Key Points
-
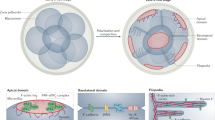
Expressed abundantly and differentially, intermediate filaments provide each cell type with a unique cytoskeletal architecture, thereby providing a mechanism for cell-type-specific cytoskeletal crosstalk. Intermediate filaments form extensive networks within the cytoplasm that extend radially in all directions from the nucleus to the cell surface, conferring an obvious advantage for intermediate filaments in coordinating cytoskeletal crosstalk in all areas of the cytoplasm.
-
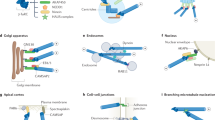
Different intermediate-filament types have preferential interactions with either microtubules or microfilaments. Among the main mediators of crosstalk between intermediate filaments and the other cytoskeletal systems are the molecular motors kinesin, dynein and myosin Va. The assembly and maintenance of an intermediate-filament network depends on microtubule- and microfilament-based motility and this motility can be regulated by phosphorylation of intermediate filaments as well as by intermediate-filament-associated proteins, such as plectin and bullous pemphigoid antigens, and microtubule-associated proteins like tau.
-
Intermediate-filament-mediated cytoskeletal crosstalk might provide specialized cells with mechanisms that are related to their different physiological activities and might be related to the determination and maintenance of the diverse cell shapes. Different types of intermediate filament are expressed during the development and regeneration of nerve cells, at which time the shape of the cells undergoes rapid and numerous changes. Targeted disruption of intermediate filaments, which leads to significant alterations in both microtubule and microfilament networks, also causes marked changes in cell shape.
-
The severely compromised wound-healing capacity of vimentin-deficient (vim−/−) mice and the reduction in motility of their fibroblasts imply a role for intermediate filaments and intermediate-filament-based cytoskeletal crosstalk in cell motility. With findings that vimentin interacts with adhesion-complex components such as fimbrin and plectin, both of which are known to interact with other cytoskeletal elements like actin, there is increasing evidence for a role for intermediate filaments in mediating cytoskeletal crosstalk at these crucial cellular structures. Plectin-deficient cells also have impaired motility.
-
Phosphorylation is intimately linked to the assembly/disassembly of intermediate-filament networks, indicating a role for intermediate filaments as highly sensitive mediators of cytoskeletal crosstalk through signalling. Intermediate filaments are important factors in signalling pathways that regulate both microtubule and microfilament function and organization during various cellular processes such as cell movement and cell division. This appears to be related to the regulation of certain activities involving key components in signal transduction such as Rho kinase α and the 14-3-3 proteins.
Abstract
Intermediate filaments, actin-containing microfilaments and microtubules are the three main cytoskeletal systems of vertebrate and many invertebrate cells. Although these systems are composed of distinctly different proteins, they are in constant and intimate communication with one another. Understanding the molecular basis of this cytoskeletal crosstalk is essential for determining the mechanisms that underlie many cell-biological phenomena. Recent studies have revealed that intermediate filaments and their associated proteins are important components in mediating this crosstalk.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Parry, D. A. & Steinert, P. M. Intermediate filaments: molecular architecture, assembly, dynamics and polymorphism. Q. Rev. Biophys. 32, 99–187 (1999).
Helfand, B. T., Chang, L. & Goldman, R. D. The dynamic and motile properties of intermediate filaments. Annu. Rev. Cell Dev. Biol. 19, 445–467 (2003).
Zimek, A., Stick, R. & Weber, K. Genes coding for intermediate filament proteins: common features and unexpected differences in the genomes of humans and the teleost fish Fugu rubripes. J. Cell Sci. 116, 2295–2302 (2003).
Herrmann, H., Hesse, M., Reichenzeller, M., Aebi, U. & Magin, T. M. Functional complexity of intermediate filament cytoskeletons: from structure to assembly to gene ablation. Int. Rev. Cytol. 223, 83–175 (2003). An excellent, recent review on many aspects of the intermediate-filament field of research.
Strelkov, S. V., Herrmann, H. & Aebi U. Molecular architecture of intermediate filaments. Bioessays 25, 243–251 (2003).
Kumar, S., Yin, X., Trapp, B. D., Hoh, J. H. & Paulaitis, M. E. Relating interactions between neurofilaments to the structure of axonal neurofilament distributions through polymer brush models. Biophys. J. 82, 2360–2372 (2002).
Zackroff, R. V. & Goldman, R. D. In vitro assembly of intermediate filaments from baby hamster kidney (BHK-21) cells. Proc. Natl Acad. Sci. USA 76, 6226–6230 (1979).
Vikstrom, K. L., Lim, S. S., Goldman, R. D. & Borisy, G. G. Steady state dynamics of intermediate filament networks. J. Cell Biol. 118, 121–129 (1992).
Prahlad, V., Yoon, M., Moir, R. D., Vale, R. D. & Goldman, R. D. Rapid movements of vimentin on microtubule tracks: kinesin-dependent assembly of intermediate filament networks. J. Cell Biol. 143, 159–170 (1998).
Yoon, M., Moir, R. D., Prahlad, V. & Goldman, R. D. Motile properties of vimentin intermediate filament networks in living cells. J. Cell Biol. 143, 147–157 (1998).
Vikstrom, K. L., Borisy, G. G. & Goldman, R. D. Dynamic aspects of intermediate filament networks in BHK-21 cells. Proc. Natl Acad. Sci. USA 86, 549–553 (1989).
Helfand, B. T., Loomis, P., Yoon, M. & Goldman, R. D. Rapid transport of neural intermediate filament protein. J. Cell Sci. 116, 2345–2359 (2003). Provides an overview of the transport of the different forms of intermediate-filament protein.
Helfand, B. T., Mikami, A., Vallee, R. B. & Goldman, R. D. A requirement for cytoplasmic dynein and dynactin in intermediate filament network assembly and organization. J. Cell Biol. 157, 795–806 (2002).
Goldman, R. D. The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J. Cell Biol. 51, 752–762 (1971).
Gurland, G. & Gundersen, G. G. Stable, detyrosinated microtubules function to localize vimentin intermediate filaments in fibroblasts. J. Cell Biol. 131, 1275–1290 (1995).
Kreitzer, G., Liao, G. & Gundersen, G. G. Detyrosination of tubulin regulates the interaction of intermediate filaments with microtubules in vivo via a kinesin-dependent mechanism. Mol. Biol. Cell 10, 1105–1118 (1999).
Yoon, K. H. et al. Insights into the dynamic properties of keratin intermediate filaments in living epithelial cells. J. Cell Biol. 153, 503–516 (2001).
Ho, C. L., Martys, J. L., Mikhailov, A., Gundersen, G. G. & Liem, R. K. Novel features of intermediate filament dynamics revealed by green fluorescent protein chimeras. J. Cell Sci. 111, 1767–1778 (1998).
Windoffer, R. & Leube, R. E. Detection of cytokeratin dynamics by time-lapse fluorescence microscopy in living cells. J. Cell Sci. 112, 4521–4534 (1999).
Roy, S. et al. Neurofilaments are transported rapidly but intermittently in axons: implications for slow axonal transport. J. Neurosci. 20, 6849–6861 (2000).
Wang, L. & Brown, A. Rapid intermittent movement of axonal neurofilaments observed by fluorescence photobleaching. Mol. Biol. Cell 12, 3257–3267 (2001).
Wang, L., Ho, C. L., Sun, D., Liem, R. K. & Brown, A. Rapid movement of axonal neurofilaments interrupted by prolonged pauses. Nature Cell Biol. 2, 137–141 (2000).
Shah, J. V., Flanagan, L. A., Janmey, P. A. & Leterrier, J. F. Bidirectional translocation of neurofilaments along microtubules mediated in part by dynein/dynactin. Mol. Biol. Cell 11, 3495–3508 (2000).
Gyoeva, F. K. & Gelfand, V. I. Coalignment of vimentin intermediate filaments with microtubules depends on kinesin. Nature 353, 445–448 (1991). The first description of an interaction between a microtubule-based motor and intermediate filaments.
Navone, F. et al. Cloning and expression of a human kinesin heavy chain gene: interaction of the COOH-terminal domain with cytoplasmic microtubules in transfected CV-1 cells. J. Cell Biol. 117, 1263–1275 (1992).
Prahlad, V., Helfand, B. T., Langford, G. M., Vale, R. D. & Goldman, R. D. Fast transport of neurofilament protein along microtubules in squid axoplasm. J. Cell Sci. 113, 3939–3946 (2000).
Yabe, J. T., Jung, C., Chan, W. K. & Shea, T. B. Phospho-dependent association of neurofilament proteins with kinesin in situ. Cell Motil. Cytoskeleton 45, 249–262 (2000).
Yabe, J. T., Pimenta, A. & Shea, T. B. Kinesin-mediated transport of neurofilament protein oligomers in growing axons. J. Cell Sci. 112, 3799–3814 (1999).
Liao, G. & Gundersen, G. G. Kinesin is a candidate for cross-bridging microtubules and intermediate filaments. Selective binding of kinesin to detyrosinated tubulin and vimentin. J. Biol. Chem. 273, 9797–9803 (1998).
Avsiuk, A., Minin, A. A. & Gyoeva, F. K. Kinesin associated with vimentin intermediate filaments contains a specific light chain. Doklady Biol. Sci. 345, 644–646 (1995).
Allan, V. Dynactin. Curr. Biol. 10, R432 (2000).
King, S. M. Organization and regulation of the dynein microtubule motor. Cell Biol. Int. 27, 213–215 (2003).
LaMonte, B. H. et al. Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron 34, 715–727 (2002).
Burkhardt, J. K., Echeverri, C. J., Nilsson, T. & Vallee, R. B. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 139, 467–484 (1997).
Echeverri, C. J., Paschal, B. M., Vaughan, K. T. & Vallee, R. B. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 132, 617–633 (1996).
Osborn, M., Franke, W. & Weber, K. Direct demonstration of the presence of two immunologically distinct intermediate-sized filament systems in the same cell by double immunofluorescence microscopy. Vimentin and cytokeratin fibers in cultured epithelial cells. Exp. Cell Res. 125, 37–46 (1980).
Windoffer, R. & Leube, R. E. De novo formation of cytokeratin filament networks originates from the cell cortex in A-431 cells. Cell Motil. Cytoskeleton 50, 33–44 (2001).
Hollenbeck, P. J., Bershadsky, A. D., Pletjushkina, O. Y., Tint, I. S. & Vasiliev, J. M. Intermediate filament collapse is an ATP-dependent and actin-dependent process. J. Cell Sci. 92, 621–631 (1989). An early clue that intermediate filaments interact with actin.
Tint, I. S., Hollenbeck, P. J., Verkhovsky, A. B., Surgucheva, I. G. & Bershadsky, A. D. Evidence that intermediate filament reorganization is induced by ATP-dependent contraction of the actomyosin cortex in permeabilized fibroblasts. J. Cell Sci. 98, 375–384 (1991).
Green, K. J., Talian, J. C. & Goldman, R. D. Relationship between intermediate filaments and microfilaments in cultured fibroblasts: evidence for common foci during cell spreading. Cell Motil. Cytoskeleton 6, 408–418 (1986).
Green, K. J., Geiger, B., Jones, J. C., Talian, J. C. & Goldman, R. D. The relationship between intermediate filaments and microfilaments before and during the formation of desmosomes and adherens-type junctions in mouse epidermal keratinocytes. J. Cell Biol. 104, 1389–1402 (1987).
Weber, K. L. & Bement, W. M. F-actin serves as a template for cytokeratin organization in cell free extracts. J. Cell Sci. 115, 1373–1382 (2002).
Rao, M. V. et al. Myosin Va binding to neurofilaments is essential for correct myosin Va distribution and transport and neurofilament density. J. Cell Biol. 159, 279–290 (2002).
Hisanaga, S. & Hirokawa, N. Structure of the peripheral domains of neurofilaments revealed by low angle rotary shadowing. J. Mol. Biol. 202, 297–305 (1988).
Miyasaka, H., Okabe, S., Ishiguro, K., Uchida, T. & Hirokawa, N. Interaction of the tail domain of high molecular weight subunits of neurofilaments with the COOH-terminal region of tubulin and its regulation by tau protein kinase II. J. Biol. Chem. 268, 22695–22702 (1993).
Julien, J. P., Cote, F. & Collard, J. F. Mice overexpressing the human neurofilament heavy gene as a model of ALS. Neurobiol. Aging 16, 487–490 (1995).
Collard, J. F., Cote, F. & Julien, J. P. Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature 375, 61–64 (1995).
Marszalek, J. R. et al. Neurofilament subunit NF-H modulates axonal diameter by selectively slowing neurofilament transport. J. Cell Biol. 135, 711–724 (1996).
Zhu, Q., Lindenbaum, M., Levavasseur, F., Jacomy, H. & Julien, J. P. Disruption of the NF-H gene increases axonal microtubule content and velocity of neurofilament transport: relief of axonopathy resulting from the toxin β, β′-iminodipropionitrile. J. Cell Biol. 143, 183–193 (1998).
Eyer, J. & Leterrier, J. F. Influence of the phosphorylation state of neurofilament proteins on the interactions between purified filaments in vitro. Biochem. J. 252, 655–660 (1988).
Gotow, T., Tanaka, T., Nakamura, Y. & Takeda, M. Dephosphorylation of the largest neurofilament subunit protein influences the structure of crossbridges in reassembled neurofilaments. J. Cell Sci. 107, 1949–1957 (1994).
Gotow, T. & Tanaka, J. Phosphorylation of neurofilament H subunit as related to arrangement of neurofilaments. J. Neurosci. Res. 37, 691–713 (1994).
Leterrier, J. F., Kas, J., Hartwig, J., Vegners, R. & Janmey, P. A. Mechanical effects of neurofilament cross-bridges. Modulation by phosphorylation, lipids, and interactions with F-actin. J. Biol. Chem. 271, 15687–15694 (1996).
Sanchez, I. et al. Local control of neurofilament accumulation during radial growth of myelinating axons in vivo. Selective role of site-specific phosphorylation. J. Cell Biol. 151, 1013–1024 (2000).
Hunt, A. J., Gittes, F. & Howard, J. The force exerted by a single kinesin molecule against a viscous load. Biophys. J. 67, 766–781 (1994).
Bulinski, J. C., McGraw, T. E., Gruber, D., Nguyen, H. L. & Sheetz, M. P. Overexpression of MAP4 inhibits organelle motility and trafficking in vivo. J. Cell Sci. 110, 3055–3064 (1997).
Ebneth, A. et al. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer's disease. J. Cell Biol. 143, 777–794 (1998).
Trinczek, B., Ebneth, A., Mandelkow, E. M. & Mandelkow, E. Tau regulates the attachment/detachment but not the speed of motors in microtubule-dependent transport of single vesicles and organelles. J. Cell Sci. 112, 2355–2367 (1999).
Stamer, K., Vogel, R., Thies, E., Mandelkow, E. & Mandelkow, E. M. Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J. Cell Biol. 156, 1051–1063 (2002).
Bloom, G. S. & Vallee, R. B. Association of microtubule-associated protein 2 (MAP 2) with microtubules and intermediate filaments in cultured brain cells. J. Cell Biol. 96, 1523–1531 (1983). An early indication that MAPs bind to intermediate filaments.
Seitz, A. et al. Single-molecule investigation of the interference between kinesin, tau and MAP2c. EMBO J. 21, 4896–4905 (2002).
Paschal, B. M., Obar, R. A. & Vallee, R. B. Interaction of brain cytoplasmic dynein and MAP2 with a common sequence at the C terminus of tubulin. Nature 342, 569–572 (1989).
Svitkina, T. M., Verkhovsky, A. B. & Borisy, G. G. Plectin sidearms mediate interaction of intermediate filaments with microtubules and other components of the cytoskeleton. J. Cell Biol. 135, 991–1007 (1996). A vivid demonstration of the intermediate-filament-associated protein, plectin, forming bridges with microtubules and microfilaments.
Leung, C. L., Green, K. J. & Liem, R. K. Plakins: a family of versatile cytolinker proteins. Trends Cell Biol. 12, 37–45 (2002).
Bernier, G. et al. Dystonin expression in the developing nervous system predominates in the neurons that degenerate in dystonia musculorum mutant mice. Mol. Cell Neurosci. 6, 509–520 (1995).
Guo, L. et al. Gene targeting of BPAG1: abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell 81, 233–243 (1995).
Leung, C. L., Sun, D. & Liem, R. K. The intermediate filament protein peripherin is the specific interaction partner of mouse BPAG1-n (dystonin) in neurons. J. Cell Biol. 144, 435–446 (1999).
Shea, T. B., Beermann, M. L. & Fischer, I. Transient requirement for vimentin in neuritogenesis: intracellular delivery of anti-vimentin antibodies and antisense oligonucleotides inhibit neurite initiation but not elongation of existing neurites in neuroblastoma. J. Neurosci Res. 36, 66–76 (1993).
Cochard, P. & Paulin, D. Initial expression of neurofilaments and vimentin in the central and peripheral nervous system of the mouse embryo in vivo. J. Neurosci. 4, 2080–2094 (1984).
Troy, C. M., Muma, N. A., Greene, L. A., Price, D. L. & Shelanski, M. L. Regulation of peripherin and neurofilament expression in regenerating rat motor neurons. Brain Res. 529, 232–238 (1990).
Hoffman, P. N. et al. Neurofilament gene expression: a major determinant of axonal caliber. Proc. Natl Acad. Sci. USA 84, 3472–3476 (1987).
Lasek, R. J., Oblinger, M. M. & Drake, P. F. Molecular biology of neuronal geometry: expression of neurofilament genes influences axonal diameter. Cold Spring Harb. Symp. Quant. Biol. 48, 731–744 (1983).
Gervasi, C., Stewart, C. B. & Szaro, B. G. Xenopus laevis peripherin (XIF3) is expressed in radial glia and proliferating neural epithelial cells as well as in neurons. J. Comp. Neurol. 423, 512–531 (2000).
Troy, C. M., Brown, K., Greene, L. A. & Shelanski, M. L. Ontogeny of the neuronal intermediate filament protein, peripherin, in the mouse embryo. Neuroscience 36, 217–237 (1990).
Undamatla, J. & Szaro, B. G. Differential expression and localization of neuronal intermediate filament proteins within newly developing neurites in dissociated cultures of Xenopus laevis embryonic spinal cord. Cell Motil. Cytoskeleton 49, 16–32 (2001).
Aletta, J. M. et al. Relationship between the nerve growth factor-regulated clone 73 gene product and the 58-kilodalton neuronal intermediate filament protein (peripherin). J. Neurochem. 51, 1317–1320 (1988).
Aletta, J. M., Shelanski, M. L. & Greene, L. A. Phosphorylation of the peripherin 58-kDa neuronal intermediate filament protein. Regulation by nerve growth factor and other agents. J. Biol. Chem. 264, 4619–4627 (1989).
Leonard, D. G., Gorham, J. D., Cole, P., Greene, L. A. & Ziff, E. B. A nerve growth factor-regulated messenger RNA encodes a new intermediate filament protein. J. Cell Biol. 106, 181–193 (1988).
Leonard, D. G., Ziff, E. B. & Greene, L. A. Identification and characterization of mRNAs regulated by nerve growth factor in PC12 cells. Mol. Cell Biol. 7, 3156–3167 (1987).
Oblinger, M. M., Wong, J. & Parysek, L. M. Axotomy-induced changes in the expression of a type III neuronal intermediate filament gene. J. Neurosci. 9, 3766–3775 (1989).
Hoffman, P. N., Thompson, G. W., Griffin, J. W. & Price, D. L. Changes in neurofilament transport coincide temporally with alterations in the caliber of axons in regenerating motor fibers. J. Cell Biol. 101, 1332–1340 (1985).
Wong, J. & Oblinger, M. M. Differential regulation of peripherin and neurofilament gene expression in regenerating rat DRG neurons. J. Neurosci. Res. 27, 332–341 (1990).
Helfand, B. T., Mendez, M. G., Pugh, J., Delsert, C. & Goldman, R. D. A role for intermediate filaments in determining and maintaining the shape of nerve cells. Mol. Biol. Cell. 14, 5069–5081 (2003).
Weinstein, D. E., Shelanski, M. L. & Liem, R. K. Suppression by antisense mRNA demonstrates a requirement for the glial fibrillary acidic protein in the formation of stable astrocytic processes in response to neurons. J. Cell Biol. 112, 1205–1213 (1991).
Lepekhin, E. A. et al. Intermediate filaments regulate astrocyte motility. J. Neurochem. 79, 617–625 (2001).
Goldman, R. D., Khuon, S., Chou, Y. H., Opal, P. & Steinert, P. M. The function of intermediate filaments in cell shape and cytoskeletal integrity. J. Cell Biol. 134, 971–983 (1996). Shows the central role that intermediate filaments have in stabilizing other cytoskeletal networks and in maintaining cell shape.
Eckes, B. et al. Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J. Cell Sci. 111, 1897–1907 (1998).
Eckes, B. et al. Impaired wound healing in embryonic and adult mice lacking vimentin. J. Cell Sci. 113, 2455–2462 (2000). One of the first papers showing that the deletion of vimentin intermediate filaments has a profound phenotype in mice.
Pekny, M. et al. Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J. Cell Biol. 145, 503–514 (1999).
Geiger, B., Bershadsky, A., Pankov, R. & Yamada, K. M. Transmembrane extracellular matrix–cytoskeleton crosstalk. Nature Rev. Mol. Cell Biol. 2, 793–805 (2001).
Nobes, C. D. & Hall, A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53–62 (1995).
Allen, W. E., Jones, G. E., Pollard, J. W. & Ridley, A. J. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J. Cell Sci. 110, 707–720 (1997).
Rottner, K., Hall, A. & Small, J. V. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr. Biol. 9, 640–648 (1999).
Kaverina, I., Krylyshkina, O. & Small, J. V. Regulation of substrate adhesion dynamics during cell motility. Int. J. Biochem. Cell Biol. 34, 746–761 (2002).
Webb, D. J., Parsons, J. T. & Horwitz, A. F. Adhesion assembly, disassembly and turnover in migrating cells — over and over and over again. Nature Cell Biol. 4, E97–E100 (2002).
Tsuruta, D. & Jones, J. C. The vimentin cytoskeleton regulates focal contact size and adhesion of endothelial cells subjected to shear stress. J. Cell Sci. 116, 4977–4984 (2003). Shows the role of intermediate filaments in regulating focal-contact structure and the stability of cell–substrate adhesion.
Correia, I., Chu, D., Chou, Y. H., Goldman, R. D. & Matsudaira, P. Integrating the actin and vimentin cytoskeletons: adhesion-dependent formation of fimbrin–vimentin complexes in macrophages. J. Cell Biol. 146, 831–842 (1999).
Mabuchi, K., Li, B., Ip, W. & Tao, T. Association of calponin with desmin intermediate filaments. J. Biol. Chem. 272, 22662–22666 (1997).
Gonzales, M. et al. Structure and function of a vimentin-associated matrix adhesion in endothelial cells. Mol. Biol. Cell 12, 85–100 (2001).
Steinbock, F. A. & Wiche, G. Plectin: a cytolinker by design. Biol. Chem. 380, 151–158 (1999).
Andra, K., Nikolic, B., Stocher, M., Drenckhahn, D. & Wiche, G. Not just scaffolding: plectin regulates actin dynamics in cultured cells. Genes Dev. 12, 3442–3451 (1998).
Wiche, G. Role of plectin in cytoskeleton organization and dynamics. J. Cell Sci. 111, 2477–2486 (1998).
Herrmann, H. & Wiche, G. Specific in situ phosphorylation of plectin in detergent-resistant cytoskeletons from cultured Chinese hamster ovary cells. J. Biol. Chem. 258, 14610–14618 (1983).
Gimona, M., Djinovic-Carugo, K., Kranewitter, W. J. & Winder, S. J. Functional plasticity of CH domains. FEBS Lett. 513, 98–106 (2002).
Eriksson, J. E. et al. Specific in vivo phosphorylation sites determine the assembly dynamics of vimentin intermediate filaments. J. Cell Sci. 117, 919–932 (2003).
Muslin, A. J. & Xing, H. 14-3-3 proteins: regulation of subcellular localization by molecular interference. Cell Signal. 12, 703–709 (2000).
Meyer, G. & Feldman, E. L. Signaling mechanisms that regulate actin-based motility processes in the nervous system. J. Neurochem. 83, 490–503 (2002).
Wittmann, T. & Waterman-Storer, C. M. Cell motility: can Rho GTPases and microtubules point the way? J. Cell Sci. 114, 3795–3803 (2001).
Valgeirsdottir, S. et al. PDGF induces reorganization of vimentin filaments. J. Cell Sci. 111, 1973–1980 (1998).
Meriane, M. et al. Cdc42Hs and Rac1 GTPases induce the collapse of the vimentin intermediate filament network. J. Biol. Chem. 275, 33046–33052 (2000).
Chan, W. et al. Vimentin intermediate filament reorganization by Cdc42: involvement of PAK and p70 S6 kinase. Eur. J. Cell Biol. 81, 692–701 (2002).
Schlessinger, J. & Lemmon, M. A. SH2 and PTB domains in tyrosine kinase signaling. Sci. STKE 191, RE12 (2003).
Goto, H. et al. Phosphorylation and reorganization of vimentin by p21-activated kinase (PAK). Genes Cells 7, 91–97 (2002).
Wittmann, T., Bokoch, G. M. & Waterman-Storer, C. M. Regulation of leading edge microtubule and actin dynamics downstream of Rac1. J. Cell Biol. 161, 845–851 (2003).
Sin, W. C., Chen, X. Q., Leung, T. & Lim, L. RhoA-binding kinase α translocation is facilitated by the collapse of the vimentin intermediate filament network. Mol. Cell Biol. 18, 6325–6339 (1998). Shows that the frequently observed 'collapse' of intermediate filaments, which is induced on activation of Rho GTPases, actually regulates ROKα activity.
Kosako, H. et al. Specific accumulation of Rho-associated kinase at the cleavage furrow during cytokinesis: cleavage furrow-specific phosphorylation of intermediate filaments. Oncogene 18, 2783–2788 (1999).
Yasui, Y. et al. Protein kinases required for segregation of vimentin filaments in mitotic process. Oncogene 20, 2868–2876 (2001).
Goto, H. et al. Phosphorylation of vimentin by Rho-associated kinase at a unique amino-terminal site that is specifically phosphorylated during cytokinesis. J. Biol. Chem. 273, 11728–11736 (1998).
Liao, J. & Omary, M. B. 14-3-3 proteins associate with phosphorylated simple epithelial keratins during cell cycle progression and act as a solubility cofactor. J. Cell Biol. 133, 345–357 (1996).
Ku, N. O., Liao, J. & Omary, M. B. Phosphorylation of human keratin 18 serine 33 regulates binding to 14-3-3 proteins. EMBO J. 17, 1892–1906 (1998).
Ku, N. O., Michie, S., Resurreccion, E. Z., Broome, R. L. & Omary, M. B. Keratin binding to 14-3-3 proteins modulates keratin filaments and hepatocyte mitotic progression. Proc. Natl Acad. Sci. USA 99, 4373–3478 (2002).
Toivola, D. M. et al. Disturbances in hepatic cell-cycle regulation in mice with assembly-deficient keratins 8/18. Hepatology 34, 1174–1183 (2001). Presents evidence that keratin intermediate-filament interactions with the signalling factor 14-3-3 are involved in the regulation of cell-cycle progression in hepatocytes.
Reichelt, J. & Magin, T. M. Hyperproliferation, induction of c-Myc and 14-3-3σ, but no cell fragility in keratin-10-null mice. J. Cell Sci. 115, 2639–2650 (2002).
Tzivion, G., Luo, Z. J. & Avruch, J. Calyculin A-induced vimentin phosphorylation sequesters 14-3-3 and displaces other 14-3-3 partners in vivo. J. Biol. Chem. 275, 29772–29778 (2000). Shows the importance of vimentin phosphorylation in the regulation of 14-3-3 activity.
Eriksson, J. E. et al. Cytoskeletal integrity in interphase cells requires protein phosphatase activity. Proc. Natl Acad. Sci. USA 89, 11093–11097 (1992).
Gohla, A. & Bokoch, G. M. 14-3-3 regulates actin dynamics by stabilizing phosphorylated cofilin. Curr. Biol. 12, 1704–1710 (2002).
Birkenfeld, J., Betz, H. & Roth, D. Identification of cofilin and LIM-domain-containing protein kinase 1 as novel interaction partners of 14-3-3ζ. Biochem. J. 369, 45–54 (2003).
Hashiguchi, M., Sobue, K. & Paudel, H. K. 14-3-3ζ is an effector of tau protein phosphorylation. J. Biol. Chem. 275, 25247–25254 (2000).
Agarwal-Mawal, A. et al. 14-3-3 connects glycogen synthase kinase-3β to tau within a brain microtubule-associated tau phosphorylation complex. J. Biol. Chem. 278, 12722–12728 (2003).
Ichimura, T. et al. Phosphorylation-dependent interaction of kinesin light chain 2 and the 14-3-3 protein. Biochemistry 41, 5566–5572 (2002).
Bray, D. Cell Movements. From Molecules to Motility. 2nd edn (Garland Publishing, New York, USA, 2001).
Herrmann, H. & Aebi, U. Intermediate filament assembly: fibrillogenesis is driven by decisive dimer–dimer interactions. Curr. Opin. Struct. Biol. 8, 177–185 (1998).
Etienne-Manneville, S. & Hall, A. Rho GTPases in cell biology. Nature 420, 629–635 (2002).
Acknowledgements
Figure 2 was kindly provided by H. Herrmann (German Cancer Research Center, Heidelberg, Germany) and U. Aebi (Maurice E. Müller Institute for Structural Biology, Biozentrum Basel, Switzerland). The authors would like to acknowledge the support of a MERIT Award from the National Institute of General Medical Sciences, and grants from the National Institute of Dental Research, and the National Heart, Lung and Blood Institute.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Glossary
- KERATIN
-
Obligate heteropolymer intermediate filaments that are composed of type-I and type-II intermediate-filament proteins, which are expressed mainly in epithelial cells.
- VIMENTIN
-
Type-III, homopolymer intermediate filaments that are expressed in mesenchymal and some ectodermal cells.
- NEUROFILAMENT
-
Type-IV, heteropolymer intermediate filaments that are composed of three neurofilament subunits (NF-L, NF-M and NF-H), which are expressed mainly in neurons.
- SPREADING PROCESS
-
The process of cells spreading after trypsinization and re-plating. Visualization of intermediate-filament particles and squiggles in the peripheral regions of the cells is optimized during the early stages of cell spreading.
- CYTOPLASMIC DYNEIN
-
A microtubule motor that can bind to and move towards the minus end of microtubules.
- FRAP
-
(fluorescence recovery after photobleaching). A live-cell imaging technique used to study the mobility of fluorescent molecules. A pulse of high intensity light is used to irreversibly photobleach a population of fluorophores in a target region. Recovery of fluorescence in the bleached region represents movement of fluorophores into that region.
- PERIPHERIN
-
Type-III, homopolymer intermediate filaments that are expressed in peripheral and enteric neurons, as well as in PC12 cells.
- CONVENTIONAL KINESIN
-
A microtubule motor that can bind to and move towards the plus ends of microtubules.
- DYNAMITIN
-
A component of dynactin, a large complex thought to be involved in dynein–cargo interactions. Overexpression of dynamitin leads to disruption of dynein function.
- WOUND-SCRAPE ASSAY
-
An assay used to assess cell migration. A scrape is made in a confluent monolayer of cells, leaving an area devoid of cells, followed by microscopy to monitor the migration of cells into the wound.
- FOCAL COMPLEXES
-
Small (1 μm diameter), dot-like adhesion structures that are present mainly at the edges of the lamellipodium.
- LAMELLIPODIA
-
Broad, flat protrusions at the leading edge of a moving cell that are enriched with a branched network of actin filaments.
- FILOPODIA
-
Thin cellular processes containing long, unbranched, parallel bundles of actin filaments.
- PODOSOMES
-
Extracellular-matrix adhesions that are found in various malignant cells and in some normal cells, including macrophages and osteoclasts. Podosomes are small (0.5 μm diameter) cylindrical structures containing typical focal-adhesion proteins, such as vinculin and paxillin.
- 14-3-3 PROTEINS
-
A family of proteins and protein domains that bind to serine/threonine-phosphorylated residues in a context-specific manner. They bind and regulate key proteins involved in various physiological processes.
- RHO-GTPase FAMILY
-
Ras-related small GTPases that function as molecular switches to control signal-transduction pathways. They have been traditionally characterized to function as regulators of actin and, to a lesser extent, microtubule dynamics.
Rights and permissions
About this article
Cite this article
Chang, L., Goldman, R. Intermediate filaments mediate cytoskeletal crosstalk. Nat Rev Mol Cell Biol 5, 601–613 (2004). https://doi.org/10.1038/nrm1438
Issue Date:
DOI: https://doi.org/10.1038/nrm1438
This article is cited by
-
Autophagosome–lysosome fusion is facilitated by plectin-stabilized actin and keratin 8 during macroautophagic process
Cellular and Molecular Life Sciences (2022)
-
Genome-wide occupancy reveals the localization of H1T2 (H1fnt) to repeat regions and a subset of transcriptionally active chromatin domains in rat spermatids
Epigenetics & Chromatin (2021)
-
Vimentin intermediate filaments stabilize dynamic microtubules by direct interactions
Nature Communications (2021)
-
Mechanical programming of arterial smooth muscle cells in health and ageing
Biophysical Reviews (2021)
-
Vimentin expression is retained in erythroid cells differentiated from human iPSC and ESC and indicates dysregulation in these cells early in differentiation
Stem Cell Research & Therapy (2019)