Key Points
-
Nearly every aspect of neuronal function depends on the accurate localization of polarized membrane proteins to the axon or dendrites.
-
Selective sorting, selective transport and selective delivery are the key events for maintaining neuronal polarity.
-
Some of the sorting signals and sorting adaptors that mediate the targeting of dendritic proteins have been identified. By contrast, little is known about the mechanisms underlying axonal protein sorting.
-
Selective microtubule-based transport is crucial for the targeting of both axonal and dendritic proteins. The basis for selective transport depends on the regulation of motor proteins, but the underlying mechanisms remain controversial.
-
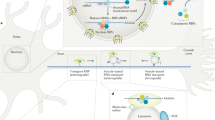
There must be links between the process of protein sorting, which determines the cargoes present in a vesicle and hence the identity of a vesicle, and the recruitment of the correct motors, SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) and tethers, which determine the fate of a vesicle. Almost nothing is yet known about these links.
Abstract
As polarized cells, neurons maintain different sets of resident plasma membrane proteins in their axons and dendrites, which is consistent with the different roles that these neurites have in electrochemical signalling. Axonal and dendritic proteins are synthesized together within the somatodendritic domain; this raises a fundamental question: what is the nature of the intracellular trafficking machinery that ensures that these proteins reach the correct domain? Recent studies have advanced our understanding of the processes underlying the selective sorting and selective transport of axonal and dendritic proteins and have created potential avenues for future progress.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Craig, A. M. & Banker, G. Neuronal polarity. Annu. Rev. Neurosci. 17, 267–310 (1994).
Peters, A., Palay, S. L. & Webster, H. D. The Fine Structure of the Nervous System: The Neurons and Supporting Cells (Oxford Univ. Press, 1991).
Roy, S. Seeing the unseen: the hidden world of slow axonal transport. Neuroscientist 20, 71–81 (2014).
Blackstone, C., O'Kane, C. J. & Reid, E. Hereditary spastic paraplegias: membrane traffic and the motor pathway. Nat. Rev. Neurosci. 12, 31–42 (2011).10.1038/nrn2946
Encalada, S. E. & Goldstein, L. S. B. Biophysical challenges to axonal transport: motor-cargo deficiencies and neurodegeneration. Annu. Rev. Biophys. 43, 141–169 (2014).
Chevalier-Larsen, E. & Holzbaur, E. L. F. Axonal transport and neurodegenerative disease. Biochim. Biophys. Acta 1762, 1094–1108 (2006).
Hunn, B. H. M., Cragg, S. J., Bolam, J. P., Spillantini, M. G. & Wade-Martins, R. Impaired intracellular trafficking defines early Parkinson's disease. Trends Neurosci. 38, 178–188 (2015).
Guo, Y., Sirkis, D. W. & Schekman, R. Protein sorting at the trans-Golgi network. Annu. Rev. Cell Dev. Biol. 30, 169–206 (2014).
Chia, P. Z. C. & Gleeson, P. A. Membrane tethering. F1000Prime Rep. 6, 74 (2014).
Sztul, E. & Lupashin, V. Role of vesicle tethering factors in the ER–Golgi membrane traffic. FEBS Lett. 583, 3770–3783 (2009).
Jahn, R. & Scheller, R. H. SNAREs — engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 7, 631–643 (2006).
Vale, R. D. The molecular motor toolbox for intracellular transport. Cell 112, 467–480 (2003).
Hirokawa, N., Noda, Y., Tanaka, Y. & Niwa, S. Kinesin superfamily motor proteins and intracellular transport. Nat. Rev. Mol. Cell Biol. 10, 682–696 (2009).
Vallee, R. B., McKenney, R. J. & Ori-McKenney, K. M. Multiple modes of cytoplasmic dynein regulation. Nat. Cell Biol. 14, 224–230 (2012).
Bhabha, G., Johnson, G. T., Schroeder, C. M. & Vale, R. D. How dynein moves along microtubules. Trends Biochem. Sci. 41, 94–105 (2015).
Akhmanova, A. & Hammer, J. A. III. Linking molecular motors to membrane cargo. Curr. Opin. Cell Biol. 22, 479–487 (2010).
Hutagalung, A. H. & Novick, P. J. Role of rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 91, 119–149 (2011).
Julie, G. Donaldson, C. L. J. Arf family G proteins and their regulators: roles in membrane transport, development and disease. Nat. Rev. Mol. Cell Biol. 12, 362–375 (2011).
Burack, M. A., Silverman, M. A. & Banker, G. The role of selective transport in neuronal protein sorting. Neuron 26, 465–472 (2000).
Al-Bassam, S., Xu, M., Wandless, T. J. & Arnold, D. B. Differential trafficking of transport vesicles contributes to the localization of dendritic proteins. Cell Rep. 2, 89–100 (2012). This paper presents evidence in support of the myosin hypothesis for selective dendritic transport.
Farías, G. G. et al. Signal-mediated, AP-1/clathrin-dependent sorting of transmembrane receptors to the somatodendritic domain of hippocampal neurons. Neuron 75, 810–823 (2012). This study shows that AP1 mediates the sorting of several dendritically polarized proteins.
Jensen, C. S. et al. Specific sorting and post-Golgi trafficking of dendritic potassium channels in living neurons. J. Biol. Chem. 289, 10566–10581 (2014).
Nakata, T. & Hirokawa, N. Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J. Cell Biol. 162, 1045–1055 (2003).
Sampo, B., Kaech, S., Kunz, S. & Banker, G. Two distinct mechanisms target membrane proteins to the axonal surface. Neuron 37, 611–624 (2003).
Lee, K. H. et al. KIF21A-mediated axonal transport and selective endocytosis underlie the polarized targeting of NCKX2. J. Neurosci. 32, 4102–4117 (2012).
Bel, C., Oguievetskaia, K., Pitaval, C., Goutebroze, L. & Faivre-Sarrailh, C. Axonal targeting of Caspr2 in hippocampal neurons via selective somatodendritic endocytosis. J. Cell Sci. 122, 3403–3413 (2009).
Wisco, D. et al. Uncovering multiple axonal targeting pathways in hippocampal neurons. J. Cell Biol. 162, 1317–1328 (2003).
MacGillavry, H. D., Kerr, J. M. & Blanpied, T. A. Lateral organization of the postsynaptic density. Mol. Cell Neurosci. 48, 321–331 (2011).
Dzhashiashvili, Y. et al. Nodes of Ranvier and axon initial segments are ankyrin G-dependent domains that assemble by distinct mechanisms. J. Cell Biol. 177, 857–870 (2007).
West, A. E., Neve, R. L. & Buckley, K. M. Identification of a somatodendritic targeting signal in the cytoplasmic domain of the transferrin receptor. J. Neurosci. 17, 6038–6047 (1997).
Jareb, M. & Banker, G. The polarized sorting of membrane proteins expressed in cultured hippocampal neurons using viral vectors. Neuron 20, 855–867 (1998).
Silverman, M. A. et al. Motifs that mediate dendritic targeting in hippocampal neurons: a comparison with basolateral targeting signals. Mol. Cell Neurosci. 29, 173–180 (2005).
Owen, D. J., Collins, B. M. & Evans, P. R. Adaptors for clathrin coats: structure and function. Annu. Rev. Cell Dev. Biol. 20, 153–191 (2004).
Robinson, M. S. Adaptable adaptors for coated vesicles. Trends Cell Biol. 14, 167–174 (2004).
Bonifacino, J. S. Adaptor proteins involved in polarized sorting. J. Cell Biol. 204, 7–17 (2014).
Kirchhausen, T., Owen, D. & Harrison, S. C. Molecular structure, function, and dynamics of clathrin-mediated membrane traffic. Cold Spring Harb. Perspect. Biol. 6, a016725 (2014).
Sang Yoon, P. & Xiaoli, G. Adaptor protein complexes and intracellular transport. Biosci. Rep. 34, e00123 (2014).
Popova, N. V., Deyev, I. E. & Petrenko, A. G. Clathrin-mediated endocytosis and adaptor proteins. Acta Naturae 5, 62–73 (2013).
Fields, I. C., King, S. M., Shteyn, E., Kang, R. S. & Folsch, H. Phosphatidylinositol 3,4,5-trisphosphate localization in recycling endosomes is necessary for AP-1B–dependent sorting in polarized epithelial cells. Mol. Biol. Cell 21, 95–105 (2010).
Shteyn, E., Pigati, L. & Folsch, H. Arf6 regulates AP-1B–dependent sorting in polarized epithelial cells. J. Cell Biol. 194, 873–887 (2011).
Matsuda, S. et al. Accumulation of AMPA receptors in autophagosomes in neuronal axons lacking adaptor protein AP-4. Neuron 57, 730–745 (2008). This report shows that AP4 mediates the sorting of AMPA receptors.
Brown, M. D., Banker, G. A., Hussaini, I. M., Gonias, S. L. & VandenBerg, S. R. Low density lipoprotein receptor-related protein is expressed early and becomes restricted to a somatodendritic domain during neuronal differentiation in culture. Brain Res. 747, 313–317 (1997).
Kang, R. S. & Folsch, H. ARH cooperates with AP-1B in the exocytosis of LDLR in polarized epithelial cells. J. Cell Biol. 193, 51–60 (2011).
Donoso, M. et al. Polarized traffic of LRP1 involves AP1B and SNX17 operating on Y-dependent sorting motifs in different pathways. Mol. Biol. Cell 20, 481–497 (2009).
Mameza, M. G. et al. Characterization of the adaptor protein ARH expression in the brain and ARH molecular interactions. J. Neurochem. 103, 927–941 (2007).
Rivera, J. F., Ahmad, S., Quick, M. W., Liman, E. R. & Arnold, D. B. An evolutionarily conserved dileucine motif in Shal K+ channels mediates dendritic targeting. Nat. Neurosci. 6, 243–250 (2003).
Diao, F., Chaufty, J., Waro, G. & Tsunoda, S. SIDL interacts with the dendritic targeting motif of Shal (Kv4) K+ channels in Drosophila. Mol. Cell Neurosci. 45, 75–83 (2010).
Kozik, P., Francis, R. W., Seaman, M. N. J. & Robinson, M. S. A. Screen for endocytic motifs. Traffic 11, 843–855 (2010).
Yap, C. C. et al. Pathway selection to the axon depends on multiple targeting signals in NgCAM. J. Cell Sci. 121, 1514–1525 (2008).
Yap, C. C. C., Lasiecka, Z. M., Caplan, S. & Winckler, B. Alterations of EHD1/EHD4 protein levels interfere with L1/NgCAM endocytosis in neurons and disrupt axonal targeting. J. Neurosci. 30, 6646–6657 (2010).
Lasiecka, Z. M., Yap, C. C., Caplan, S. & Winckler, B. Neuronal early endosomes require EHD1 for L1/NgCAM trafficking. J. Neurosci. 30, 16485–16497 (2010).
Gu, C. & Barry, J. Function and mechanism of axonal targeting of voltage-sensitive potassium channels. Prog. Neurobiol. 94, 115–132 (2011).
Gu, C., Jan, Y. N. & Jan, L. Y. A conserved domain in axonal targeting of Kv1 (Shaker) voltage-gated potassium channels. Science 301, 646–649 (2003).
Rivera, J. F., Chu, P. J. & Arnold, D. B. The T1 domain of Kv1.3 mediates intracellular targeting to axons. Eur. J. Neurosci. 22, 1853–1862 (2005).
Gu, C. et al. The microtubule plus-end tracking protein EB1 is required for Kv1 voltage-gated K+ channel axonal targeting. Neuron 52, 803–816 (2006).
Li, P., Merrill, S. A., Jorgensen, E. M. & Shen, K. Two clathrin adaptor protein complexes instruct axon-dendrite polarity. Neuron 90, 564–580 (2016).
Burton, P. R. & Paige, J. L. Polarity of axoplasmic microtubules in the olfactory nerve of the frog. Proc. Natl Acad. Sci. USA 78, 3269–3273 (1981).
Heidemann, S. R. et al. Spatial organization of axonal microtubules. J. Cell Biol. 99, 1289–1295 (1984).
Baas, P. W., Deitch, J. S., Black, M. M. & Banker, G. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc. Natl Acad. Sci. USA 85, 8335–8339 (1988).
Stepanova, T. et al. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein). J. Neurosci. 23, 2655–2664 (2003).
Baas, P. W. & Lin, S. Hooks and comets: the story of microtubule polarity orientation in the neuron. Dev. Neurobiol. 71, 403–418 (2011).
Baas, P. W., Black, M. M. & Banker, G. A. Changes in microtubule polarity orientation during the development of hippocampal neurons in culture. J. Cell Biol. 109, 3085–3094 (1989).
Yau, K. W. et al. Dendrites in vitro and in vivo contain microtubules of opposite polarity and axon formation correlates with uniform plus-end-out microtubule orientation. J. Neurosci. 36, 1071–1085 (2016).
Kapitein, L. C. & Hoogenraad, C. C. Building the neuronal microtubule cytoskeleton. Neuron 87, 492–506 (2015).
Leterrier, C. et al. End-binding proteins EB3 and EB1 link microtubules to ankyrin G in the axon initial segment. Proc. Natl Acad. Sci. USA 108, 8826–8831 (2011).
van Beuningen, S. F. B. et al. TRIM46 controls neuronal polarity and axon specification by driving the formation of parallel microtubule arrays. Neuron 88, 1208–1226 (2015).
Silverman, M. A. et al. Expression of kinesin superfamily genes in cultured hippocampal neurons. Cytoskeleton (Hoboken) 67, 784–795 (2010).
Roberts, A. J., Kon, T., Knight, P. J., Sutoh, K. & Burgess, S. A. Functions and mechanics of dynein motor proteins. Nat. Rev. Mol. Cell Biol. 14, 713–726 (2013).
Jacobson, C., Schnapp, B. & Banker, G. A. A change in the selective translocation of the Kinesin-1 motor domain marks the initial specification of the axon. Neuron 49, 797–804 (2006).
Yang, R., Bentley, M., Huang, C. F. & Banker, G. Analyzing kinesin motor domain translocation in cultured hippocampal neurons. Methods Cell Biol. 131, 217–232 (2015).
Vale, R. D. et al. Direct observation of single kinesin molecules moving along microtubules. Nature 380, 451–453 (1996).
Friedman, D. S. & Vale, R. D. Single-molecule analysis of kinesin motility reveals regulation by the cargo-binding tail domain. Nat. Cell Biol. 1, 293–297 (1999).
Cai, D., McEwen, D. P., Martens, J. R., Meyhofer, E. & Verhey, K. J. Single molecule imaging reveals differences in microtubule track selection between kinesin motors. PLoS Biol. 7, e1000216 (2009).
Soppina, V. et al. Dimerization of mammalian kinesin-3 motors results in superprocessive motion. Proc. Natl Acad. Sci. USA 111, 5562–5567 (2014).
Huang, C. F. & Banker, G. The translocation selectivity of the kinesins that mediate neuronal organelle transport. Traffic 13, 549–564 (2012). This systematic screen of kinesin motor domains shows that no motors are dendrite selective.
Kapitein, L. C. et al. Probing intracellular motor protein activity using an inducible cargo trafficking assay. Biophys. J. 99, 2143–2152 (2010).
Kapitein, L. C. et al. Mixed microtubules steer dynein-driven cargo transport into dendrites. Curr. Biol. 20, 290–299 (2010). This study, which introduced the peroxisome assay for evaluating motor selectivity, shows that cargoes can be targeted selectively into dendrites and provides evidence for the dynein hypothesis for selective dendritic transport.
Ally, S., Larson, A. G., Barlan, K., Rice, S. E. & Gelfand, V. I. Opposite-polarity motors activate one another to trigger cargo transport in live cells. J. Cell Biol. 187, 1071–1082 (2009).
Lipka, J., Kapitein, L. C., Jaworski, J. & Hoogenraad, C. C. Microtubule-binding protein doublecortin-like kinase 1 (DCLK1) guides kinesin-3-mediated cargo transport to dendrites. EMBO J. 35, 302–318 (2016).
Setou, M., Nakagawa, T., Seog, D. H. & Hirokawa, N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science 288, 1796–1802 (2000).
Guillaud, L., Setou, M. & Hirokawa, N. KIF17 dynamics and regulation of NR2B trafficking in hippocampal neurons. J. Neurosci. 23, 131–140 (2003).
Chu, P. J., Rivera, J. F. & Arnold, D. B. A role for Kif17 in transport of Kv4.2. J. Biol. Chem. 281, 365–373 (2006).
Saxton, W. M. & Hollenbeck, P. J. The axonal transport of mitochondria. J. Cell Sci. 125, 2095–2104 (2012).
Watanabe, K. et al. Networks of polarized actin filaments in the axon initial segment provide a mechanism for sorting axonal and dendritic proteins. Cell Rep. 2, 1546–1553 (2012).
Kapitein, L. C. et al. Myosin-V opposes microtubule-based cargo transport and drives directional motility on cortical actin. Curr. Biol. 23, 828–834 (2013).
Konishi, Y. & Setou, M. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat. Neurosci. 12, 559–567 (2009).
Verhey, K. J. & Gaertig, J. The tubulin code. Cell Cycle 6, 2152–2160 (2007).
Hammond, J. W. et al. Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol. Biol. Cell 21, 572–583 (2010).
Kaul, N., Soppina, V. & Verhey, K. J. Effects of α-tubulin K40 acetylation and detyrosination on kinesin-1 motility in a purified system. Biophys. J. 106, 2636–2643 (2014).
Janke, C. & Bulinski, J. C. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat. Rev. Mol. Cell Biol. 12, 773–786 (2011).
Magiera, M. M. & Janke, C. Post-translational modifications of tubulin. Curr. Biol. 24, R351–R354 (2014).
Ludueña, R. F. A hypothesis on the origin and evolution of tubulin. Int. Rev. Cell. Mol. Biol. 302, 41–185 (2013).
Szyk, A. et al. Molecular basis for age-dependent microtubule acetylation by tubulin acetyltransferase. Cell 157, 1405–1415 (2014).
Janke, C. The tubulin code: molecular components, readout mechanisms, and functions. J. Cell Biol. 206, 461–472 (2014).
Reed, N. A. et al. Microtubule acetylation promotes kinesin-1 binding and transport. Curr. Biol. 16, 2166–2172 (2006).
Sirajuddin, M., Rice, L. M. & Vale, R. D. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat. Cell Biol. 16, 335–344 (2014).
Nakata, T., Niwa, S., Okada, Y., Perez, F. & Hirokawa, N. Preferential binding of a kinesin-1 motor to GTP-tubulin-rich microtubules underlies polarized vesicle transport. J. Cell Biol. 194, 245–255 (2011).
Morikawa, M. et al. X-Ray and Cryo-EM structures reveal mutual conformational changes of Kinesin and GTP-state microtubules upon binding. EMBO J. 34, 1270–1286 (2015).
Petersen, J. D., Kaech, S. & Banker, G. Selective microtubule-based transport of dendritic membrane proteins arises in concert with axon specification. J. Neurosci. 34, 4135–4147 (2014). This paper, which characterizes selective dendritic transport, shows that dendritically polarized vesicles reverse direction at the proximal part of the axon initial segment.
Black, M. M. & Baas, P. W. The basis of polarity in neurons. Trends Neurosci. 12, 211–214 (1989).
Lewis, T. L., Mao, T., Svoboda, K. & Arnold, D. B. Myosin-dependent targeting of transmembrane proteins to neuronal dendrites. Nat. Neurosci. 12, 568–576 (2009).
Song, A. H. et al. A selective filter for cytoplasmic transport at the axon initial segment. Cell 136, 1148–1160 (2009).
van Spronsen, M. et al. TRAK/Milton motor-adaptor proteins steer mitochondrial trafficking to axons and dendrites. Neuron 77, 485–502 (2013).
Setou, M. et al. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature 417, 83–87 (2002).
Jenkins, B., Decker, H., Bentley, M., Luisi, J. & Banker, G. A novel split kinesin assay identifies motor proteins that interact with distinct vesicle populations. J. Cell Biol. 198, 749–761 (2012).
Silverman, M. A. et al. Sorting and directed transport of membrane proteins during development of hippocampal neurons in culture. Proc. Natl Acad. Sci. USA 98, 7051–7057 (2001).
Kuijpers, M. et al. Dynein regulator NDEL1 controls polarized cargo transport at the axon initial segment. Neuron 89, 461–471 (2016).
Arnold, D. B. Actin and microtubule-based cytoskeletal cues direct polarized targeting of proteins in neurons. Sci. Signal. 2, pe49 (2009).
Setou, M., Hayasaka, T. & Yao, I. Axonal transport versus dendritic transport. J. Neurobiol. 58, 201–206 (2004).
Fu, M. M. & Holzbaur, E. L. F. Integrated regulation of motor-driven organelle transport by scaffolding proteins. Trends Cell Biol. 24, 564–574 (2014).
Gibbs, K. L., Greensmith, L. & Schiavo, G. Regulation of axonal transport by protein kinases. Trends Biochem. Sci. 40, 597–610 (2015).
Akin, E. J., Solé, L., Dib-Hajj, S. D., Waxman, S. G. & Tamkun, M. M. Preferential targeting of Nav1.6 voltage-gated Na+ channels to the axon initial segment during development. PLoS ONE 10, e0124397 (2015).
Mostov, K. E., Verges, M. & Altschuler, Y. Membrane traffic in polarized epithelial cells. Curr. Opin. Cell Biol. 12, 483–490 (2000).
Beest, ter, M. B. A., Chapin, S. J., Avrahami, D. & Mostov, K. E. The role of syntaxins in the specificity of vesicle targeting in polarized epithelial cells. Mol. Biol. Cell 16, 5784–5792 (2005).
Barnes, A. P. & Polleux, F. Establishment of axon-dendrite polarity in developing neurons. Annu. Rev. Neurosci. 32, 347–381 (2009).
Cheng, P. L. & Poo, M. M. Early events in axon/dendrite polarization. Annu. Rev. Neurosci. 35, 181–201 (2012).
Caceres, A., Ye, B. & Dotti, C. G. Neuronal polarity: demarcation, growth and commitment. Curr. Opin. Cell Biol. 24, 547–553 (2012).
Liu, Z., Lavis, L. D. & Betzig, E. Imaging live-cell dynamics and structure at the single-molecule level. Mol. Cell 58, 644–659 (2015).
Heidenreich, M. & Zhang, F. Applications of CRISPR–Cas systems in neuroscience. Nat. Rev. Neurosci. 17, 36–44 (2015).
Hsu, P. D., Lander, E. S. & Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 (2014).
Kaech, S., Huang, C.-F. & Banker, G. Short-term high-resolution imaging of developing hippocampal neurons in culture. Cold Spring Harb. Protoc. 2012, 340–343 (2012).
Kaech, S., Huang, C.-F. & Banker, G. Long-term time-lapse imaging of developing hippocampal neurons in culture. Cold Spring Harb. Protoc. 2012, 335–339 (2012).
Hayashi, K., Kawai-Hirai, R., Harada, A. & Takata, K. Inhibitory neurons from fetal rat cerebral cortex exert delayed axon formation and active migration in vitro. J. Cell Sci. 116, 4419–4428 (2003).
Mitsui, S., Saito, M., Hayashi, K., Mori, K. & Yoshihara, Y. A novel phenylalanine-based targeting signal directs telencephalin to neuronal dendrites. J. Neurosci. 25, 1122–1131 (2005).
Rolls, M. M. Neuronal polarity in Drosophila: sorting out axons and dendrites. Dev. Neurobiol. 71, 419–429 (2011).
Rolls, M. M. & Jegla, T. J. Neuronal polarity: an evolutionary perspective. J. Exp. Biol. 218, 572–580 (2015).
Ou, C. Y. & Shen, K. Neuronal polarity in C. elegans. Dev. Neurobiol. 71, 554–566 (2011).
Chua, J. J. E., Jahn, R. & Klopfenstein, D. R. Managing intracellular transport. Worm 2, e21564 (2013).
Sato, K., Norris, A., Sato, M. & Grant, B. D. in WormBook (ed. The C. elegans Research Community) 1–47 (2014).
Drerup, C. M. & Nechiporuk, A. V. JNK-interacting protein 3 mediates the retrograde transport of activated c-Jun N-terminal kinase and lysosomes. PLoS Genet. 9, e1003303 (2013).
Stowers, R. S., Megeath, L. J., Górska-Andrzejak, J., Meinertzhagen, I. A. & Schwarz, T. L. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron 36, 1063–1077 (2002).
Guo, X. et al. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron 47, 379–393 (2005).
Glater, E. E., Megeath, L. J., Stowers, R. S. & Schwarz, T. L. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J. Cell Biol. 173, 545–557 (2006).
Russo, G. J. et al. Drosophila Miro is required for both anterograde and retrograde axonal mitochondrial transport. J. Neurosci. 29, 5443–5455 (2009).
Nguyen, M. M. et al. γ-Tubulin controls neuronal microtubule polarity independently of Golgi outposts. Mol. Biol. Cell 25, 2039–2050 (2014).
Kern, J. V., Zhang, Y. V., Kramer, S., Brenman, J. E. & Rasse, T. M. The kinesin-3, Unc-104 regulates dendrite morphogenesis and synaptic development in Drosophila. Genetics 195, 59–72 (2013).
Klopfenstein, D. R. & Vale, R. D. The lipid binding pleckstrin homology domain in UNC-104 kinesin is necessary for synaptic vesicle transport in Caenorhabditis elegans. Mol. Biol. Cell 15, 3729–3739 (2004).
Pilling, A. D., Horiuchi, D., Lively, C. M. & Saxton, W. M. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol. Biol. Cell 17, 2057–2068 (2006).
Hoerndli, F. J. et al. Kinesin-1 regulates synaptic strength by mediating the delivery, removal, and redistribution of AMPA receptors. Neuron 80, 1421–1437 (2013).
Hoerndli, F. J. et al. Neuronal activity and CaMKII regulate kinesin-mediated transport of synaptic AMPARs. Neuron 86, 457–474 (2015).
Choquet, D. & Triller, A. The dynamic synapse. Neuron 80, 691–703 (2013).
Das, S. S. & Banker, G. A. The role of protein interaction motifs in regulating the polarity and clustering of the metabotropic glutamate receptor mGluR1a. J. Neurosci. 26, 8115–8125 (2006).
Cheng, C., Glover, G., Banker, G. & Amara, S. G. A novel sorting motif in the glutamate transporter excitatory amino acid transporter 3 directs its targeting in Madin–Darby canine kidney cells and hippocampal neurons. J. Neurosci. 22, 10643–10652 (2002).
Coombs, J. S., Curtis, D. R. & Eccles, J. C. The interpretation of spike potentials of motoneurones. J. Physiol. (Lond.) 139, 198–231 (1957).
Palay, S. L., Sotelo, C., Peters, A. & Orkand, P. M. The axon hillock and the initial segment. J. Cell Biol. 38, 193–201 (1968).
Winckler, B., Forscher, P. & Mellman, I. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature 397, 698–701 (1999).
Nakada, C. et al. Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat. Cell Biol. 5, 626–632 (2003).
Fujiwara, T., Ritchie, K., Murakoshi, H., Jacobson, K. & Kusumi, A. Phospholipids undergo hop diffusion in compartmentalized cell membrane. J. Cell Biol. 157, 1071–1081 (2002).
Jones, S. L., Korobova, F. & Svitkina, T. Axon initial segment cytoskeleton comprises a multiprotein submembranous coat containing sparse actin filaments. J. Cell Biol. 2, 89 (2014).
Hedstrom, K. L., Ogawa, Y. & Rasband, M. N. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J. Cell Biol. 183, 635–640 (2008).
Jenkins, P. M. et al. Giant ankyrin-G: a critical innovation in vertebrate evolution of fast and integrated neuronal signaling. Proc. Natl Acad. Sci. USA 112, 957–964 (2014). This paper shows that the loss of the axon initial segment does not disrupt selective dendritic transport.
Sobotzik, J. M. et al. AnkyrinG is required to maintain axo-dendritic polarity in vivo. Proc. Natl Acad. Sci. USA 106, 17564–17569 (2009).
Gallon, M. & Cullen, P. J. Retromer and sorting nexins in endosomal sorting. Biochem. Soc. Trans. 43, 33–47 (2015).
Kardon, J. R. & Vale, R. D. Regulators of the cytoplasmic dynein motor. Nat. Rev. Mol. Cell Biol. 10, 854–865 (2009).
Hong, W. SNAREs and traffic. Biochim. Biophys. Acta 1744, 120–144 (2005).
Mattera, R., Farías, G. G., Mardones, G. A. & Bonifacino, J. S. Co-assembly of viral envelope glycoproteins regulates their polarized sorting in neurons. PLoS Pathog. 10, e1004107 (2014).
Eiraku, M., Hirata, Y., Takeshima, H., Hirano, T. & Kengaku, M. Delta/notch-like epidermal growth factor (EGF)-related receptor, a novel EGF-like repeat-containing protein targeted to dendrites of developing and adult central nervous system neurons. J. Biol. Chem. 277, 25400–25407 (2002).
Xu, J., Zhu, Y. & Heinemann, S. F. Identification of sequence motifs that target neuronal nicotinic receptors to dendrites and axons. J. Neurosci. 26, 9780–9793 (2006).
Rosales, C. R., Osborne, K. D., Zuccarino, G. V., Scheiffele, P. & Silverman, M. A. A cytoplasmic motif targets neuroligin-1 exclusively to dendrites of cultured hippocampal neurons. Eur. J. Neurosci. 22, 2381–2386 (2005).
Acknowledgements
The work in the authors' laboratory is supported by the US National Institutes of Health (NIH MH066179). The authors thank S. Kaech for her comments on the manuscript and S. Henderson for help in creating the figures. The images in Figure 2, panels b and c, are supplied by M.B. and G.B.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (movie)
A movie of a cultured neuron co-expressing axonal and dendritic proteins. Movie shows proteins being transported within the neuron. (M.B. and G.B., unpublished observations). (MOV 1071 kb)
Glossary
- Polarized cells
-
Cells composed of two distinct domains, which differ in molecular composition. Examples include epithelial cells, oocytes and neurons.
- Cargoes
-
In the context of protein trafficking, cargoes refer to membrane proteins that must be moved from one compartment to another.
- Endosomes
-
Intracellular organelles involved in the trafficking of proteins internalized from the plasma membrane.
- Coat proteins
-
Protein components that bind to the cytosolic face of membranes, serving to concentrate cargoes into new vesicles and to induce bud formation. Some coat proteins form an electron-dense 'coat' that is visible by electron microscopy, hence the name.
- Glycoproteins
-
Proteins that are post-translationally modified by the addition of sugar chains to ectodomain amino acid residues.
- Tethering proteins
-
Also known as tethering factors. Proteins that initiate the first interaction between a vesicle and the target membrane where the vesicle will undergo fusion.
- Motor adaptors
-
Proteins that link kinesin or dynein motors to vesicles or other cargoes.
- Submembranous scaffolding proteins
-
Proteins that form complexes located just beneath the plasma membrane that bind to and concentrate specific sets of plasma membrane and cytosolic proteins.
- Canonical sorting motifs
-
Sorting sequences that share a common three-dimensional structure that allows them to interact with identified binding pockets in heterotetrameric adaptor proteins.
Rights and permissions
About this article
Cite this article
Bentley, M., Banker, G. The cellular mechanisms that maintain neuronal polarity. Nat Rev Neurosci 17, 611–622 (2016). https://doi.org/10.1038/nrn.2016.100
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn.2016.100
This article is cited by
-
Endocytosis in the axon initial segment maintains neuronal polarity
Nature (2022)
-
ER – lysosome contacts at a pre-axonal region regulate axonal lysosome availability
Nature Communications (2021)
-
In vivo and in vitro sex differences in the dendritic morphology of developing murine hippocampal and cortical neurons
Scientific Reports (2017)
-
The impact of cytoskeletal organization on the local regulation of neuronal transport
Nature Reviews Neuroscience (2017)
-
The nano-architecture of the axonal cytoskeleton
Nature Reviews Neuroscience (2017)