneration might result from a combination of a loss of normal tau function (primarily the microtubule (MT)-stabilizing function of tau) with gains of pathological functions of hyperphosphorylated tau, and the filaments formed thereof.
-
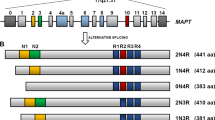
The primary function of tau is to stabilize the MTs. Under physiological conditions tau is in a tightly regulated dynamic equilibrium both on and off the MTs. This equilibrium, which is post-translationally regulated primarily by the phosphorylation state of tau, is thought to have a central role in maintaining effective axonal transport.
-
Under pathological conditions, an excessive disengagement of tau from the MTs takes place. This abnormal disengagement is likely to cause axonal transport defects. Furthermore, the increased cytosolic concentration of unbound tau renders the tau more likely to undergo misfolding and aggregation.
-
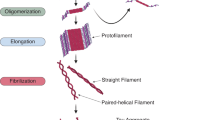
The aggregation of tau, leading to the formation of neurofibrillary tangles (NFTs), is likely to be associated with toxic gains-of-function. For example, NFTs may contribute to disease progression by further sequestering functional tau, thereby amplifying the loss of normal tau function. At the same time, relatively large NFTs may represent a direct physical obstacle to vesicles and other cargoes moving along the axons.
-
Direct causes of the pathological disengagement of tau from the MTs include tau gene mutations and an imbalance between tau kinases and phosphatases. Other pathological events, such as Aβ-mediated neurotoxicity, oxidative stress and inflammation, may also be able to initiate or contribute directly or indirectly to tau mediated neurodegeneration; however, their precise positioning in the cascade of events that leads to neuronal loss remains unclear.
-
A schematic overview of the various tau-directed therapeutic approaches currently under investigation is provided, along with an overview of the different transgenic mouse models that are available.
Abstract
Advances in our understanding of the mechanisms of tau-mediated neurodegeneration in Alzheimer's disease (AD) and related tauopathies, which are characterized by prominent CNS accumulations of fibrillar tau inclusions, are rapidly moving this previously underexplored disease pathway to centre stage for disease-modifying drug discovery efforts. However, controversies abound concerning whether or not the deleterious effects of tau pathologies result from toxic gains-of-function by pathological tau or from critical losses of normal tau function in the disease state. This Review summarizes the most recent advances in our knowledge of the mechanisms of tau-mediated neurodegeneration to forge an integrated concept of those tau-linked disease processes that drive the onset and progression of AD and related tauopathies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Buee, L., Bussiere, T., Buee-Scherrer, V., Delacourte, A. & Hof, P. R. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Rev. 33, 95–130 (2000).
Brandt, R., Leger, J. & Lee, G. Interaction of tau with the neural plasma membrane mediated by tau's amino-terminal projection domain. J. Cell Biol. 131, 1327–1340 (1995).
Maas, T., Eidenmuller, J. & Brandt, R. Interaction of tau with the neural membrane cortex is regulated by phosphorylation at sites that are modified in paired helical filaments. J. Biol. Chem. 275, 15733–15740 (2000).
Fulga, T. A. et al. Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nature Cell Biol. 9, 139–148 (2007).
Lee, G. Tau and src family tyrosine kinases. Biochim. Biophys. Acta 1739, 323–330 (2005).
Lee, G., Neve, R. L. & Kosik, K. S. The microtubule binding domain of tau protein. Neuron 2, 1615–1624 (1989).
Binder, L. I., Frankfurter, A. & Rebhun, L. I. The distribution of tau in the mammalian central nervous system. J. Cell Biol. 101, 1371–1378 (1985).
Hong, M. et al. Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science 282, 1914–1917 (1998).
Amos, L. A. Microtubule structure and its stabilisation. Org. Biomol. Chem. 2, 2153–2160 (2004).
Kar, S., Fan, J., Smith, M. J., Goedert, M. & Amos, L. A. Repeat motifs of tau bind to the insides of microtubules in the absence of taxol. EMBO J. 22, 70–77 (2003).
Kar, S., Florence, G. J., Paterson, I. & Amos, L. A. Discodermolide interferes with the binding of tau protein to microtubules. FEBS Lett. 539, 34–36 (2003).
Kampers, T., Pangalos, M., Geerts, H., Wiech, H. & Mandelkow, E. Assembly of paired helical filaments from mouse tau: implications for the neurofibrillary pathology in transgenic mouse models for Alzheimer's disease. FEBS Lett. 451, 39–44 (1999).
Takashima, A. et al. Presenilin 1 associates with glycogen synthase kinase-3β and its substrate tau. Proc. Natl Acad. Sci. USA 95, 9637–9641 (1998).
Kuret, J. et al. Evaluating triggers and enhancers of tau fibrillization. Microsc. Res. Tech. 67, 141–155 (2005). This review provides a model to rationalize the multistep pathway to tau fibril formation, as well as experimental methods for tau fibrillization assays.
Mazanetz, M. P. & Fischer, P. M. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nature Rev. Drug Discov. 6, 464–479 (2007). An up-to-date account of the role of specific kinases in tau-mediated neurodegeneration and their significance as targets for therapeutic intervention.
Arnold, C. S. et al. The microtubule-associated protein tau is extensively modified with O-linked N-acetylglucosamine. J. Biol. Chem. 271, 28741–28744 (1996).
Li, X., Lu, F., Wang, J. -Z. & Gong, C. -X. Concurrent alterations of O-GlcNAcylation and phosphorylation of tau in mouse brains during fasting. Eur. J. Neurosci. 23, 2078–2086 (2006).
Liu, F., Iqbal, K., Grundke-Iqbal, I., Hart, G. W. & Gong, C. -X. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer's disease. Proc. Natl Acad. Sci. USA 101, 10804–10809 (2004).
Gong, C. X., Liu, F., Grundke-Iqbal, I. & Iqbal, K. Post-translational modifications of tau protein in Alzheimer's disease. J. Neural Transm. 112, 813–838 (2005).
Münch, G., Deuther-Conrad, W. & Gasic-Milenkovic, J. Glycoxidative stress creates a vicious cycle of neurodegeneration in Alzheimer's disease – a target for neuroprotective treatment strategies? J. Neural Transm. 62 (Suppl.), 303–307 (2002).
Cripps, D. et al. Alzheimer disease-specific conformation of hyperphosphorylated paired helical filament-tau is polyubiquitinated through Lys-48, Lys-11, and Lys-6 ubiquitin conjugation. J. Biol. Chem. 281, 10825–10838 (2006).
Dorval, V. & Fraser, P. E. Small ubiquitin-like modifier (SUMO) modification of natively unfolded proteins tau and α-synuclein. J. Biol. Chem. 281, 9919–9924 (2006).
Dorval, V. & Fraser, P. E. SUMO on the road to neurodegeneration. Biochim. Biophys. Acta 1773, 694–706 (2007).
Mailliot, C., Trojanowski, J. Q. & Lee, V. M. Impaired tau protein function following nitration-induced oxidative stress in vitro and in vivo. Neurobiol. Aging 23 (Suppl. 1), 415 (2002).
Johnson, G. Tau phosphorylation and proteolysis: insights and perspectives. J. Alzheimers Dis. 9, 243–250 (2006).
Roy, S., Zhang, B., Lee, V. M. -Y. & Trojanowski, J. Q. Axonal transport defects: a common theme in neurodegenerative diseases. Acta Neuropathol. 109, 5–13 (2005). Reviews the biology of axonal transport and its role in neurodegenerative disease.
Kuret, J. et al. Pathways of tau fibrillization. Biochim. Biophys. Acta 1739, 167–178 (2005).
Ross, C. A. & Poirier, M. A. Protein aggregation and neurodegenerative disease. Nature Med. 10, S10–S17 (2004).
Galvan, M., David, J. P., Delacourte, A., Luna, J. & Mena, R. Sequence of neurofibrillary changes in aging and Alzheimer's disease: a confocal study with phospho-tau antibody, AD2. J. Alzheimers Dis. 3, 417–425 (2001).
Maeda, S. et al. Granular tau oligomers as intermediates of tau filaments. Biochemistry 46, 3856–3861 (2007).
Maeda, S. et al. Increased levels of granular tau oligomers: an early sign of brain aging and Alzheimer's disease. Neurosci. Res. 54, 197–201 (2006).
Goedert, M. & Jakes, R. Mutations causing neurodegenerative tauopathies. Biochim. Biophys. Acta 1739, 240–250 (2005).
von Bergen, M. et al. Mutations of tau protein in frontotemporal dementia promote aggregation of paired helical filaments by enhancing local β-structure. J. Biol. Chem. 276, 48165–48174 (2001).
Nacharaju, P. et al. Accelerated filament formation from tau protein with specific FTDP-17 missense mutations. FEBS Lett. 447, 195–199 (1999).
Alonso Adel, C., Mederlyova, A., Novak, M., Grundke-Iqbal, I. & Iqbal, K. Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. J. Biol. Chem. 279, 34873–34881 (2004).
Dayanandan, R. et al. Mutations in tau reduce its microtubule binding properties in intact cells and affect its phosphorylation. FEBS Lett. 446, 228–232 (1999).
Churcher, I. Tau therapeutic strategies for the treatment of Alzheimer's disease. Curr. Top. Med. Chem. 6, 579–595 (2006).
Noble, W. et al. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc. Natl Acad. Sci. USA 102, 6990–6995 (2005). Study that validates GSK3β as a target for tau-directed therapies.
Phiel, C. J., Wilson, C. A., Lee, V. M. Y. & Klein, P. S. GSK-3α regulates production of Alzheimer's disease amyloid-β peptides. Nature 423, 435–439 (2003).
Tian, Q. & Wang, J. Role of serine/threonine protein phosphatase in Alzheimer's disease. Neurosignals 11, 262–269 (2002).
Andersen, J. K. Oxidative stress in neurodegeneration: cause or consequence? Nature Med. 5, S18–S25 (2004).
Moreira, P. I. et al. Oxidative stress and neurodegeneration. Ann. NY Acad. Sci. 1043, 545–552 (2005).
King, M. E. et al. Tau-dependent microtubule disassembly initiated by prefibrillar β-amyloid. J. Cell Biol. 175, 541–546 (2006).
Rapoport, M., Dawson, H. N., Binder, L. I., Vitek, M. P. & Ferreira, A. Tau is essential to β-amyloid-induced neurotoxicity. Proc. Natl Acad. Sci. USA 99, 6364–6369 (2002). Study that substantiates the notion that tau may be a mediator of upstream pathological events, namely Aβ-induced neurotoxicity.
Liu, Q. et al. Tau modifiers as therapeutic targets for Alzheimer's disease. Biochim. Biophys. Acta 1739, 211–215 (2005).
Schweers, O., Mandelkow, E., Biernat, J. & Mandelkow, E. Oxidation of cysteine-322 in the repeat domain of microtubule-associated protein τ controls the in vitro assembly of paired helical filaments. Proc. Natl Acad. Sci. USA 92, 8463–8467 (1995).
David, D. C. et al. Proteomic and functional analyses reveal a mitochondrial dysfunction in P301L tau transgenic mice. J. Biol. Chem. 280, 23802–23814 (2005).
Blurton-Jones, M. & LaFerla, F. M. Pathways by which Aβ facilitates tau pathology. Curr. Alzheimer Res. 3, 437–448 (2006).
Oddo, S. et al. Temporal profile of amyloid-β (Aβ) oligomerization in an in vivo model of Alzheimer disease: a link between Aβ and tau pathology. J. Biol. Chem. 281, 1599–1604 (2006).
Roberson, E. D. et al. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer's disease mouse model. Science 316, 750–754 (2007). Study that suggests the role tau might have as a mediator of neurodegeneration. This study also suggests that tau reduction may be therapeutically beneficial.
Ikegami, S., Harada, A. & Hirokawa, N. Muscle weakness, hyperactivity, and impairment in fear conditioning in tau-deficient mice. Neurosci. Lett. 279, 129–132 (2000).
Forman, M. S., Trojanowski, J. Q. & Lee, V. M. -Y. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nature Med. 10, 1055–1063 (2004).
Trojanowski, J. Q. & Mattson, M. P. Overview of protein aggregation in single, double, and triple neurodegenerative brain amyloidoses. Neuromolecular Med. 4, 1–6 (2003).
Mitchell, T. W. et al. Novel method to quantify neuropil threads in brains from elders with or without cognitive impairment. J. Histochem. Cytochem. 48, 1627–1638 (2000).
Trojanowski, J. Q., Smith, A. B., Huryn, D. & Lee, V. M. -Y. Microtubule-stabilizing drugs for therapy of Alzheimer's disease and other neurodegenerative disorders with axonal transport impairments. Expert Opin. Pharmacother. 6, 683–686 (2005).
Ishihara, T. et al. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron 24, 751–762 (1999).
Zhang, B. et al. Microtubule-binding drugs offset tau sequestration by stabilizing microtubules and reversing fast axonal transport deficits in a tauopathy model. Proc. Natl Acad. Sci. USA 102, 227–231 (2005).
Arriagada, P. V., Growdon, J. H., Hedley-Whyte, E. T. & Hyman, B. T. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology 42, 631–639 (1992).
Arriagada, P. V., Marzloff, K. & Hyman, B. T. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology 42, 1681–1688 (1992).
Santacruz, K. et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 309, 476–481 (2005). Interesting study that shows that suppression of tau improves memory function even though NFTs continue to grow.
Trojanowski, J. Q. & Lee, V. M. Pathological tau: a loss of normal function or a gain in toxicity? Nature Neurosci. 8, 1136–1137 (2005).
Stokin, G. B. et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer's disease. Science 307, 1282–1288 (2005). Together with reference 61, this study shows that axonal transport defects may be early pathological events in tau-mediated neurodegeneration.
Yoshiyama, Y. et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53, 337–351 (2007). Paper demonstrating that microgliosis and synaptic pathology may be the earliest manifestation of neurodegenerative tauopathies. The paper also suggests that abrogation of tau-induced microglial activation may be therapeutically beneficial.
Lee, V. M.-Y., Kenyon, T. K. & Trojanowski, J. Q. Transgenic animal models of tauopathies. Biochim. Biophys. Acta 1739, 251–259 (2005).
Shaw, L. M., Korecka, M., Clark, C. M., Lee, V. M. & Trojanowski, J. Q. Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nature Rev. Drug Discov. 6, 295–303 (2007).
Necula, M., Chirita, C. N. & Kuret, J. Cyanine dye N744 inhibits tau fibrillization by blocking filament extension: implications for the treatment of tauopathic neurodegenerative diseases. Biochemistry 44, 10227–10237 (2005).
Pickhardt, M. et al. Screening for inhibitors of tau polymerization. Curr. Alzheimer Res. 2, 219–226 (2005).
Pickhardt, M. et al. Anthraquinones inhibit tau aggregation and dissolve Alzheimer's paired helical filaments in vitro and in cells. J. Biol. Chem. 280, 3628–3635 (2005).
Taniguchi, S. et al. Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins. J. Biol. Chem. 280, 7614–7623 (2005).
Frid, P., Anisimov, S. V. & Popovic, N. Congo red and protein aggregation in neurodegenerative diseases. Brain Res. Rev. 53, 135–160 (2007).
Chirita, C., Necula, M. & Kuret, J. Ligand-dependent inhibition and reversal of tau filament formation. Biochemistry 43, 2879–2887 (2004).
Liu, M., Ni, J., Kosik, K. S. & Yeh, L. A. Development of a fluorescent high throughput assay for tau aggregation. Assay Drug Dev. Technol. 2, 609–619 (2004).
Wischik, C. M., Edwards, P. C., Lai, R. Y. K., Roth, M. & Harrington, C. R. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc. Natl Acad. Sci. USA 93, 11213–11218 (1996).
Ignatova, Z. & Gierasch, L. M. Inhibition of protein aggregation in vitro and in vivo by a natural osmoprotectant. Proc. Natl Acad. Sci. USA 103, 13357–13361 (2006).
Gotz, J. et al. A decade of tau transgenic animal models and beyond. Brain Pathol. 17, 91–103 (2007). An up-to-date account of tau transgenic animal models.
McGowan, E., Eriksen, J. & Hutton, M. A decade of modeling Alzheimer's disease in transgenic mice. Trends Genet. 22, 281–289 (2006).
Van Dam, D. & De Deyn, P. P. Drug discovery in dementia: the role of rodent models. Nature Rev. Drug Discov. 5, 956–970 (2006).
Schindowski, K. et al. Alzheimer's disease-like tau neuropathology leads to memory deficits and loss of functional synapses in a novel mutated tau transgenic mouse without any motor deficits. Am. J. Pathol. 169, 599–616 (2006).
Melnikova, I. Therapies for Alzheimer's disease. Nature Rev. Drug Discov. 6, 341–342 (2007).
Okamura, N. et al. Quinoline and benzimidazole derivatives: candidate probes for in vivo imaging of tau pathology in Alzheimer's disease. J. Neurosci. 25, 10857–10862 (2005).
Pardridge, W. M. The blood–brain barrier: bottleneck in brain drug development. NeuroRx 2, 3–14 (2005).
Gotz, J. et al. Somatodendritic localization and hyperphosphorylation of tau protein in transgenic mice expressing the longest human brain tau isoform. EMBO J. 14, 1304–1313 (1995).
Spittaels, K. et al. Prominent axonopathy in the brain and spinal cord of transgenic mice overexpressing four-repeat human tau protein. Am. J. Pathol. 155, 2153–2165 (1999).
Probst, A. et al. Axonopathy and amyotrophy in mice transgenic for human four-repeat tau protein. Acta Neuropathol. 99, 469–481 (2000).
Ishihara, T. et al. Age-dependent induction of congophilic neurofibrillary tau inclusions in tau transgenic mice. Am. J. Pathol. 158, 555–562 (2001).
Lewis, J. et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nature Genet. 25, 402–405 (2000).
Gotz, J., Chen, F., Barmettler, R. & Nitsch, R. M. Tau filament formation in transgenic mice expressing P301L tau. J. Biol. Chem. 276, 529–534 (2001).
Duff, K. et al. Characterization of pathology in transgenic mice over-expressing human genomic and cDNA tau transgenes. Neurobiol. Dis. 7, 87–98 (2000).
Gotz, J. et al. Oligodendroglial tau filament formation in transgenic mice expressing G272V tau. Eur. J. Neurosci. 13, 2131–2140 (2001).
Allen, B. et al. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J. Neurosci. 22, 9340–9351 (2002).
Lim, F. et al. FTDP-17 mutations in tau transgenic mice provoke lysosomal abnormalities and tau filaments in forebrain. Mol. Cell Neurosci. 18, 702–714 (2001).
Tanemura, K. et al. Neurodegeneration with tau accumulation in a transgenic mouse expressing V337M human tau. J. Neurosci. 22, 133–141 (2002).
Tatebayashi, Y. et al. Tau filament formation and associative memory deficit in aged mice expressing mutant (R406W) human tau. Proc. Natl Acad. Sci. USA 99, 13896–13901 (2002).
Tesseur, I. et al. Prominent axonopathy and disruption of axonal transport in transgenic mice expressing human apolipoprotein E4 in neurons of brain and spinal cord. Am. J. Pathol. 157, 1495–1510 (2000).
Ahlijanian, M. K. et al. Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc. Natl Acad. Sci. USA 97, 2910–2915 (2000).
Capsoni, S. et al. Alzheimer-like neurodegeneration in aged antinerve growth factor transgenic mice. Proc. Natl Acad. Sci. USA 97, 6826–6831 (2000).
Oddo, S., Caccamo, A., Kitazawa, M., Tseng, B. P. & LaFerla, F. M. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer's disease. Neurobiol. Aging 24, 1063–1070 (2003).
Oddo, S. et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron 39, 409–421 (2003).
Crowe, A., Ballatore, C., Hyde, E., Trojanowski, J. Q. & Lee, V. M. -Y. High throughput screening for small molecule inhibitors of heparin-induced tau fibril formation. Biochem. Biophys. Res. Commun. 358, 1–6 (2007).
Dickey, C. A. et al. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J. Clin. Invest. 117, 648–658 (2007).
Goryunov, D. & Liem, R. K. H. CHIP-ping away at tau. J. Clin. Invest. 117, 590–592 (2007).
Matsuoka, Y. et al. Intranasal NAP administration reduces accumulation of amyloid peptide and tau hyperphosphorylation in a transgenic mouse model of Alzheimer's disease at early pathological stage. J. Mol. Neurosci. 31, 165–170 (2007).
Pasinetti, G. M. From epidemiology to therapeutic trials with anti-inflammatory drugs in Alzheimer's disease: the role of NSAIDs and cyclooxygenase in β-amyloidosis and clinical dementia. J. Alzheimers Dis. 4, 435–445 (2002).
Klegeris, A. & McGeer, P. L. Non-steroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory agents in the treatment of neurodegenerative disease. Curr. Alzheimer Res. 2, 355–365 (2005).
Townsend, K. P. & Pratico, D. Novel therapeutic opportunities for Alzheimer's disease: focus on nonsteroidal anti-inflammatory drugs. FASEB J. 19, 1592–1601 (2005).
Acknowledgements
We thank our colleagues for their contributions to the work summarized here, which has been supported by grants from the US National Institutes of Health (P01 AG09215, P30 AG10124, P01 AG11542, P01 AG14382, P01 AG14449, P01 AG17586, PO1 AG19724, P01 NS-044233, UO1 AG24904), and the Marian S. Ware Alzheimer Program. Finally, we are indebted to our patients and their families, whose commitment to research has made our work possible.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
OMIM
FURTHER INFORMATION
Glossary
- Senile plaque
-
A site of Aβ accumulation and dystrophic neurites in the brains of mouse models and patients with Alzheimer's disease.
- Alternative splicing
-
The process by which introns are excised from RNA after transcription and the cut ends of the RNA are rejoined to form a continuous message. Alternative splicing allows the production of different messages from the same DNA molecule.
- Oxidative stress
-
A disturbance in the pro-oxidant-antioxidant balance in favour of the pro-oxidant, leading to potential cellular damage. Indicators of oxidative stress include damaged DNA bases, protein oxidation and lipid peroxidation products.
- Dystrophic neurites
-
The processes (axons and dendrites) of neurons that are damaged or degenerating in AD.
- Microglia
-
A non-neuronal cell type that is present in the spinal cord and the brain (it is the resident CNS macrophage) and is characterized by its ramified morphology.
Rights and permissions
About this article
Cite this article
Ballatore, C., Lee, VY. & Trojanowski, J. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci 8, 663–672 (2007). https://doi.org/10.1038/nrn2194
Issue Date:
DOI: https://doi.org/10.1038/nrn2194
This article is cited by
-
Liquid–liquid phase separation in Alzheimer’s disease
Journal of Molecular Medicine (2024)
-
p-tau Ser356 is associated with Alzheimer’s disease pathology and is lowered in brain slice cultures using the NUAK inhibitor WZ4003
Acta Neuropathologica (2024)
-
The HSP40 family chaperone isoform DNAJB6b prevents neuronal cells from tau aggregation
BMC Biology (2023)
-
Intra-hippocampal cis-P tau microinjection induces long-term changes in behavior and synaptic plasticity in mice
Behavioral and Brain Functions (2023)
-
Quantitative proteomics of cerebrospinal fluid from African Americans and Caucasians reveals shared and divergent changes in Alzheimer’s disease
Molecular Neurodegeneration (2023)