Key Points
-
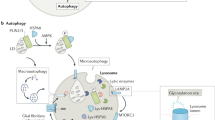
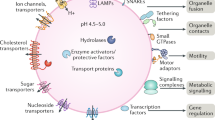
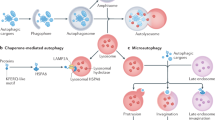
Lysosomes are membrane-bound organelles that contain digestive enzymes that are responsible for the degradation of macromolecules such as proteins, lipids and carbohydrates.
-
Lysosomal storage disorders (LSDs) are a class of inherited metabolic diseases. They are caused by the defective activity of soluble lysosomal enzymes or integral membrane proteins, which results in the intra-lysosomal accumulation of undegraded metabolites.
-
A number of new proteins that are involved in LSDs have recently been identified, including an enzyme that is required for optimal sulphatase activity (defects in which cause multiple sulphatase deficiency) and soluble and integral membrane proteins that are involved in the ceroid lipofuscinoses.
-
Little is known about how and why the intra-lysosomal accumulation of undegraded metabolites causes disease pathology, but the extensive range of disease symptoms indicates that many secondary biochemical and cellular pathways are activated in different LSDs.
-
Potential changes in lysosomal stability and integrity, in intracellular trafficking and intracellular signalling, as well as alterations in secondary biochemical pathways and in gene expression, could be involved in LSD pathology.
-
The most effective treatment for LSDs would be somatic gene therapy, but the prospect of this becoming available soon is remote. At present, treatments are mainly limited to enzyme-replacement therapy and substrate-reduction therapy.
-
New therapies should arise as the secondary biochemical and cellular pathways that are altered in LSDs are elucidated.
Abstract
Lysosomal storage disorders, of which more than 40 are known, are caused by the defective activity of lysosomal proteins, which results in the intra-lysosomal accumulation of undegraded metabolites. Despite years of study of the genetic and molecular bases of lysosomal storage disorders, little is known about the events that lead from this intra-lysosomal accumulation to pathology. Here, we summarize the biochemistry of lysosomal storage disorders. We then discuss downstream cellular pathways that are potentially affected in these disorders and that might help us to delineate their pathological mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
de Duve, C. Exploring cells with a centrifuge. Science 189, 186–194 (1975). This classic paper describes the discovery of various intracellular organelles, including lysosomes, that led to de Duve being awarded a Nobel prize in 1974.
Sandhoff, K. & Kolter, T. Topology of glycosphingolipid degradation. Trends Cell Biol. 6, 98–103 (1996).
Journet, A., Chapel, A., Kieffer, S., Roux, F. & Garin, J. Proteomic analysis of human lysosomes: application to monocytic and breast cancer cells. Proteomics 2, 1026–1040 (2002).
Eskelinen, E. L., Tanaka, Y. & Saftig, P. At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol. 13, 137–145 (2003).
Mancini, G. M., Havelaar, A. C. & Verheijen, F. W. Lysosomal transport disorders. J. Inherit. Metab. Dis. 23, 278–292 (2000).
Kornfeld, S. & Sly, W. S. in The Metabolic and Molecular Bases of Inherited Disease (eds Scriver, C. R. et al.) 3469–3482 (McGraw–Hill Inc., Columbus, USA, 2001).
Callahan, J. W. Molecular basis of GM1 gangliosidosis and Morquio disease, type B. Structure–function studies of lysosomal β-galactosidase and the non-lysosomal β-galactosidase-like protein. Biochim. Biophys. Acta 1455, 85–103 (1999).
Li, Y. T., Maskos, K., Chou, C. W., Cole, R. B. & Li, S. C. Presence of an unusual GM2 derivative, taurine-conjugated GM2, in Tay–Sachs brain. J. Biol. Chem. 278, 35286–35291 (2003).
Hopwood, J. J. & Ballabio, A. in The Metabolic and Molecular Bases of Inherited Disease (eds Scriver, C. R. et al.) 3725–3732 (McGraw–Hill Inc., Columbus, USA, 2001).
Schmidt, B., Selmer, T., Ingendoh, A. & von Figura, K. A novel amino acid modification in sulfatases that is defective in multiple sulfatase deficiency. Cell 82, 271–278 (1995).
Cosma, M. P. et al. The multiple sulfatase deficiency gene encodes an essential and limiting factor for the activity of sulfatases. Cell 113, 445–456 (2003).
Dierks, T. et al. Multiple sulfatase deficiency is caused by mutations in the gene encoding the human Cα-formylglycine generating enzyme. Cell 113, 435–444 (2003). An excellent study in which the gene that is affected in MSD was first described and mutations that cause this disease were identified.
Ostrowska, H., Krukowska, K., Kalinowska, J., Orlowska, M. & Lengiewicz, I. Lysosomal high molecular weight multienzyme complex. Cell Mol. Biol. Lett. 8, 19–24 (2003).
Zhou, X. Y. et al. Mouse model for the lysosomal disorder galactosialidosis and correction of the phenotype with overexpressing erythroid precursor cells. Genes Dev. 9, 2623–2634 (1995).
Leimig, T. et al. Functional amelioration of murine galactosialidosis by genetically modified bone marrow hematopoietic progenitor cells. Blood 99, 3169–3178 (2002).
Jolly, R. D., Brown, S., Das, A. M. & Walkley, S. U. Mitochondrial dysfunction in the neuronal ceroid-lipofuscinoses (Batten disease). Neurochem. Int. 40, 565–571 (2002).
Hofmann, S. L. & Peltonen, L. in The Metabolic and Molecular Bases of Inherited Disease (eds Scriver, C. R. et al.) 3877–3894 (McGraw–Hill Inc., Columbus, USA, 2001).
Cooper, J. D. Progress towards understanding the neurobiology of Batten disease or neuronal ceroid lipofuscinosis. Curr. Opin. Neurol. 16, 121–128 (2003).
Linder, M. E. & Deschenes, R. J. New insights into the mechanisms of protein palmitoylation. Biochemistry 42, 4311–4320 (2003).
Hofmann, S. L. et al. Neuronal ceroid lipofuscinoses caused by defects in soluble lysosomal enzymes (CLN1 and CLN2). Curr. Mol. Med. 2, 423–437 (2002).
Gupta, P. et al. Disruption of PPT2 in mice causes an unusual lysosomal storage disorder with neurovisceral features. Proc. Natl Acad. Sci. USA 100, 12325–12330 (2003).
Kim, Y., Ramirez-Montealegre, D. & Pearce, D. A. A role in vacuolar arginine transport for yeast Btn1p and for human CLN3, the protein defective in Batten disease. Proc. Natl Acad. Sci. USA 100, 15458–15462 (2003). A recent study in which the function of the CLN3 protein was first identified on the basis of analysing the function of the CLN3 orthologue Btn1 in S. cerevisiae . An S. cerevisiae transport defect that results from the ablation of the gene for Btn1 could be reversed by expressing either Btn1 or CLN3.
Gao, H. et al. Mutations in a novel CLN6-encoded transmembrane protein cause variant neuronal ceroid lipofuscinosis in man and mouse. Am. J. Hum. Genet. 70, 324–335 (2002).
Wheeler, R. B. et al. The gene mutated in variant late-infantile neuronal ceroid lipofuscinosis (CLN6) and in nclf mutant mice encodes a novel predicted transmembrane protein. Am. J. Hum. Genet. 70, 537–542 (2002).
Isosomppi, J., Vesa, J., Jalanko, A. & Peltonen, L. Lysosomal localization of the neuronal ceroid lipofuscinosis CLN5 protein. Hum. Mol. Genet. 11, 885–891 (2002).
Verheijen, F. W. et al. A new gene, encoding an anion transporter, is mutated in sialic acid storage diseases. Nature Genet. 23, 462–465 (1999).
Town, M. et al. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nature Genet. 18, 319–324 (1998).
Simons, K. & Gruenberg, J. Jamming the endosomal system: lipid rafts and lysosomal storage diseases. Trends Cell Biol. 10, 459–462 (2000).
Nishino, I. et al. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease). Nature 406, 906–910 (2000). Although LAMP2 was known to be an abundant lysosomal structural protein, this study was the first to link defects in this protein to a known LSD.
Sandhoff, K., Kolter, T. & Harzer, K. in The Metabolic and Molecular Bases of Inherited Disease (eds Scriver, C. R. et al.) 3371–3388 (McGraw–Hill Inc., Columbus, USA, 2001).
Dittmer, F. et al. Alternative mechanisms for trafficking of lysosomal enzymes in mannose 6-phosphate receptor-deficient mice are cell type-specific. J. Cell Sci. 112, 1591–1597 (1999).
Kaufman, R. J. Orchestrating the unfolded protein response in health and disease. J. Clin. Invest. 110, 1389–1398 (2002).
Ferri, K. F. & Kroemer, G. Organelle-specific initiation of cell death pathways. Nature Cell Biol. 3, E255–E263 (2001).
Castino, R., Demoz, M. & Isidoro, C. Destination 'lysosome': a target organelle for tumour cell killing? J. Mol. Recognit. 16, 337–348 (2003).
Yang, A. J., Chandswangbhuvana, D., Margol, L. & Glabe, C. G. Loss of endosomal/lysosomal membrane impermeability is an early event in amyloid Aβ1–42 pathogenesis. J. Neurosci. Res. 52, 691–698 (1998).
Zhang, F. et al. Characterization of ABCB9, an ATP binding cassette protein associated with lysosomes. J. Biol. Chem. 275, 23287–23294 (2000).
Vulevic, B. et al. Cloning and characterization of human adenosine 5′-triphosphate-binding cassette, sub-family A, transporter 2 (ABCA2). Cancer Res. 61, 3339–3347 (2001).
Raggers, R. J., Pomorski, T., Holthuis, J. C., Kalin, N. & van Meer, G. Lipid traffic: the ABC of transbilayer movement. Traffic 1, 226–234 (2000).
Schmitz, G. & Kaminski, W. E. ABCA2: a candidate regulator of neural transmembrane lipid transport. Cell Mol. Life Sci. 59, 1285–1295 (2002).
Choi, H. Y. et al. Impaired ABCA1-dependent lipid efflux and hypoalphalipoproteinemia in human Niemann–Pick type C disease. J. Biol. Chem. 278, 32569–32577 (2003).
Jaiswal, J. K., Andrews, N. W. & Simon, S. M. Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J. Cell Biol. 159, 625–635 (2002). A study that showed a link between intracellular Ca2+ levels and the fusion of secretory lysosomes with the plasma membrane, which might have ramifications for understanding the pathology of LSDs.
Andrews, N. W. Regulated secretion of conventional lysosomes. Trends Cell Biol. 10, 316–321 (2000).
Reddy, A., Caler, E. V. & Andrews, N. W. Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell 106, 157–169 (2001).
Linke, M., Herzog, V. & Brix, K. Trafficking of lysosomal cathepsin B–green fluorescent protein to the surface of thyroid epithelial cells involves the endosomal/lysosomal compartment. J. Cell Sci. 115, 4877–4889 (2002).
Marks, D. L. & Pagano, R. E. Endocytosis and sorting of glycosphingolipids in sphingolipid storage disease. Trends Cell Biol. 12, 605–613 (2002).
Sillence, D. J. & Platt, F. M. Storage diseases: new insights into sphingolipid functions. Trends Cell Biol. 13, 195–203 (2003).
Chen, C. S., Patterson, M. C., Wheatley, C. L., O'Brien, J. F. & Pagano, R. E. Broad screening test for sphingolipid-storage diseases. Lancet 354, 901–905 (1999).
Sillence, D. J. et al. Glucosylceramide modulates membrane traffic along the endocytic pathway. J. Lipid Res. 43, 1837–1845 (2002).
Puri, V. et al. Cholesterol modulates membrane traffic along the endocytic pathway in sphingolipid-storage diseases. Nature Cell Biol. 1, 386–388 (1999).
Choudhury, A. et al. Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann–Pick C cells. J. Clin. Invest. 109, 1541–1550 (2002).
Brown, D. & London, E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275, 17221–17224 (2000).
Lai, E. C. Lipid rafts make for slippery platforms. J. Cell Biol. 162, 365–370 (2003).
Gondre-Lewis, M. C., McGlynn, R. & Walkley, S. U. Cholesterol accumulation in NPC1-deficient neurons is ganglioside dependent. Curr. Biol. 13, 1324–1329 (2003). This paper offers a new view of the cause of NPC disease by proposing that the NPC1 protein might be more closely linked to the homeostatic control of glycosphingolipid transport rather than to cholesterol transport.
Pfrieger, F. W. Cholesterol homeostasis and function in neurons of the central nervous system. Cell Mol. Life Sci. 60, 1158–1171 (2003).
Buccoliero, R., Bodennec, J. & Futerman, A. H. The role of sphingolipids in neuronal development: lessons from models of sphingolipid storage diseases. Neurochem. Res. 27, 565–574 (2002).
Lefrancois, S., Zeng, J., Hassan, A. J., Canuel, M. & Morales, C. R. The lysosomal trafficking of sphingolipid activator proteins (SAPs) is mediated by sortilin. EMBO J. 22, 6430–6437 (2003). This recent study showed that the transport pathway of sphingolipid-activator proteins to lysosomes occurs through a new pathway that involves sortilin receptors.
Pearce, D. A., Ferea, T., Nosel, S. A., Das, B. & Sherman, F. Action of BTN1, the yeast orthologue of the gene mutated in Batten disease. Nature Genet. 22, 55–58 (1999).
Chattopadhyay, S., Roberts, P. M. & Pearce, D. A. The yeast model for Batten disease: a role for Btn2p in the trafficking of the Golgi-associated vesicular targeting protein, Yif1p. Biochem. Biophys. Res. Commun. 302, 534–538 (2003).
Fares, H. & Greenwald, I. Regulation of endocytosis by CUP-5, the Caenorhabditis elegans mucolipin-1 homolog. Nature Genet. 28, 64–68 (2001).
Hersh, B. M., Hartwieg, E. & Horvitz, H. R. The Caenorhabditis elegans mucolipin-like gene cup-5 is essential for viability and regulates lysosomes in multiple cell types. Proc. Natl Acad. Sci. USA 99, 4355–4360 (2002).
Raychowdhury, M. K. et al. Molecular pathophysiology of mucolipidosis type IV: pH dysregulation of the mucolipin-1 cation channel. Hum. Mol. Genet. 13, 617–627 (2004).
Futerman, A. H. (ed.) Ceramide Signaling (Kluwer Academic/Plenum Publishers, New York, 2003).
Hannun, Y. A. & Obeid, L. M. The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J. Biol. Chem. 277, 25847–25850 (2002).
Garcia-Barros, M. et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 300, 1155–1159 (2003).
Lozano, J. et al. Cell autonomous apoptosis defects in acid sphingomyelinase knockout fibroblasts. J. Biol. Chem. 276, 442–448 (2001).
Li, C. M. et al. Insertional mutagenesis of the mouse acid ceramidase gene leads to early embryonic lethality in homozygotes and progressive lipid storage disease in heterozygotes. Genomics 79, 218–224 (2002).
Hollak, C. E. M., van Weely, S., van Oers, M. H. J. & Aerts, J. M. F. G. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J. Clin. Invest. 93, 1288–1292 (1994).
Boot, R. G. et al. Marked elevation of the chemokine CCL18/PARC in Gaucher disease: a novel surrogate marker for assessing therapeutic intervention. Blood 103, 33–39 (2004).
Suzuki, K. et al. Neuronal accumulation of α- and β-synucleins in the brain of a GM2 gangliosidosis mouse model. Neuroreport 14, 551–554 (2003).
Kakela, R., Somerharju, P. & Tyynela, J. Analysis of phospholipid molecular species in brains from patients with infantile and juvenile neuronal-ceroid lipofuscinosis using liquid chromatography-electrospray ionization mass spectrometry. J. Neurochem. 84, 1051–1065 (2003).
Bodennec, J., Pelled, D., Riebeling, C., Trajkovic, S. & Futerman, A. H. Phosphatidylcholine synthesis is elevated in neuronal models of Gaucher disease due to direct activation of CTP:phosphocholine cytidylyltransferase by glucosylceramide. FASEB J. 16, 1814–1816 (2002).
Lloyd-Evans, E. et al. Glucosylceramide and glucosylsphingosine modulate calcium mobilization from brain microsomes via different mechanisms. J. Biol. Chem. 278, 23594–23599 (2003).
Pelled, D. et al. Inhibition of calcium uptake via the sarco/endoplasmic reticulum Ca2+-ATPase in a mouse model of Sandhoff disease and prevention by treatment with N-butyldeoxynojirimycin. J. Biol. Chem. 278, 29496–29501 (2003).
LaPlante, J. M. et al. Identification and characterization of the single channel function of human mucolipin-1 implicated in mucolipidosis type IV, a disorder affecting the lysosomal pathway. FEBS Lett. 532, 183–187 (2002).
Berridge, M. J., Bootman, M. D. & Lipp, P. Calcium a life and death signal. Nature 395, 645–648 (1998).
Wada, R., Tifft, C. J. & Proia, R. L. Microglial activation precedes acute neurodegeneration in Sandhoff disease and is suppressed by bone marrow transplantation. Proc. Natl Acad. Sci. USA 97, 10954–10959 (2000). One of the first studies to delineate the biochemical and cellular sequence of events that lead from the accumulation of an undegraded substrate (in this case, GM2 ganglioside) to cellular, tissue and organ pathology.
Myerowitz, R. et al. Molecular pathophysiology in Tay–Sachs and Sandhoff diseases as revealed by gene expression profiling. Hum. Mol. Genet. 11, 1343–1350 (2002).
Jeyakumar, M. et al. Central nervous system inflammation is a hallmark of pathogenesis in mouse models of GM1 and GM2 gangliosidosis. Brain 126, 974–987 (2003).
Ohmi, K. et al. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc. Natl Acad. Sci. USA 100, 1902–1907 (2003).
Brooks, A. I., Chattopadhyay, S., Mitchison, H. M., Nussbaum, R. L. & Pearce, D. A. Functional categorization of gene expression changes in the cerebellum of a Cln3-knockout mouse model for Batten disease. Mol. Genet. Metab. 78, 17–30 (2003).
Meikle, P. J. & Hopwood, J. J. Lysosomal storage disorders: emerging therapeutic options require early diagnosis. Eur. J. Pediatr. 162 (Suppl. 1), S34–S37 (2003).
D'Azzo, A. Gene transfer strategies for correction of lysosomal storage disorders. Acta Haematol. 110, 71–85 (2003).
Cheng, S. H. & Smith, A. E. Gene therapy progress and prospects: gene therapy of lysosomal storage disorders. Gene Ther. 10, 1275–1281 (2003).
Grabowski, G. A. & Hopkin, R. J. Enzyme therapy for lysosomal storage disease: principles, practice, and prospects. Annu. Rev. Genomics Hum. Genet. 4, 403–436 (2003).
Zhu, Y., Li, X., Schuchman, E. H., Desnick, R. J. & Cheng, S. H. Dexamethasone-mediated upregulation of the mannose receptor improves the delivery of recombinant glucocerebrosidase to Gaucher macrophages. J. Pharmacol. Exp. Ther. 308, 705–711 (2004).
Dhami, R. & Schuchman, E. H. Mannose-6-phosphate receptor-mediated uptake is defective in acid sphingomyelinase-deficient macrophages: implications for Niemann–Pick disease enzyme replacement therapy. J. Biol. Chem. 279, 1526–1532 (2003).
Bengtsson, B. A., Johansson, J. O., Hollak, C., Linthorst, G. & FeldtRasmussen, U. Enzyme replacement in Anderson–Fabry disease. Lancet 361, 352 (2003).
Germain, D. P. Fabry disease: recent advances in enzyme replacement therapy. Expert Opin. Investig. Drugs 11, 1467–1476 (2002).
Desnick, R. J. & Schuchman, E. H. Enzyme replacement and enhancement therapies: lessons from lysosomal disorders. Nature Rev. Genet. 3, 954–966 (2002).
Fan, J. Q. A contradictory treatment for lysosomal storage disorders: inhibitors enhance mutant enzyme activity. Trends Pharmacol. Sci. 24, 355–360 (2003).
Futerman, A. H., Sussman, J. L., Horowitz, M., Silman, I. & Zimran, A. New directions in the treatment of Gaucher disease. Trends Pharmacol. Sci. 25, 147–151 (2004).
Dvir, H. et al. X-ray structure of human acid-β-glucosidase, the defective enzyme in Gaucher disease. EMBO Rep. 4, 704–709 (2003).
Mark, B. L. et al. Crystal structure of human β-hexosaminidase B: understanding the molecular basis of Sandhoff and Tay–Sachs disease. J. Mol. Biol. 327, 1093–1109 (2003).
Platt, F. M. et al. Prevention of lysosomal storage disease in Tay–Sachs mice treated with N-butyldeoxynojirimycin. Science 276, 428–431 (1997).
Cox, T. et al. Novel oral treatment of Gaucher's disease with N-butyldeoxynojirimycin (OGT 918) to decrease substrate biosynthesis. Lancet 355, 1481–1485 (2000). This paper provides a clinical description of Gaucher disease patients that had been treated using SRT — the first new treatment for Gaucher disease in over a decade.
Lachmann, R. H. Miglustat. Oxford GlycoSciences/Actelion. Curr. Opin. Investig. Drugs 4, 472–479 (2003).
Gahl, W. A., Thoene, J. G. & Schneider, J. A. Cystinosis. N. Engl. J. Med. 347, 111–121 (2002).
Gravel, R. A. et al. in The Metabolic and Molecular Bases of Inherited Disease (eds Scriver, C. R. et al.) 3827–3876 (McGraw–Hill Inc., Columbus, USA, 2001).
Beutler, E. & Grabowski, G. A. in The Metabolic and Molecular Bases of Inherited Disease (eds Scriver, C. R. et al.) 3635–3668 (McGraw–Hill Inc., Columbus, USA, 2001).
Brady, R. O. in Gaucher's Disease. (ed. Zimran, A.) 621–634 (Bailliere Tindall, London, 1997).
Luzio, J. P. et al. Membrane dynamics and the biogenesis of lysosomes. Mol. Membr. Biol. 20, 141–154 (2003).
Stahl, P. D. & Barbieri, M. A. Multivesicular bodies and multivesicular endosomes: the 'ins and outs' of endosomal traffic. Sci. STKE 141, PE32 (2002).
Hirsch, J. G., Fedorko, M. E. & Cohn, Z. A. Vesicle fusion and formation at the surface of pinocytic vacuoles in macrophages. J. Cell Biol. 38, 629–632 (1968).
Chow, A., Toomre, D., Garrett, W. & Mellman, I. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature 418, 988–994 (2002).
Blott, E. J. & Griffiths, G. M. Secretory lysosomes. Nature Rev. Mol. Cell Biol. 3, 122–131 (2002).
Murk, J. L. et al. Endosomal compartmentalization in three dimensions: implications for membrane fusion. Proc. Natl Acad. Sci. USA 100, 13332–13337 (2003).
Sawkar, A. R. et al. Chemical chaperones increase the cellular activity of N370S β-glucosidase: a therapeutic strategy for Gaucher disease. Proc. Natl Acad. Sci. USA 99, 15428–15433 (2002).
Scriver, C. R. et al. (eds) The Metabolic and Molecular Bases of Inherited Disease (McGraw–Hill Inc., Columbus, USA, 2001).
Wraith, J. E. Lysosomal disorders. Semin. Neonatol. 7, 75–83 (2002).
Meikle, P. J., Hopwood, J. J., Clague, A. E. & Carey, W. F. Prevalence of lysosomal storage disorders. J. Am. Med. Soc. 281, 249–254 (1999).
Altmann, S. W. et al. Niemann–Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science 303, 1201–1204 (2004).
Sleat, D. E. et al. Genetic evidence for nonredundant functional cooperativity between NPC1 and NPC2 in lipid transport. Proc. Natl Acad. Sci. USA 101, 5886–5891 (2004).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Related links
Related links
DATABASES
Nobel e-museum
Christian de Duve — Nobel lecture
Saccharomyces genome database
Swiss-Prot
Glossary
- MANNOSE-6-PHOSPHATE RECEPTORS
-
Receptors that are located at the trans-Golgi network and, at low levels, at the plasma membrane. They are responsible for targeting several soluble lysosomal hydrolases from the Golgi, through endosomes, to lysosomes.
- SPHINGOMYELIN
-
A sphingolipid that contains a phosphorylcholine headgroup.
- GLYCOSAMINOGLYCANS
-
Long, linear, charged polysaccharides that are composed of a repeating pair of sugars, of which one is an amino sugar.
- SPHINGOLIPIDS
-
Membrane lipids containing a ceramide backbone. Ceramide consists of a long chain sphingoid base, to which a fatty acid is linked through the amino group at C2. A headgroup is linked to the hydroxyl at C1.
- GANGLIOSIDE
-
A sialic-acid-containing glycosphingolipid that accumulates in the gangliosidoses.
- ABC TRANSPORTERS
-
A family of membrane transport proteins that use the energy of ATP hydrolysis to transport various molecules across the membrane.
- UNFOLDED PROTEIN RESPONSE
-
A cellular response that is triggered by the accumulation of misfolded proteins in the endoplasmic reticulum (ER) and that results in the transcriptional upregulation of ER chaperones and degradative enzymes.
- LYSOSOMOTRPHIC AGENTS
-
Molecules that move to the lysosome: mostly weak bases that diffuse across the lysosomal membrane as uncharged molecules and are trapped inside in their protonated form due to the low pH.
- EXOSOMES
-
50–80-nm membrane vesicles that are secreted into the extracellular milieu as a consequence of multivesicular-body fusion with the plasma membrane.
- GLYCOSPHINGOLIPID
-
A sphingolipid with an oligosaccharide headgroup. The sugar that is linked to the ceramide lipid backbone is generally glucose, but is galactose in galactosylceramide and sulphatide.
Rights and permissions
About this article
Cite this article
Futerman, A., van Meer, G. The cell biology of lysosomal storage disorders. Nat Rev Mol Cell Biol 5, 554–565 (2004). https://doi.org/10.1038/nrm1423
Issue Date:
DOI: https://doi.org/10.1038/nrm1423
This article is cited by
-
Tubular lysosome induction couples animal starvation to healthy aging
Nature Aging (2023)
-
Evaluation of oxidative stress and mitochondrial function in a type II mucopolysaccharidosis cellular model: in vitro effects of genistein and coenzyme Q10
Metabolic Brain Disease (2023)
-
Elucidating the functional impact of G137V and G144R variants in Maroteaux Lamy’s Syndrome by Molecular Dynamics Simulation
Molecular Diversity (2023)
-
Targeting neuronal lysosomal dysfunction caused by β-glucocerebrosidase deficiency with an enzyme-based brain shuttle construct
Nature Communications (2023)
-
Context-dependent transcriptional regulations of YAP/TAZ in stem cell and differentiation
Stem Cell Research & Therapy (2022)