Key Points
-
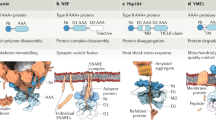
AAA+ proteins are ATPases associated with various cellular activities.
-
AAA+ proteins contain structurally conserved ATP-binding domains (AAA+ domains) that consist of N-terminal α/β and C-terminal α-helical subdomains. AAA+ domains are attached to various other domains and, in some cases, interact with adaptor proteins to generate the structural and functional diversity of the family. AAA+ domains function as oligomeric rings.
-
Mutations of conserved residues in AAA+ domains have predictable effects on nucleotide binding or hydrolysis, and have been successfully used to probe AAA+-protein function.
-
AAA+ proteins undergo conformational changes on nucleotide binding and hydrolysis; these changes can be large and seem to be responsible for the effects of these enzymes on their substrates.
-
AAA+ proteins are involved in many cellular processes, but seem to share the common behaviour of inducing conformational changes in target proteins. These conformational changes lead to substrate remodelling and, in some cases, perturb protein structure sufficiently to promote unfolding.
-
Key categories of AAA+-protein-mediated reactions include unfolding for proteolysis, the disassembly of protein aggregates, and the disassembly of otherwise stable protein complexes. All of these processes require the enzyme to hydrolyse ATP.
-
Mutations in AAA+ proteins are directly responsible for a number of inherited human diseases, including Zellweger's spectrum peroxisome-biogenesis disorders, early-onset torsion dystonia, hereditary spastic paraplegia, and inclusion-body myopathy with early-onset Paget's disease and frontotemporal dementia.
Abstract
The AAA+ (ATPases associated with various cellular activities) family is a large and functionally diverse group of enzymes that are able to induce conformational changes in a wide range of substrate proteins. The family's defining feature is a structurally conserved ATPase domain that assembles into oligomeric rings and undergoes conformational changes during cycles of nucleotide binding and hydrolysis. Here, we review the structural organization of AAA+ proteins, the conformational changes they undergo, the range of different reactions they catalyse, and the diseases associated with their dysfunction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Neuwald, A. F., Aravind, L., Spouge, J. L. & Koonin, E. V. AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 9, 27–43 (1999).
Iyer, L. M., Leipe, D. D., Koonin, E. V. & Aravind, L. Evolutionary history and higher order classification of AAA+ ATPases. J. Struct. Biol. 146, 11–31 (2004). A recent, definitive sequence and structure-based analysis of the AAA+-protein family.
Ogura, T. & Wilkinson, A. J. AAA+ superfamily ATPases: common structure — diverse function. Genes Cells 6, 575–597 (2001).
Lupas, A. N. & Martin, J. AAA proteins. Curr. Opin. Struct. Biol. 12, 746–753 (2002).
Sauer, R. T. et al. Sculpting the proteome with AAA(+) proteases and disassembly machines. Cell 119, 9–18 (2004).
Frickey, T. & Lupas, A. N. Phylogenetic analysis of AAA proteins. J. Struct. Biol. 146, 2–10 (2004).
Beyer, A. Sequence analysis of the AAA protein family. Protein Sci. 6, 2043–2058 (1997).
Bochtler, M. et al. The structures of HsIU and the ATP-dependent protease HsIU–HsIV. Nature 403, 800–805 (2000).
Sousa, M. C. et al. Crystal and solution structures of an HslUV protease–chaperone complex. Cell 103, 633–643 (2000).
Smith, G. R., Contreras-Moreira, B., Zhang, X. & Bates, P. A. A link between sequence conservation and domain motion within the AAA+ family. J. Struct. Biol. 146, 189–204 (2004).
Weibezahn, J., Schlieker, C., Bukau, B. & Mogk, A. Characterization of a trap mutant of the AAA+ chaperone ClpB. J. Biol. Chem. 278, 32608–32617 (2003).
Dalal, S., Rosser, M. F., Cyr, D. M. & Hanson, P. I. Distinct roles for the AAA ATPases NSF and p97 in the secretory pathway. Mol. Biol. Cell 15, 637–648 (2004).
Babst, M., Wendland, B., Estepa, E. J. & Emr, S. D. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 17, 2982–2993 (1998).
Tomoyasu, T. et al. The Escherichia coli FtsH protein is a prokaryotic member of a protein family of putative ATPases involved in membrane functions, cell cycle control, and gene expression. J. Bacteriol. 175, 1344–1351 (1993).
Ogura, T., Whiteheart, S. W. & Wilkinson, A. J. Conserved arginine residues implicated in ATP hydrolysis, nucleotide-sensing, and inter-subunit interactions in AAA and AAA+ ATPases. J. Struct. Biol. 146, 106–112 (2004).
Steel, G. J., Harley, C., Boyd, A. & Morgan, A. A screen for dominant negative mutants of SEC18 reveals a role for the AAA protein consensus sequence in ATP hydrolysis. Mol. Biol. Cell 11, 1345–1356 (2000).
Scheffzek, K. et al. The Ras–RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science 277, 333–338 (1997).
Joshi, S. A., Baker, T. A. & Sauer, R. T. C-terminal domain mutations in ClpX uncouple substrate binding from an engagement step required for unfolding. Mol. Microbiol. 48, 67–76 (2003).
Wang, J. et al. Crystal structures of the HslVU peptidase–ATPase complex reveal an ATP-dependent proteolysis mechanism. Structure 9, 177–184 (2001). First study to highlight the importance of the conserved GYVG motif in the central pore of AAA+ proteins.
Song, H. K. et al. Mutational studies on HslU and its docking mode with HslV. Proc. Natl Acad. Sci. USA 97, 14103–14108 (2000).
Yamada-Inagawa, T., Okuno, T., Karata, K., Yamanaka, K. & Ogura, T. Conserved pore residues in the AAA protease FtsH are important for proteolysis and its coupling to ATP hydrolysis. J. Biol. Chem. 278, 50182–50187 (2003).
Siddiqui, S. M., Sauer, R. T. & Baker, T. A. Role of the processing pore of the ClpX AAA+ ATPase in the recognition and engagement of specific protein substrates. Genes Dev. 18, 369–374 (2004).
Lum, R., Tkach, J. M., Vierling, E. & Glover, J. R. Evidence for an unfolding/threading mechanism for protein disaggregation by Saccharomyces cerevisiae Hsp104. J. Biol. Chem. 279, 29139–29146 (2004).
Schlieker, C. et al. Substrate recognition by the AAA+ chaperone ClpB. Nature Struct. Mol. Biol. 11, 607–615 (2004).
Wang, J. et al. Nucleotide-dependent conformational changes in a protease-associated ATPase HsIU. Structure 9, 1107–1116 (2001).
Wang, J. Nucleotide-dependent domain motions within rings of the RecA/AAA(+) superfamily. J. Struct. Biol. 148, 259–267 (2004).
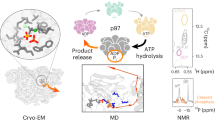
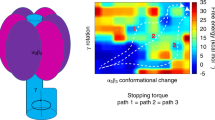
Rouiller, I. et al. Conformational changes of the multifunction p97 AAA ATPase during its ATPase cycle. Nature Struct. Biol. 9, 950–957 (2002).
DeLaBarre, B. & Brunger, A. T. Nucleotide dependent motion and mechanism of action of p97/VCP. J. Mol. Biol. 347, 437–452 (2005).
Davies, J. M., Tsuruta, H., May, A. P. & Weis, W. I. Conformational changes of p97 during nucleotide hydrolysis determined by small-angle X-ray scattering. Structure 13, 183–195 (2005).
DeLaBarre, B. & Brunger, A. T. Complete structure of p97/valosin-containing protein reveals communication between nucleotide domains. Nature Struct. Biol. 10, 856–863 (2003). First complete crystal structure of a hexameric AAA+ protein that contains tandem AAA+ domains.
Burgess, S. A., Walker, M. L., Sakakibara, H., Knight, P. J. & Oiwa, K. Dynein structure and power stroke. Nature 421, 715–718 (2003).
Davey, M. J., Jeruzalmi, D., Kuriyan, J. & O'Donnell, M. Motors and switches: AAA+ machines within the replisome. Nature Rev. Mol. Cell Biol. 3, 826–835 (2002).
Pickart, C. M. & Cohen, R. E. Proteasomes and their kin: proteases in the machine age. Nature Rev. Mol. Cell Biol. 5, 177–187 (2004).
Wang, J., Hartling, J. A. & Flanagan, J. M. The structure of ClpP at 2.3 Å resolution suggests a model for ATP-dependent proteolysis. Cell 91, 447–456 (1997).
Ishikawa, T. et al. Translocation pathway of protein substrates in ClpAP protease. Proc. Natl Acad. Sci. USA 98, 4328–4333 (2001).
Ortega, J., Lee, H. S., Maurizi, M. R. & Steven, A. C. ClpA and ClpX ATPases bind simultaneously to opposite ends of ClpP peptidase to form active hybrid complexes. J. Struct. Biol. 146, 217–226 (2004).
Kim, Y. I. et al. Molecular determinants of complex formation between Clp/Hsp100 ATPases and the ClpP peptidase. Nature Struct. Biol. 8, 230–233 (2001). Defines the conserved IGF motif that is required for the interaction of AAA+ proteins with ClpP.
Joshi, S. A., Hersch, G. L., Baker, T. A. & Sauer, R. T. Communication between ClpX and ClpP during substrate processing and degradation. Nature Struct. Mol. Biol. 11, 404–411 (2004).
Keiler, K. C., Waller, P. R. & Sauer, R. T. Role of a peptide tagging system in degradation of proteins synthesized from damaged messenger RNA. Science 271, 990–993 (1996).
Flynn, J. M., Neher, S. B., Kim, Y. I., Sauer, R. T. & Baker, T. A. Proteomic discovery of cellular substrates of the ClpXP protease reveals five classes of ClpX-recognition signals. Mol. Cell 11, 671–683 (2003). Proteomics study that greatly expanded the known repertoire of ClpXP substrates.
Neher, S. B., Flynn, J. M., Sauer, R. T. & Baker, T. A. Latent ClpX-recognition signals ensure LexA destruction after DNA damage. Genes Dev. 17, 1084–1089 (2003).
Ishikawa, T., Maurizi, M. R. & Steven, A. C. The N-terminal substrate-binding domain of ClpA unfoldase is highly mobile and extends axially from the distal surface of ClpAP protease. J. Struct. Biol. 146, 180–188 (2004).
Xia, D., Esser, L., Singh, S. K., Guo, F. & Maurizi, M. R. Crystallographic investigation of peptide binding sites in the N-domain of the ClpA chaperone. J. Struct. Biol. 146, 166–179 (2004).
Smith, C. K., Baker, T. A. & Sauer, R. T. Lon and Clp family proteases and chaperones share homologous substrate-recognition domains. Proc. Natl Acad. Sci. USA 96, 6678–6682 (1999).
Leonhard, K., Stiegler, A., Neupert, W. & Langer, T. Chaperone-like activity of the AAA domain of the yeast Yme1 AAA protease. Nature 398, 348–351 (1999).
Wickner, S. et al. A molecular chaperone, ClpA, functions like DnaK and DnaJ. Proc. Natl Acad. Sci. USA 91, 12218–12222 (1994).
Weber-Ban, E. U., Reid, B. G., Miranker, A. D. & Horwich, A. L. Global unfolding of a substrate protein by the Hsp100 chaperone ClpA. Nature 401, 90–93 (1999).
Kim, Y. I., Burton, R. E., Burton, B. M., Sauer, R. T. & Baker, T. A. Dynamics of substrate denaturation and translocation by the ClpXP degradation machine. Mol. Cell 5, 639–648 (2000).
Kenniston, J. A., Baker, T. A., Fernandez, J. M. & Sauer, R. T. Linkage between ATP consumption and mechanical unfolding during the protein processing reactions of an AAA+ degradation machine. Cell 114, 511–520 (2003). A study of ATP utilization during substrate denaturation by ClpXP indicates that denaturation proceeds by the repeated application of a constant denaturing force.
Reid, B. G., Fenton, W. A., Horwich, A. L. & Weber-Ban, E. U. ClpA mediates directional translocation of substrate proteins into the ClpP protease. Proc. Natl Acad. Sci. USA 98, 3768–3772 (2001).
Burton, R. E., Siddiqui, S. M., Kim, Y. I., Baker, T. A. & Sauer, R. T. Effects of protein stability and structure on substrate processing by the ClpXP unfolding and degradation machine. EMBO J. 20, 3092–3100 (2001).
Lee, C., Schwartz, M. P., Prakash, S., Iwakura, M. & Matouschek, A. ATP-dependent proteases degrade their substrates by processively unraveling them from the degradation signal. Mol. Cell 7, 627–637 (2001). This paper shows, using well characterized model substrates, that ClpAP-mediated unfolding and degradation is controlled by the structure(s) adjacent to the degradation signal, rather than by global thermodynamic stability.
Kenniston, J. A., Burton, R. E., Siddiqui, S. M., Baker, T. A. & Sauer, R. T. Effects of local protein stability and the geometric position of the substrate degradation tag on the efficiency of ClpXP denaturation and degradation. J. Struct. Biol. 146, 130–140 (2004).
Prakash, S., Tian, L., Ratliff, K. S., Lehotzky, R. E. & Matouschek, A. An unstructured initiation site is required for efficient proteasome-mediated degradation. Nature Struct. Mol. Biol. 11, 830–837 (2004). Identification of a role for unstructured regions of polypeptides in the initiation of degradation by the proteasome.
Braun, B. C. et al. The base of the proteasome regulatory particle exhibits chaperone-like activity. Nature Cell Biol. 1, 221–226 (1999).
Liu, C. W. et al. Conformational remodeling of proteasomal substrates by PA700, the 19S regulatory complex of the 26S proteasome. J. Biol. Chem. 277, 26815–26820 (2002).
Ye, Y., Meyer, H. H. & Rapoport, T. A. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature 414, 652–656 (2001).
Ye, Y., Shibata, Y., Yun, C., Ron, D. & Rapoport, T. A. A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature 429, 841–847 (2004).
Lilley, B. N. & Ploegh, H. L. A membrane protein required for dislocation of misfolded proteins from the ER. Nature 429, 834–840 (2004). References 58 and 59 identify a receptor for p97 in the ER membrane.
Lee, R. J. et al. Uncoupling retro-translocation and degradation in the ER-associated degradation of a soluble protein. EMBO J. 23, 2206–2215 (2004).
Langer, T., Kaser, M., Klanner, C. & Leonhard, K. AAA proteases of mitochondria: quality control of membrane proteins and regulatory functions during mitochondrial biogenesis. Biochem. Soc. Trans. 29, 431–436 (2001).
Sanchez, Y. & Lindquist, S. L. HSP104 required for induced thermotolerance. Science 248, 1112–1115 (1990).
Parsell, D. A., Kowal, A. S., Singer, M. A. & Lindquist, S. Protein disaggregation mediated by heat-shock protein Hsp104. Nature 372, 475–478 (1994).
Glover, J. R. & Tkach, J. M. Crowbars and ratchets: hsp100 chaperones as tools in reversing protein aggregation. Biochem. Cell Biol. 79, 557–568 (2001).
Chernoff, Y. O., Lindquist, S. L., Ono, B., Inge-Vechtomov, S. G. & Liebman, S. W. Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [PSI+]. Science 268, 880–884 (1995).
Shorter, J. & Lindquist, S. Hsp104 catalyzes formation and elimination of self-replicating Sup35 prion conformers. Science 304, 1793–1797 (2004).
Glover, J. R. & Lindquist, S. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell 94, 73–82 (1998). First in vitro demonstration of Hsp104 activity.
Goloubinoff, P., Mogk, A., Zvi, A. P., Tomoyasu, T. & Bukau, B. Sequential mechanism of solubilization and refolding of stable protein aggregates by a bichaperone network. Proc. Natl Acad. Sci. USA 96, 13732–13737 (1999).
Weibezahn, J. et al. Thermotolerance requires refolding of aggregated proteins by substrate translocation through the central pore of ClpB. Cell 119, 653–665 (2004). This paper shows that ClpB, like ClpA and ClpX, threads substrates through its central pore.
Hattendorf, D. A. & Lindquist, S. L. Cooperative kinetics of both Hsp104 ATPase domains and interdomain communication revealed by AAA sensor-1 mutants. EMBO J. 21, 12–21 (2002).
Lee, S. et al. The structure of ClpB: a molecular chaperone that rescues proteins from an aggregated state. Cell 115, 229–240 (2003).
Mizuuchi, M., Baker, T. A. & Mizuuchi, K. Assembly of phage Mu transpososomes: cooperative transitions assisted by protein and DNA scaffolds. Cell 83, 375–385 (1995).
Surette, M. G., Buch, S. J. & Chaconas, G. Transpososomes: stable protein–DNA complexes involved in the in vitro transposition of bacteriophage Mu DNA. Cell 49, 253–262 (1987).
Levchenko, I., Luo, L. & Baker, T. A. Disassembly of the Mu transposase tetramer by the ClpX chaperone. Genes Dev. 9, 2399–2408 (1995).
Levchenko, I., Yamauchi, M. & Baker, T. A. ClpX and MuB interact with overlapping regions of Mu transposase: implications for control of the transposition pathway. Genes Dev. 11, 1561–1572 (1997).
Burton, B. M., Williams, T. L. & Baker, T. A. ClpX-mediated remodeling of Mu transpososomes: selective unfolding of subunits destabilizes the entire complex. Mol. Cell 8, 449–454 (2001).
Burton, B. M. & Baker, T. A. Mu transpososome architecture ensures that unfolding by ClpX or proteolysis by ClpXP remodels but does not destroy the complex. Chem. Biol. 10, 463–472 (2003). References 76 and 77 establish the mechanistic basis for ClpX function in transposome remodelling.
Jahn, R., Lang, T. & Sudhof, T. C. Membrane fusion. Cell 112, 519–533 (2003).
Rothman, J. E. Lasker Basic Medical Research Award. The machinery and principles of vesicle transport in the cell. Nature Med. 8, 1059–1062 (2002).
Whiteheart, S. W., Schraw, T. & Matveeva, E. A. N-ethylmaleimide sensitive factor (NSF) structure and function. Int. Rev. Cytol. 207, 71–112 (2001).
Hohl, T. M. et al. Arrangement of subunits in 20S particles consisting of NSF, SNAPs, and SNARE complexes. Mol. Cell 2, 539–548 (1998).
Whiteheart, S. W. & Matveeva, E. A. Multiple binding proteins suggest diverse functions for the N-ethylmaleimide sensitive factor. J. Struct. Biol. 146, 32–43 (2004).
Pak, M. & Wickner, S. Mechanism of protein remodeling by ClpA chaperone. Proc. Natl Acad. Sci. USA 94, 4901–4906 (1997).
Konieczny, I. & Liberek, K. Cooperative action of Escherichia coli ClpB protein and DnaK chaperone in the activation of a replication initiation protein. J. Biol. Chem. 277, 18483–18488 (2002).
Hartman, J. J. et al. Katanin, a microtubule-severing protein, is a novel AAA ATPase that targets to the centrosome using a WD40-containing subunit. Cell 93, 277–287 (1998).
Evans, K. J., Gomes, E. R., Reisenweber, S. M., Gundersen, G. G. & Lauring, B. P. Linking axonal degeneration to microtubule remodeling by Spastin-mediated microtubule severing. J. Cell Biol. 168, 599–606 (2005).
Babst, M. A protein's final ESCRT. Traffic 6, 2–9 (2005).
Reuber, B. E. et al. Mutations in PEX1 are the most common cause of peroxisome biogenesis disorders. Nature Genet. 17, 445–448 (1997).
Watts, G. D. et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nature Genet. 36, 377–381 (2004).
Goodchild, R. E. & Dauer, W. T. Mislocalization to the nuclear envelope: an effect of the dystonia-causing torsinA mutation. Proc. Natl Acad. Sci. USA 101, 847–852 (2004).
Naismith, T. V., Heuser, J. E., Breakefield, X. O. & Hanson, P. I. TorsinA in the nuclear envelope. Proc. Natl Acad. Sci. USA 101, 7612–7617 (2004).
Yamamoto, S. et al. Expression level of valosin-containing protein is strongly associated with progression and prognosis of gastric carcinoma. J. Clin. Oncol. 21, 2537–2544 (2003).
Mirnics, K., Middleton, F. A., Marquez, A., Lewis, D. A. & Levitt, P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron 28, 53–67 (2000).
Guan, Z. et al. A spontaneous recurrent seizure-related Rattus NSF gene identified by linker capture subtraction. Brain Res. Mol. Brain Res. 87, 117–123 (2001).
Grote, E., Carr, C. M. & Novick, P. J. Ordering the final events in yeast exocytosis. J. Cell Biol. 151, 439–452 (2000).
Littleton, J. T. et al. SNARE-complex disassembly by NSF follows synaptic-vesicle fusion. Proc. Natl Acad. Sci. USA 98, 12233–12238 (2001).
Matsushita, K. et al. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell 115, 139–150 (2003).
Matveeva, E. A., Whiteheart, S. W., Vanaman, T. C. & Slevin, J. T. Phosphorylation of the N-ethylmaleimide-sensitive factor is associated with depolarization-dependent neurotransmitter release from synaptosomes. J. Biol. Chem. 276, 12174–12181 (2001).
Huynh, H. et al. Control of vesicle fusion by a tyrosine phosphatase. Nature Cell Biol. 6, 831–839 (2004).
Rabouille, C., Levine, T. P., Peters, J. M. & Warren, G. An NSF-like ATPase, p97, and NSF mediate cisternal regrowth from mitotic Golgi fragments. Cell 82, 905–914 (1995).
Hetzer, M. et al. Distinct AAA-ATPase p97 complexes function in discrete steps of nuclear assembly. Nature Cell Biol. 3, 1086–1091 (2001).
Woodman, P. G. p97, a protein coping with multiple identities. J. Cell Sci. 116, 4283–4290 (2003).
Lenzen, C. U., Steinmann, D., Whiteheart, S. W. & Weis, W. I. Crystal structure of the hexamerization domain of N-ethylmaleimide-sensitive fusion protein. Cell 94, 525–536 (1998).
Beuron, F. et al. At sixes and sevens: characterization of the symmetry mismatch of the ClpAP chaperone-assisted protease. J. Struct. Biol. 123, 248–259 (1998).
Matveeva, E. A., He, P. & Whiteheart, S. W. N-ethylmaleimide-sensitive fusion protein contains high and low affinity ATP-binding sites that are functionally distinct. J. Biol. Chem. 272, 26413–26418 (1997).
Karata, K., Inagawa, T., Wilkinson, A. J., Tatsuta, T. & Ogura, T. Dissecting the role of a conserved motif (the second region of homology) in the AAA family of ATPases. Site-directed mutagenesis of the ATP-dependent protease FtsH. J. Biol. Chem. 274, 26225–26232 (1999).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Glossary
- WALKER-A AND -B MOTIFS
-
Conserved sequence elements that are characteristic of nucleotide-binding folds.
- P-LOOP
-
A loop element of the Walker-A motif that is associated with the phoshates of bound nucleotides.
- ROSSMAN FOLD
-
An α/β fold that is characteristic of nucleotide-binding domains.
- HslU
-
The AAA+-protein component of the heat-shock-locus HslVU protease of bacteria and archaea.
- NSF
-
(N-ethylmaleimide-sensitive factor or N-ethylmaleimide-sensitive fusion protein). It was originally discovered on the basis of its essential activity in transport between Golgi stacks.
- p97/VCP
-
(97-kDa protein/valosin-containing protein). p97/VCP has been implicated in numerous cellular processes, but most clearly in handling ubiquitylated proteins en route to the proteasome.
- THERMOTOLERANCE
-
A transient state of enhanced heat resistance that is induced by exposure to mild heat shock.
- CHAMBERED PROTEASE
-
A barrel-shaped oligomeric proteolytic complex with restricted access to internal active sites.
- ClpXP
-
Caseinolytic protease found in bacteria, which consists of a proteolytic ClpP barrel and a 'gatekeeping' AAA+ hexamer of ClpX.
- ClpAP
-
ClpAP is similar to ClpXP and is also found in bacteria, but the 'gatekeeping' AAA+ hexamer is composed of ClpA.
- FtsH
-
A membrane-anchored AAA protease in bacteria, which contains AAA and protease domains within a single polypeptide chain.
- Lon
-
A soluble AAA protease in bacteria, which contains AAA and protease domains within a single polypeptide chain. It is important in cell stress responses.
- 26S PROTEASOME
-
The primary chambered protease in eukaryotes. Its name reflects its approximate sedimentation coefficient. It is composed of multicatalytic proteolytic (20S) and regulatory (19S) subcomplexes.
- ClpP
-
The proteolytic component of bacterial chambered proteases, which consists of 14 proteolytic subunits.
- GroEL TRAP
-
A mutant of the GroEL chaperonin that binds unfolded proteins but does not release them.
- PRION
-
A proteinaceous infectious particle, which was discovered by Stanley Prusiner for its role in the transmission of infectious neurodegenerative disorders. Prions are conformationally modified proteins.
- [PSI+]
-
An ordered aggregate of the yeast translation terminator Sup35, which is referred to as a yeast prion.
- Hsp70 CHAPERONE SYSTEM
-
(70-kDa heat-shock-protein chaperone system). Conserved family of ∼70-kDa ATPases that are involved in protein folding. They cooperate with Hsp40 and nucleotide-exchange factors. Members include DnaK, DnaJ and GrpE in Escherichia coli and related proteins in higher organisms.
- DNA TRANSPOSITION
-
The movement of mobile DNA elements or transposons by recombination.
- Mu TRANSPOSOSOME
-
A complex containing the MuA transposase tetramer and the two ends of the Mu genomic DNA.
- MuA TRANSPOSASE
-
A 75-kDa multidomain enzyme of a bacterial virus. It forms a homotetramer and promotes DNA recombination by catalysing donor DNA cleavage and strand transfer and joining at the target site.
- SNAREs
-
(soluble N-ethylmaleimide-sensitive fusion protein (NSF) attachment protein receptors). Coiled-coil-forming proteins that are found on cellular membranes and that promote intracellular membrane fusion.
- α-SNAP
-
(α-soluble N-ethylmaleimide-sensitive fusion protein (NSF) attachment protein). Recruits NSF to membranes and binds to SNARE proteins.
- MULTIVESICULAR BODY
-
An endosome, usually a late endosome, that contains lumenal vesicles. The internal vesicles are thought to form by invagination and budding from the limiting membrane.
Rights and permissions
About this article
Cite this article
Hanson, P., Whiteheart, S. AAA+ proteins: have engine, will work. Nat Rev Mol Cell Biol 6, 519–529 (2005). https://doi.org/10.1038/nrm1684
Issue Date:
DOI: https://doi.org/10.1038/nrm1684
This article is cited by
-
KdpD is a tandem serine histidine kinase that controls K+ pump KdpFABC transcriptionally and post-translationally
Nature Communications (2024)
-
Autoinhibition of a clamp-loader ATPase revealed by deep mutagenesis and cryo-EM
Nature Structural & Molecular Biology (2024)
-
A 5+1 assemble-to-activate mechanism of the Lon proteolytic machine
Nature Communications (2023)
-
ClpP/ClpX deficiency impairs mitochondrial functions and mTORC1 signaling during spermatogenesis
Communications Biology (2023)
-
TRIP 13-dependent pathways promote the development of gastric cancer
Functional & Integrative Genomics (2023)