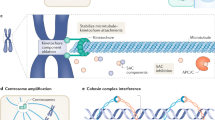
Key Points
-
The mitotic checkpoint is a major cell cycle control mechanism that acts to guard against chromosome missegregation and the subsequent production of aneuploid daughter cells. Aneuploidy is a remarkably common feature of human tumours and was proposed more than 100 years ago to promote cancer.
-
Aneuploidy is often caused as a result of an underlying chromosomal instability, characterized by the frequent gains and losses of chromosomes during division. Increased kinetochore malorientation is a primary cause of the chromosomal instability that is found in cancer cells.
-
Mice with overexpressed or reduced levels of mitotic checkpoint components display elevated aneuploidy. In many cases, this elevated aneuploidy correlates with an increased susceptibility to spontaneous and/or carcinogen-induced tumours.
-
Although aneuploidy has been implicated in driving cancer, recent evidence has revealed that in vitro, aneuploidy hampers the growth of cells. Aneuploidy has also been found to antagonize tumorigenesis in certain genetic contexts and cell types.
-
The effect of aneuploidy on tumorigenesis is context dependent and is not driven by a particular combination of chromosomes per se, but rather by the specific interaction of the karyotype with the genetic context and microenvironment of the cell.
Abstract
The mitotic checkpoint is a major cell cycle control mechanism that guards against chromosome missegregation and the subsequent production of aneuploid daughter cells. Most cancer cells are aneuploid and frequently missegregate chromosomes during mitosis. Indeed, aneuploidy is a common characteristic of tumours, and, for over 100 years, it has been proposed to drive tumour progression. However, recent evidence has revealed that although aneuploidy can increase the potential for cellular transformation, it also acts to antagonize tumorigenesis in certain genetic contexts. A clearer understanding of the tumour suppressive function of aneuploidy might reveal new avenues for anticancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Boveri, T. Über mehrpolige mitosen als mittel zur analyse des zellkerns. Verh. Phys. Med. Ges. Würzburg 35, 67–90 (1902) (in German).
Hansemann, D. Über asymmetrische zelltheilung in epithelkrebsen und deren biologische bedeutung. Arch. Pathol. Anat. Physiol. Klin. Medicin. 119, 299–326 (1890) (in German).
Boveri, T. in Zur Frage der Entstehung Maligner Tumoren. 1–64 (Gustav Fischer, Jena, 1914) (in German).
Weaver, B. A. & Cleveland, D. W. Does aneuploidy cause cancer? Curr. Opin. Cell Biol. 18, 658–667 (2006).
Keen-Kim, D., Nooraie, F. & Rao, P. N. Cytogenetic biomarkers for human cancer. Front. Biosci. 13, 5928–5949 (2008).
Weaver, B. A. & Cleveland, D. W. Aneuploidy: instigator and inhibitor of tumorigenesis. Cancer Res. 67, 10103–10105 (2007).
Kops, G. J., Weaver, B. A. & Cleveland, D. W. On the road to cancer: aneuploidy and the mitotic checkpoint. Nature Rev. Cancer 5, 773–785 (2005).
Musacchio, A. & Salmon, E. D. The spindle-assembly checkpoint in space and time. Nature Rev. Mol. Cell Biol. 8, 379–393 (2007).
Buffin, E., Emre, D. & Karess, R. E. Flies without a spindle checkpoint. Nature Cell Biol. 9, 565–572 (2007).
Hoyt, M. A., Totis, L. & Roberts, B. T. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell 66, 507–517 (1991).
Li, R. & Murray, A. W. Feedback control of mitosis in budding yeast. Cell 66, 519–531 (1991).
Michel, L. S. et al. MAD2 haplo-insufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature 409, 355–359 (2001).
Dobles, M., Liberal, V., Scott, M. L., Benezra, R. & Sorger, P. K. Chromosome missegregation and apoptosis in mice lacking the mitotic checkpoint protein Mad2. Cell 101, 635–645 (2000).
Kalitsis, P., Earle, E., Fowler, K. J. & Choo, K. H. Bub3 gene disruption in mice reveals essential mitotic spindle checkpoint function during early embryogenesis. Genes Dev. 14, 2277–2282 (2000).
Kitagawa, R. & Rose, A. M. Components of the spindle-assembly checkpoint are essential in Caenorhabditis elegans. Nature Cell Biol. 1, 514–521 (1999).
Kops, G. J., Foltz, D. R. & Cleveland, D. W. Lethality to human cancer cells through massive chromosome loss by inhibition of the mitotic checkpoint. Proc. Natl Acad. Sci. USA 101, 8699–8704 (2004).
Rieder, C. L., Cole, R. W., Khodjakov, A. & Sluder, G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J. Cell Biol. 130, 941–948 (1995).
Hanks, S. et al. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nature Genet. 36, 1159–1161 (2004).
Matsuura, S. et al. Monoallelic BUB1B mutations and defective mitotic-spindle checkpoint in seven families with premature chromatid separation (PCS) syndrome. Am. J. Med. Genet. A 140, 358–367 (2006).
Barber, T. D. et al. Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proc. Natl Acad. Sci. USA 105, 3443–3448 (2008).
Zhang, N. et al. Overexpression of separase induces aneuploidy and mammary tumorigenesis. Proc. Natl Acad. Sci. USA 105, 13033–13038 (2008).
Pei, L. & Melmed, S. Isolation and characterization of a pituitary tumor-transforming gene (PTTG). Mol. Endocrinol. 11, 433–441 (1997).
Yu, R., Lu, W., Chen, J., McCabe, C. J. & Melmed, S. Overexpressed pituitary tumor-transforming gene causes aneuploidy in live human cells. Endocrinology 144, 4991–4998 (2003).
Zhang, X. et al. Structure, expression, and function of human pituitary tumor-transforming gene (PTTG). Mol. Endocrinol. 13, 156–166 (1999).
Cimini, D. Merotelic kinetochore orientation, aneuploidy, and cancer. Biochim. Biophys. Acta 1786, 32–40 (2008).
Cimini, D., Moree, B., Canman, J. C. & Salmon, E. D. Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J. Cell Sci. 116, 4213–4225 (2003).
Cimini, D., Fioravanti, D., Salmon, E. D. & Degrassi, F. Merotelic kinetochore orientation versus chromosome mono-orientation in the origin of lagging chromosomes in human primary cells. J. Cell Sci. 115, 507–515 (2002).
Cimini, D. et al. Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J. Cell Biol. 153, 517–527 (2001).
Brinkley, B. R. Managing the centrosome numbers game: from chaos to stability in cancer cell division. Trends Cell Biol. 11, 18–21 (2001).
Nigg, E. A. Origins and consequences of centrosome aberrations in human cancers. Int. J. Cancer 119, 2717–2723 (2006).
Lingle, W. L. et al. Centrosome amplification drives chromosomal instability in breast tumor development. Proc. Natl Acad. Sci. USA 99, 1978–1983 (2002).
Lengauer, C., Kinzler, K. W. & Vogelstein, B. Genetic instability in colorectal cancers. Nature 386, 623–627 (1997).
Cahill, D. P. et al. Mutations of mitotic checkpoint genes in human cancers. Nature 392, 300–303 (1998).
Gascoigne, K. E. & Taylor, S. S. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell 14, 111–122 (2008). This study, along with reference 35, demonstrates that chromosomal instability is not caused by mitotic checkpoint dysfunction.
Thompson, S. L. & Compton, D. A. Examining the link between chromosomal instability and aneuploidy in human cells. J. Cell Biol. 180, 665–672 (2008). The first demonstration that chromosome missegregation in CIN cells is driven by kinetochore malorientations.
Bakhoum, S. F., Thompson, S. L., Manning, A. L. & Compton, D. A. Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nature Cell Biol. 11, 27–35 (2008). Shows that increasing microtubule dynamics at the kinetochore reduces chromosome missegregation rates in chromosomally unstable cell lines.
Iwanaga, Y. et al. Heterozygous deletion of mitotic arrest-deficient protein 1 (MAD1) increases the incidence of tumors in mice. Cancer Res. 67, 160–166 (2007).
Babu, J. R. et al. Rae1 is an essential mitotic checkpoint regulator that cooperates with Bub3 to prevent chromosome missegregation. J. Cell Biol. 160, 341–353 (2003).
Perera, D. et al. Bub1 maintains centromeric cohesion by activation of the spindle checkpoint. Dev. Cell 13, 566–579 (2007).
Putkey, F. R. et al. Unstable kinetochore-microtubule capture and chromosomal instability following deletion of CENP-E. Dev. Cell 3, 351–365 (2002).
Wang, Q. et al. BUBR1 deficiency results in abnormal megakaryopoiesis. Blood 103, 1278–1285 (2004).
Baker, D. J. et al. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nature Genet. 36, 744–749 (2004).
Jeganathan, K., Malureanu, L., Baker, D. J., Abraham, S. C. & van Deursen, J. M. Bub1 mediates cell death in response to chromosome missegregation and acts to suppress spontaneous tumorigenesis. J. Cell Biol. 179, 255–267 (2007). Shows Bub1 hypomorphic mice are susceptible to spontaneous tumorigenesis.
Weaver, B. A. et al. Centromere-associated protein-E is essential for the mammalian mitotic checkpoint to prevent aneuploidy due to single chromosome loss. J. Cell Biol. 162, 551–563 (2003).
Rao, C. V. et al. Colonic tumorigenesis in BubR1+/−ApcMin/+ compound mutant mice is linked to premature separation of sister chromatids and enhanced genomic instability. Proc. Natl Acad. Sci. USA 102, 4365–4370 (2005).
Weaver, B. A., Silk, A. D., Montagna, C., Verdier-Pinard, P. & Cleveland, D. W. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell 11, 25–36 (2007). Provides clear evidence that aneuploidy can suppress tumorigenesis in certain genetic contexts and cell types.
Kalitsis, P. et al. Increased chromosome instability but not cancer predisposition in haploinsufficient Bub3 mice. Genes Chromosomes Cancer 44, 29–36 (2005).
Baker, D. J. et al. Early aging-associated phenotypes in Bub3/Rae1 haploinsufficient mice. J. Cell Biol. 172, 529–540 (2006).
Dai, W. et al. Slippage of mitotic arrest and enhanced tumor development in mice with BubR1 haploinsufficiency. Cancer Res. 64, 440–445 (2004).
Jeganathan, K. B., Malureanu, L. & van Deursen, J. M. The Rae1–Nup98 complex prevents aneuploidy by inhibiting securin degradation. Nature 438, 1036–1039 (2005).
Jeganathan, K. B., Baker, D. J. & van Deursen, J. M. Securin associates with APCCdh1 in prometaphase but its destruction is delayed by Rae1 and Nup98 until the metaphase/anaphase transition. Cell Cycle 5, 366–370 (2006).
Baker, D. J. et al. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nature Cell Biol. 10, 825–836 (2008).
Hayama, S. et al. Activation of CDCA1–KNTC2, members of centromere protein complex, involved in pulmonary carcinogenesis. Cancer Res. 66, 10339–10348 (2006).
Tanaka, K. et al. Mitotic checkpoint protein hsMAD2 as a marker predicting liver metastasis of human gastric cancers. Jpn J. Cancer Res. 92, 952–958 (2001).
Li, G. Q., Li, H. & Zhang, H. F. Mad2 and p53 expression profiles in colorectal cancer and its clinical significance. World J. Gastroenterol. 9, 1972–1975 (2003).
Hernando, E. et al. Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature 430, 797–802 (2004).
Diaz-Rodriguez, E., Sotillo, R., Schvartzman, J. M. & Benezra, R. Hec1 overexpression hyperactivates the mitotic checkpoint and induces tumor formation in vivo. Proc. Natl Acad. Sci. USA 105, 16719–16724 (2008).
Sotillo, R. et al. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell 11, 9–23 (2007). Shows overexpression of Mad2 promotes numerical and structural chromosomal alterations along with tumorigenesis.
Chi, Y. H., Ward, J. M., Cheng, L. I., Yasunaga, J. & Jeang, K. T. Spindle assembly checkpoint and p53 deficiencies cooperate for tumorigenesis in mice. Int. J. Cancer 124, 1483–1489 (2008).
Shi, Q. & King, R. W. Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines. Nature 437, 1038–1042 (2005).
Mullins, J. M. & Biesele, J. J. Terminal phase of cytokinesis in D-98S cells. J. Cell Biol. 73, 672–684 (1977).
Weaver, B. A., Silk, A. D. & Cleveland, D. W. Cell biology: nondisjunction, aneuploidy and tetraploidy. Nature 442, E9–E10 (2006).
Steigemann, P. et al. Aurora B-mediated abscission checkpoint protects against tetraploidization. Cell 136, 473–484 (2009).
Norden, C. et al. The NoCut pathway links completion of cytokinesis to spindle midzone function to prevent chromosome breakage. Cell 125, 85–98 (2006).
Mendoza, M. et al. A mechanism for chromosome segregation sensing by the NoCut checkpoint. Nature Cell Biol. 11, 477–483 (2009).
Fujiwara, T. et al. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 437, 1043–1047 (2005). A clear demonstration that tetraploidy can promote transformation as well as numerical and structural chromosomal alterations.
Duelli, D. M., Hearn, S., Myers, M. P. & Lazebnik, Y. A primate virus generates transformed human cells by fusion. J. Cell Biol. 171, 493–503 (2005).
Duelli, D. M. et al. A virus causes cancer by inducing massive chromosomal instability through cell fusion. Curr. Biol. 17, 431–437 (2007).
Roh, M., Franco, O. E., Hayward, S. W., van der Meer, R. & Abdulkadir, S. A. A role for polyploidy in the tumorigenicity of Pim-1-expressing human prostate and mammary epithelial cells. PLoS ONE 3, e2572 (2008).
Ganem, N. J., Storchova, Z. & Pellman, D. Tetraploidy, aneuploidy and cancer. Curr. Opin. Genet. Dev. 17, 157–162 (2007).
Galipeau, P. C. et al. 17p (p53) allelic losses, 4N (G2/tetraploid) populations, and progression to aneuploidy in Barrett's esophagus. Proc. Natl Acad. Sci. USA 93, 7081–7084 (1996).
Olaharski, A. J. et al. Tetraploidy and chromosomal instability are early events during cervical carcinogenesis. Carcinogenesis 27, 337–343 (2006).
Ornitz, D. M., Hammer, R. E., Messing, A., Palmiter, R. D. & Brinster, R. L. Pancreatic neoplasia induced by SV40 T-antigen expression in acinar cells of transgenic mice. Science 238, 188–193 (1987).
Meraldi, P., Honda, R. & Nigg, E. A. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53−/− cells. EMBO J. 21, 483–492 (2002).
Zhang, D. et al. Cre-loxP-controlled periodic Aurora-A overexpression induces mitotic abnormalities and hyperplasia in mammary glands of mouse models. Oncogene 23, 8720–8730 (2004).
Wang, X. et al. Overexpression of Aurora kinase A in mouse mammary epithelium induces genetic instability preceding mammary tumor formation. Oncogene 25, 7148–7158 (2006).
Daniels, M. J., Wang, Y., Lee, M. & Venkitaraman, A. R. Abnormal cytokinesis in cells deficient in the breast cancer susceptibility protein BRCA2. Science 306, 876–879 (2004).
Yang, X. et al. LATS1 tumour suppressor affects cytokinesis by inhibiting LIMK1. Nature Cell Biol. 6, 609–617 (2004).
Caldwell, C. M., Green, R. A. & Kaplan, K. B. APC mutations lead to cytokinetic failures in vitro and tetraploid genotypes in Min mice. J. Cell Biol. 178, 1109–1120 (2007).
Chesnokova, V., Kovacs, K., Castro, A. V., Zonis, S. & Melmed, S. Pituitary hypoplasia in Pttg−/− mice is protective for Rb+/− pituitary tumorigenesis. Mol. Endocrinol. 19, 2371–2379 (2005).
Sussan, T. E., Yang, A., Li, F., Ostrowski, M. C. & Reeves, R. H. Trisomy represses ApcMin-mediated tumours in mouse models of Down's syndrome. Nature 451, 73–75 (2008). Demonstrates that trisomy for approximately one-half of the orthologous genes on chromosome 21 suppress development of intestinal tumours in ApcMin/+ mice.
Upender, M. B. et al. Chromosome transfer induced aneuploidy results in complex dysregulation of the cellular transcriptome in immortalized and cancer cells. Cancer Res. 64, 6941–6949 (2004).
Williams, B. R. et al. Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science 322, 703–709 (2008). This elegant study demonstrates aneuploid mouse embryonic fibroblasts that are stably trisomic for one of four mouse chromosomes exhibit altered metabolism and impaired proliferation.
Torres, E. M. et al. Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science 317, 916–924 (2007).
Segal, D. J. & McCoy, E. E. Studies on Down's syndrome in tissue culture. I. Growth rates and protein contents of fibroblast cultures. J. Cell Physiol. 83, 85–90 (1974).
Torres, E. M., Williams, B. R. & Amon, A. Aneuploidy: cells losing their balance. Genetics 179, 737–746 (2008).
Yang, Q., Rasmussen, S. A. & Friedman, J. M. Mortality associated with Down's syndrome in the USA from 1983 to 1997: a population-based study. Lancet 359, 1019–1025 (2002).
Hasle, H., Clemmensen, I. H. & Mikkelsen, M. Risks of leukaemia and solid tumours in individuals with Down's syndrome. Lancet 355, 165–169 (2000).
Satge, D. et al. A tumor profile in Down syndrome. Am. J. Med. Genet. 78, 207–216 (1998).
Kulukian, A., Han, J. S. & Cleveland, D. W. Unattached kinetochores catalyze production of an anaphase inhibitor that requires a Mad2 template to prime Cdc20 for BubR1 binding. Dev. Cell 16, 105–117 (2009).
Holland, A. J. & Taylor, S. S. Many faces of separase regulation. SEB Exp. Biol. Ser. 59, 99–112 (2008).
Basto, R. et al. Centrosome amplification can initiate tumorigenesis in flies. Cell 133, 1032–1042 (2008). An elegant examination of the consequences of centrosome amplification in the context of a whole organism.
Castellanos, E., Dominguez, P. & Gonzalez, C. Centrosome dysfunction in Drosophila neural stem cells causes tumors that are not due to genome instability. Curr. Biol. 18, 1209–1214 (2008).
Yang, Z., Loncarek, J., Khodjakov, A. & Rieder, C. L. Extra centrosomes and/or chromosomes prolong mitosis in human cells. Nature Cell Biol. 10, 748–751 (2008).
Quintyne, N. J., Reing, J. E., Hoffelder, D. R., Gollin, S. M. & Saunders, W. S. Spindle multipolarity is prevented by centrosomal clustering. Science 307, 127–129 (2005).
Basto, R. et al. Flies without centrioles. Cell 125, 1375–1386 (2006).
Kwon, M. et al. Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev. 22, 2189–2203 (2008). A genome-wide RNA interference screen was used to identify novel mechanisms by which cells suppress multipolar mitotic divisions.
Habedanck, R., Stierhof, Y. D., Wilkinson, C. J. & Nigg, E. A. The Polo kinase Plk4 functions in centriole duplication. Nature Cell Biol. 7, 1140–1146 (2005).
Peloponese, J. M. Jr, Haller, K., Miyazato, A. & Jeang, K. T. Abnormal centrosome amplification in cells through the targeting of Ran-binding protein-1 by the human T cell leukemia virus type-1 Tax oncoprotein. Proc. Natl Acad. Sci. USA 102, 18974–18979 (2005).
Wang, X. et al. sSgo1, a major splice variant of Sgo1, functions in centriole cohesion where it is regulated by Plk1. Dev. Cell 14, 331–341 (2008).
Ganem, N. J., Godinho, S. A. & Pellman, D. A mechanism linking extra centrosomes to chromosomal instability. Nature 7 Jun 2009 (doi:10.1038/nature08136).
Acknowledgements
The authors thank W. Silkworth, D. Cimini, N. Ganem and D. Pellman for sharing results before publication. We apologize to all those whose work could not be cited owing to space limitations. D.W.C. receives salary support from the Ludwig Institute for Cancer Research and A.J.H. is supported by a European Molecular Biology Organization (EMBO) Long-Term Fellowship.
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- Transformation
-
The change that a normal cell undergoes when it becomes immortalized and acquires the potential to grow in an uncontrolled manner.
- Microtubule spindle
-
A dynamic array of microtubules that forms during mitosis and serves to partition the duplicated chromosomes into the daughter cells.
- Kinetochore
-
A complicated protein assembly that links the chromosomes to the microtubule-based mitotic spindle.
- Separase
-
A Cys protease that triggers anaphase by cleaving the cohesin complex that holds sister chromatids together.
- Securin
-
A chaperone that binds and inhibits the catalytic activity of separase.
- Centrosome
-
The major microtubule-organizing centre of animal cells that forms the poles of the mitotic spindle.
- Centriole
-
A short, barrel-shaped array of microtubules localized in the centrosome.
- Down's syndrome
-
A chromosomal disorder caused by trisomy of chromosome 21.
- Centromere
-
A specialized chromatin structure on which the kinetochore assembles. This occurs at the constricted point at which the two chromatids that form the chromosome are joined together.
- Hypomorphic
-
A mutant that produces less than the normal amount of a gene product.
- Benign
-
A tumour that does not grow in an uncontrolled manner, invade surrounding tissues or metastasize to other parts of the body.
- RAE1
-
A protein initially charcterized as an mRNA export factor that shares sequence and structural similarity with BUB3.
- NUP98
-
A nuclear pore complex component that interacts with RAE1.
- Splenocyte
-
A type of white blood cell that is a precursor of splenic tissue.
- Apc Min
-
A truncating mutation in the adenomatous polyposis coli tumour suppressor gene. Mice that are heterozygous for this mutation develop a large number of benign colon and intestinal tumours at an early age.
- Loss of heterozygosity
-
Represents the loss of function of the remaining copy of a tumour suppressor gene in which the other allele has previously been inactivated.
- p53
-
A tumour suppressor gene that is frequently mutated in human cancer. It has an important role in cell cycle regulation and apoptosis.
- Retinoblastoma
-
A tumour suppressor gene that has an important function in the regulation of the cell cycle.
- Tetraploid
-
Possessing four times the haploid number of chromosomes.
- Endoreduplication
-
The duplication of the genome without subsequent cell division.
- Aurora B
-
A member of the Aurora kinase family that localizes to the centromere during metaphase and to the spindle midzone during anaphase. Aurora B has a role in the correction of incorrect kinetochore microtubule attachments and cytokinesis.
- Abscission
-
The separation of the two daughter cells at the end of cytokinesis.
- NoCut pathway
-
A signalling pathway identified in yeast that delays the completion of cytokinesis when chromatin is present in the spindle midzone.
- Aurora A
-
A member of the Aurora kinase family that is enriched at the poles of the spindle and has a role in bipolar spindle formation. Aurora A is frequently overexpressed in human cancers.
- BRCA2
-
(Breast cancer 2, early onset). Mutations in this protein correlate with an increased risk of breast and/or ovarian cancer.
Rights and permissions
About this article
Cite this article
Holland, A., Cleveland, D. Boveri revisited: chromosomal instability, aneuploidy and tumorigenesis. Nat Rev Mol Cell Biol 10, 478–487 (2009). https://doi.org/10.1038/nrm2718
Issue Date:
DOI: https://doi.org/10.1038/nrm2718
This article is cited by
-
The reckoning of chromosomal instability: past, present, future
Chromosome Research (2024)
-
RIT1 regulates mitosis and promotes proliferation by interacting with SMC3 and PDS5 in hepatocellular carcinoma
Journal of Experimental & Clinical Cancer Research (2023)
-
SYT7 regulates the progression of chronic lymphocytic leukemia through interacting and regulating KNTC1
Biomarker Research (2023)
-
Clonal origin and development of high hyperdiploidy in childhood acute lymphoblastic leukaemia
Nature Communications (2023)
-
KNTC1 knockdown inhibits proliferation and metastases of liver cancer
3 Biotech (2023)