Key Points
-
Interleukin-2 (IL-2) was discovered as a cytokine that supports the proliferation and differentiation of effector T cells. IL-2 initially entered clinical development based on this activity, in settings such as cancer and infectious diseases.
-
When used at high doses in patients with melanoma or renal cell carcinoma, IL-2 induces relatively rare (around 7%) but durable complete responses, at the expense of severe side effects. IL-2 has been approved by the US Food and Drug Administration for these indications.
-
Surprisingly, knockout of the genes encoding IL-2 or IL-2 receptor in mice led to severe autoimmunity, rather than the predicted immune deficiency. This was later explained by a defect in regulatory T (TReg) cells, and the discovery that IL-2 is the key cytokine for TReg cell function and survival.
-
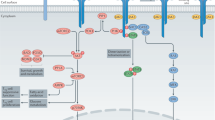
Further studies showed that IL-2 also favours the development of activated CD4+ T cells towards the T helper 1 (TH1), TH2, TH9 and peripherally induced TReg (pTReg) cell lineages, rather than the TH17 and T follicular helper (TFH) cell lineages. Thus, IL-2 contributes to tipping the immune balance towards regulation rather than inflammation, notably by favouring the differentiation of pTReg cells over TH17 cells; helps to control autoantibody generation by favouring T follicular regulatory cells over TFH cells; and helps to control autoreactive CD8+ effector T cells.
-
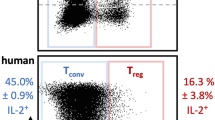
Proof-of-concept clinical trials have shown that at a low dose, IL-2 is well tolerated, induces TReg cells and mediates clinical improvements in autoimmune and inflammatory diseases; these findings have been confirmed in additional trials.
-
These trials and additional experimental work also showed that IL-2 mediates immunoregulation without immunosuppression. This opens the door for broad investigation of the therapeutic potential of low-dose IL-2 in a large number of autoimmune and inflammatory diseases.
Abstract
Depletion of regulatory T (TReg) cells in otherwise healthy individuals leads to multi-organ autoimmune disease and inflammation. This indicates that in a normal immune system, there are self-specific effector T cells that are ready to attack normal tissue if they are not restrained by TReg cells. The data imply that there is a balance between effector T cells and TReg cells in health and suggest a therapeutic potential of TReg cells in diseases in which this balance is altered. Proof-of-concept clinical trials, now supported by robust mechanistic studies, have shown that low-dose interleukin-2 specifically expands and activates TReg cell populations and thus can control autoimmune diseases and inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sakaguchi, S., Powrie, F. & Ransohoff, R. M. Re-establishing immunological self-tolerance in autoimmune disease. Nature Med. 18, 54–58 (2012).
Shevach, E. M. & Thornton, A. M. tTregs, pTregs, and iTregs: similarities and differences. Immunol. Rev. 259, 88–102 (2014).
Moraes-Vasconcelos, D., Costa-Carvalho, B. T., Torgerson, T. R. & Ochs, H. D. Primary immune deficiency disorders presenting as autoimmune diseases: IPEX and APECED. J. Clin. Immunol. 28 (Suppl. 1), 11–19 (2008).
Godfrey, V., Wilkinson, J. & Russell, L. X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am. J. Pathol. 138, 1379–1387 (1991).
Noack, M. & Miossec, P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 13, 668–677 (2014).
Kim, J. M., Rasmussen, J. P. & Rudensky, A. Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nature Immunol. 8, 191–197 (2007). T Reg cell depletion in otherwise healthy individuals triggers brisk autoimmune responses, underscoring the permanent suppression of potentially harmful effector T cells that occurs during homeostasis.
Marek-Trzonkowska, N. et al. Administration of CD4+CD25highCD127− regulatory T cells preserves β-cell function in type 1 diabetes in children. Diabetes Care 35, 1817–1820 (2012).
Malek, T. R. The biology of interleukin-2. Annu. Rev. Immunol. 26, 453–479 (2008).
Boyman, O. & Sprent, J. The role of interleukin-2 during homeostasis and activation of the immune system. Nature Rev. Immunol. 12, 180–190 (2012).
Liao, W., Lin, J. X. & Leonard, W. J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 38, 13–25 (2013).
Saadoun, D. et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N. Engl. J. Med. 365, 2067–2077 (2011). The first clinical trial showing that low-dose IL-2 is safe and clinically efficacious, and that it induces T Reg cells, in an autoimmune disease.
Koreth, J. et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N. Engl. J. Med. 365, 2055–2066 (2011). The first clinical trial showing that low-dose IL-2 is safe and clinically efficacious, and that it induces T Reg cells, in an alloimmune inflammatory disease.
Robb, R. J. Interleukin 2: the molecule and its function. Immunol. Today 5, 203–209 (1984).
Sadlack, B. et al. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 75, 253–261 (1993). The first evidence that IL-2 is involved in preventing autoimmune diseases.
Suzuki, H. et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science 268, 1472–1476 (1995).
Willerford, D. M. et al. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity 3, 521–530 (1995).
Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155, 1151–1164 (1995). The discovery of T Reg cells and their role in preventing autoimmune diseases.
Malek, T. R., Yu, A., Vincek, V., Scibelli, P. & Kong, L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice. Implications for the nonredundant function of IL-2. Immunity 17, 167–178 (2002).
Vang, K. B. et al. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J. Immunol. 181, 3285–3290 (2008).
Fontenot, J. D., Rasmussen, J. P., Gavin, M. A. & Rudensky, A. Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nature Immunol. 6, 1142–1151 (2005).
Barron, L. et al. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J. Immunol. 185, 6426–6430 (2010).
Feng, Y. et al. Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell 158, 749–763 (2014).
Ohkura, N. et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity 37, 785–799 (2012).
Williams, M. A., Tyznik, A. J. & Bevan, M. J. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 441, 890–893 (2006).
Kalia, V. et al. Prolonged interleukin-2Rα expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity 32, 91–103 (2010).
Pipkin, M. E. et al. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity 32, 79–90 (2010).
Setoguchi, R., Hori, S., Takahashi, T. & Sakaguchi, S. Homeostatic maintenance of natural Foxp3+ CD25+ CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J. Exp. Med. 201, 723–735 (2005).
Bilate, A. M. & Lafaille, J. J. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu. Rev. Immunol. 30, 733–758 (2012).
Cote-Sierra, J. et al. Interleukin 2 plays a central role in TH2 differentiation. Proc. Natl Acad. Sci. USA 101, 3880–3885 (2004).
Liao, W. et al. Opposing actions of IL-2 and IL-21 on Th9 differentiation correlate with their differential regulation of BCL6 expression. Proc. Natl Acad. Sci. USA 111, 3508–3513 (2014).
Liao, W., Lin, J.-X., Wang, L., Li, P. & Leonard, W. J. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nature Immunol. 12, 551–559 (2011).
Laurence, A. et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 26, 371–381 (2007).
Ballesteros-Tato, A. et al. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity 36, 847–856 (2012).
Linterman, M. A. et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nature Med. 17, 975–982 (2011).
Chung, Y. et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nature Med. 17, 983–988 (2011).
Wollenberg, I. et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J. Immunol. 187, 4553–4560 (2011).
Neill, D. R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 (2010).
Roediger, B. et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nature Immunol. 14, 564–573 (2013).
Van Gool, F. et al. Interleukin-5 producing group 2 innate lymphoid cells (ILC2) control eosinophilia induced by interleukin-2 therapy. Blood 124, 3572–3576 (2014).
Ribot, J. C., Ribeiro, S. T., Correia, D. V., Sousa, A. E. & Silva-Santos, B. Human γδ thymocytes are functionally immature and differentiate into cytotoxic type 1 effector T cells upon IL-2/IL-15 signaling. J. Immunol. 192, 2237–2243 (2014).
Yu, A., Zhu, L., Altman, N. H. & Malek, T. R. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity 30, 204–217 (2009). The molecular explanation for the specific effects of low-dose IL-2 on T Reg cells.
Yu, A. et al. Selective IL-2 responsiveness of regulatory T cells through multiple intrinsic mechanisms support the use of low-dose IL-2 therapy in type-1 diabetes. Diabetes http://dx.doi.org/10.2337/db14-1322 (2015).
Bensinger, S. J. et al. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J. Immunol. 172, 5287–5296 (2004).
Walsh, P. T. et al. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J. Clin. Invest. 116, 2521–2531 (2006).
Contractor, N. V. et al. Lymphoid hyperplasia, autoimmunity, and compromised intestinal intraepithelial lymphocyte development in colitis-free gnotobiotic IL-2-deficient mice. J. Immunol. 160, 385–394 (1998).
Schultz, M. et al. IL-2-deficient mice raised under germfree conditions develop delayed mild focal intestinal inflammation. Am. J. Physiol. 276, G1461–G1472 (1999).
Sharfe, N., Dadi, H. K., Shahar, M. & Roifman, C. M. Human immune disorder arising from mutation of the α chain of the interleukin-2 receptor. Proc. Natl Acad. Sci. USA 94, 3168–3171 (1997).
Smigiel, K. S. et al. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J. Exp. Med. 211, 121–136 (2014).
Gratz, I. K. et al. Cutting edge: memory regulatory T cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J. Immunol. 190, 4483–4487 (2013).
Raynor, J. et al. IL-15 fosters age-driven regulatory T cell accrual in the face of declining IL-2 levels. T Cell Biol. 4, 161 (2013).
Zier, K. S., Leo, M. M., Spielman, R. S. & Baker, L. Decreased synthesis of interleukin-2 (IL-2) in insulin-dependent diabetes mellitus. Diabetes 33, 552–555 (1984).
Kitas, G. D., Salmon, M., Farr, M., Gaston, J. S. & Bacon, P. A. Deficient interleukin 2 production in rheumatoid arthritis: association with active disease and systemic complications. Clin. Exp. Immunol. 73, 242–249 (1988).
Lieberman, L. A. & Tsokos, G. C. The IL-2 defect in systemic lupus erythematosus disease has an expansive effect on host immunity. J. Biomed. Biotechnol. 2010, 740619 (2010).
Boyman, O., Kovar, M., Rubinstein, M. P., Surh, C. D. & Sprent, J. Selective stimulation of T cell subsets with antibody–cytokine immune complexes. Science 311, 1924–1927 (2006). The first report of the properties of IL-2–antibody complexes.
Rouse, M., Nagarkatti, M. & Nagarkatti, P. S. The role of IL-2 in the activation and expansion of regulatory T-cells and the development of experimental autoimmune encephalomyelitis. Immunobiology 218, 674–682 (2013).
Hao, J. et al. Interleukin-2/interleukin-2 antibody therapy induces target organ natural killer cells that inhibit central nervous system inflammation. Ann. Neurol. 69, 721–734 (2011).
Grinberg-Bleyer, Y. et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J. Exp. Med. 207, 1871–1878 (2010).
Mizui, M. et al. IL-2 protects lupus-prone mice from multiple end-organ damage by limiting CD4−CD8− IL-17-producing T cells. J. Immunol. 193, 2168–2177 (2014).
Tang, Q. et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 28, 687–697 (2008).
Liu, R. et al. Expansion of regulatory T cells via IL-2/anti-IL-2 mAb complexes suppresses experimental myasthenia. Eur. J. Immunol. 40, 1577–1589 (2010).
Ait-Oufella, H. et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nature Med. 12, 178–180 (2006).
Mack, D. G. et al. Regulatory T cells modulate granulomatous inflammation in an HLA-DP2 transgenic murine model of beryllium-induced disease. Proc. Natl Acad. Sci. USA 111, 8553–8558 (2014).
Villalta, S. A. et al. Regulatory T cells suppress muscle inflammation and injury in muscular dystrophy. Sci. Transl. Med. 6, 258ra142 (2014).
Taniguchi, T. et al. Structure and expression of a cloned cDNA for human interleukin-2. Nature 302, 305–310 (1983).
Bindon, C. et al. Clearance rates and systemic effects of intravenously administered interleukin 2 (IL-2) containing preparations in human subjects. Br. J. Cancer 47, 123–133 (1983).
Lotze, M. T., Frana, L. W., Sharrow, S. O., Robb, R. J. & Rosenberg, S. A. In vivo administration of purified human interleukin 2. I. Half-life and immunologic effects of the Jurkat cell line-derived interleukin 2. J. Immunol. 134, 157–166 (1985).
Rosenberg, S. A. et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N. Engl. J. Med. 313, 1485–1492 (1985).
Rosenberg, S. A. et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA 271, 907–913 (1994). A large study of high-dose IL-2 in patients with cancer.
Yang, J. C. et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J. Clin. Oncol. 21, 3127–3132 (2003).
Rosenberg, S. A. IL-2: the first effective immunotherapy for human cancer. J. Immunol. 192, 5451–5458 (2014).
Ahmadzadeh, M. & Rosenberg, S. A. IL-2 administration increases CD4+ CD25hi Foxp3+ regulatory T cells in cancer patients. Blood 107, 2409–2414 (2006).
Zhang, H. et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nature Med. 11, 1238–1243 (2005).
Lemoine, F. M. et al. Massive expansion of regulatory T-cells following interleukin 2 treatment during a Phase I–II dendritic cell-based immunotherapy of metastatic renal cancer. Int. J. Oncol. 35, 569–581 (2009).
Weiss, L. et al. In vivo expansion of naive and activated CD4+CD25+FOXP3+ regulatory T cell populations in interleukin-2-treated HIV patients. Proc. Natl Acad. Sci. USA 107, 10632–10637 (2010).
Krieg, C., Letourneau, S., Pantaleo, G. & Boyman, O. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc. Natl Acad. Sci. USA 107, 11906–11911 (2010).
London, N. R. et al. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci. Transl. Med. 2, 23ra19 (2010).
Ferrara, J. L. Cytokine dysregulation as a mechanism of graft versus host disease. Curr. Opin. Immunol. 5, 794–799 (1993).
Boyer, O. et al. CD4+CD25+ regulatory T-cell deficiency in patients with hepatitis C-mixed cryoglobulinemia vasculitis. Blood 103, 3428–3430 (2004).
Saadoun, D. et al. Restoration of peripheral immune homeostasis after rituximab in mixed cryoglobulinemia vasculitis. Blood 111, 5334–5341 (2008).
Landau, D.-A. et al. Correlation of clinical and virologic responses to antiviral treatment and regulatory T cell evolution in patients with hepatitis C virus-induced mixed cryoglobulinemia vasculitis. Arthritis Rheum. 58, 2897–2907 (2008).
Alric, L. et al. Pilot study of interferon-α-ribavirin-interleukin-2 for treatment of nonresponder patients with severe liver disease infected by hepatitis C virus genotype 1. J. Viral Hepat. 13, 139–144 (2006).
Hartemann, A. et al. Low-dose interleukin 2 in patients with type 1 diabetes: a Phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 1, 295–305 (2013).
Castela, E. et al. Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatol. 150, 748–751 (2014).
Kennedy-Nasser, A. A. et al. Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin. Cancer Res. 20, 2215–2225 (2014).
Rosenzwajg, M. et al. Low-dose interleukin-2 fosters a dose-dependent regulatory-tuned milieu in T1D patients. J. Autoimmun. 58, 48–58 (2015).
Ito, S. et al. Ultra-low dose interleukin-2 promotes immune-modulating function of regulatory T cells and natural killer cells in healthy volunteers. Mol. Ther. J. Am. Soc. Gene Ther. 22, 1388–1395 (2014).
Matsuoka, K. et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci. Transl. Med. 5, 179ra43 (2013).
Aoyama, A. et al. Low-dose IL-2 for in vivo expansion of CD4+ and CD8+ regulatory T cells in nonhuman primates. Am. J. Transpl. 12, 2532–2537 (2012).
Chaput, N. et al. Identification of CD8+CD25+Foxp3+ suppressive T cells in colorectal cancer tissue. Gut 58, 520–529 (2009).
Humrich, J. Y. et al. Rapid induction of clinical remission by low-dose interleukin-2 in a patient with refractory SLE. Ann. Rheum. Dis. 74, 791–792 (2015).
Gutierrez-Ramos, J. C., Andreu, J. L., Revilla, Y., Vinuela, E. & Martinez, C. Recovery from autoimmunity of MRL/lpr mice after infection with an interleukin-2/vaccinia recombinant virus. Nature 346, 271–274 (1990).
Goudy, K. S. et al. Inducible adeno-associated virus-mediated IL-2 gene therapy prevents autoimmune diabetes. J. Immunol. 186, 3779–3786 (2011).
Churlaud, G. et al. Sustained stimulation and expansion of Tregs by IL-2 control autoimmunity without impairing immune responses to infection, vaccination and cancer. Clin. Immunol. 151, 114–126 (2014).
Yamaguchi, T., Wing, J. B. & Sakaguchi, S. Two modes of immune suppression by Foxp3+ regulatory T cells under inflammatory or non-inflammatory conditions. Semin. Immunol. 23, 424–430 (2011).
Billiard, F. et al. Regulatory and effector T cell activation levels are prime determinants of in vivo immune regulation. J. Immunol. 177, 2167–2174 (2006).
Sakaguchi, S., Vignali, D. A. A., Rudensky, A. Y., Niec, R. E. & Waldmann, H. The plasticity and stability of regulatory T cells. Nature Rev. Immunol. 13, 461–467 (2013).
Van Loosdregt, J. et al. Stabilization of the transcription factor Foxp3 by the deubiquitinase USP7 increases Treg-cell-suppressive capacity. Immunity 39, 259–271 (2013).
Chen, Z. et al. The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity 39, 272–285 (2013).
Li, Z. et al. PIM1 kinase phosphorylates the human transcription factor FOXP3 at serine 422 to negatively regulate its activity under inflammation. J. Biol. Chem. 289, 26872–26881 (2014).
Li, C. et al. MeCP2 enforces Foxp3 expression to promote regulatory T cells' resilience to inflammation. Proc. Natl Acad. Sci. USA 111, E2807–E2816 (2014).
Dietrich, T. et al. Local delivery of IL-2 reduces atherosclerosis via expansion of regulatory T cells. Atherosclerosis 220, 329–336 (2012).
Dinh, T. N. et al. Cytokine therapy with interleukin-2/anti-interleukin-2 monoclonal antibody complexes expands CD4+CD25+Foxp3+ regulatory T cells and attenuates development and progression of atherosclerosis. Circulation 126, 1256–1266 (2012).
D'Alessio, F. R. et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J. Clin. Invest. 119, 2898–2913 (2009).
Wilson, M. S. et al. Suppression of murine allergic airway disease by IL-2: anti-IL-2 monoclonal antibody-induced regulatory T cells. J. Immunol. 181, 6942–6954 (2008).
Rosenberg, H. F., Dyer, K. D. & Foster, P. S. Eosinophils: changing perspectives in health and disease. Nature Rev. Immunol. 13, 9–22 (2013).
Oliphant, C. J. et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4+ T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 41, 283–295 (2014).
Zheng, X. X. et al. Favorably tipping the balance between cytopathic and regulatory T cells to create transplantation tolerance. Immunity 19, 503–514 (2003).
Blackman, M. A. & Woodland, D. L. The narrowing of the CD8 T cell repertoire in old age. Curr. Opin. Immunol. 23, 537–542 (2011).
Thomas-Vaslin, V. et al. in Immunosuppression — Role in Health and Diseases. (eds Kapur, S. & Portela, M. B.) 125–147 (Intech, 2012).
Harvill, E. T. & Morrison, S. L. An IgG3-IL2 fusion protein activates complement, binds Fcγ RI, generates LAK activity and shows enhanced binding to the high affinity IL-2R. Immunotechnol. Int. J. Immunol. Eng. 1, 95–105 (1995).
Shanafelt, A. B. et al. A T-cell-selective interleukin 2 mutein exhibits potent antitumor activity and is well tolerated in vivo. Nature Biotech. 18, 1197–1202 (2000).
Levin, A. M. et al. Exploiting a natural conformational switch to engineer an interleukin-2 'superkine'. Nature 484, 529–533 (2012).
Rosalia, R. A., Arenas-Ramirez, N., Bouchaud, G., Raeber, M. E. & Boyman, O. Use of enhanced interleukin-2 formulations for improved immunotherapy against cancer. Curr. Opin. Chem. Biol. 23, 39–46 (2014).
Kaufman, H. L. et al. The society for immunotherapy of cancer consensus statement on tumour immunotherapy for the treatment of cutaneous melanoma. Nature Rev. Clin. Oncol. 10, 588–598 (2013).
Kasakura, S. & Lowenstein, L. A factor stimulating DNA synthesis derived from the medium of leukocyte cultures. Nature 208, 794–795 (1965).
Gordon, J. & MacLean, L. D. A lymphocyte-stimulating factor produced in vitro. Nature 208, 795–796 (1965).
Morgan, D. A., Ruscetti, F. W. & Gallo, R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science 193, 1007–1008 (1976). The first evidence of a T cell growth factor (IL-2) produced by activated T cells.
Gillis, S., Baker, P. E., Ruscetti, F. W. & Smith, K. A. Long-term culture of human antigen-specific cytotoxic T-cell lines. J. Exp. Med. 148, 1093–1098 (1978).
Smith, K. A. Interleukin-2: inception, impact, and implications. Science 240, 1169–1176 (1988).
Edwards, I. R. & Aronson, J. K. Adverse drug reactions: definitions, diagnosis, and management. Lancet 356, 1255–1259 (2000).
Prometheus Laboratories. Proleukin® (aldesleukin). [online], (2012).
Churlaud, G. et al. Human and mouse CD8+CD25+FOXP3+ regulatory T cells at steady state and during interleukin-2 therapy. Front. Immunol. http://dx.doi.org/10.3389/fimmu.2015.00171 (2015).
Acknowledgements
The work of D.K. is funded by French state funds within the Investissements d'Avenir programme (ANR-11-IDEX-0004-02; LabEx Transimmunom); the European Research Council Advanced Grant (ERC-2012-AdG, TRiPoD, Agreement number 322856); the Assistance Publique – Hopitaux de Paris, France; the Sorbonne University, Pierre and Marie Curie Medical School, Paris, France; the Institut National de la Santé et de la Recherche Médicale (INSERM); and Le Centre National de la Recherche Scientifique (CNRS).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
D.K. is an inventor of a patent application claiming low-dose IL-2 for therapy of autoimmune diseases, owned by his academic institution, and licensed to ILTOO Pharma, which he advises and in which he holds shares. A.K.A. declares no competing interests.
Related links
DATABASES
Supplementary information
Supplementary information S1 (table)
Main clinical and biological findings from high- to low-dose IL-2 clinical trials (PDF 269 kb)
Glossary
- T follicular regulatory cells
-
(TFR cells). Cells that are derived from thymus-derived regulatory T cells and share phenotypic characteristics with T follicular helper (TFH) cells, including the expression of the B cell follicle-homing receptor CXC-chemokine receptor 5. TFH cells control germinal centre B cells undergoing somatic hypermutation and selection that results in antibody affinity maturation. TFR cells reduce TFH cell and germinal centre B cell numbers.
- Type 2 innate lymphoid cells
-
(ILC2s). ILCs are rare cells from the lymphoid lineage — comprising the ILC1, ILC2 and ILC3 subsets — that produce many of the same cytokines as T cells but lack T cell antigen receptors. They have diverse roles in immune responses and inflammation. ILC2s produce type 2 cytokines, such as interleukin-5 (IL-5) and IL-13, and have key roles in anthelminthic responses and allergic lung inflammation.
- IL-2 international units
-
(IL-2 IU). The interleukin-2 (IL-2) IU is a biological activity determined by the ability to support the growth of an IL-2-dependent cell line. In practice, as the manufacture of IL-2 is standardized, 1.1 mg of IL-2 corresponds to 18 million IU.
- Immune dysregulation polyendocrinopathy enteropathy X-linked syndrome
-
(IPEX syndrome). This rare genetic autoimmune disease is caused by mutations in the FOXP3 gene (which encodes forkhead box P3). Affected males present with early-onset severe enteropathy that is usually accompanied by insulin-dependent diabetes (type 1). Other autoimmune manifestations include hypothyroidism, anaemia, thrombocytopenia and neutropenia. Increased serum IgE levels and dermatitis are often also present.
- IL-2–antibody complex
-
(IL-2c). A complex of interleukin-2 (IL-2) and IL-2-specific antibody that has a prolonged half-life compared with IL-2. Its biological activity depends on the antibody. Some complexes preferentially stimulate effector T cells, whereas others preferentially stimulate regulatory T cells.
- Vascular leak syndrome
-
(VLS). VLS (also known as capillary leak syndrome, systemic capillary leak syndrome or Clarkson disease) is characterized by a leakage of fluid from the circulatory system into the interstitial space, resulting in hypotension, haemoconcentration and hypoalbuminaemia.
- Cytokine storm
-
Also known as hypercytokinaemia. The systemic expression of a vigorous immune response resulting in the release of inflammatory mediators into the bloodstream, including cytokines, oxygen free radicals and coagulation factors. The primary symptoms of a cytokine storm are high fever, swelling and redness caused by vascular leak, extreme fatigue and nausea.
- Graft-versus-host disease
-
(GVHD). A common complication of allogeneic stem cell transplantation, in which immune cells from the graft recognize the recipient cells as foreign and attack them. The main target tissues are the liver, skin and gastrointestinal tract. It can be acute (within 100 days of transplantation) and life-threatening, or chronic.
- Hepatitis C virus-induced vasculitis
-
(HCV-induced vasculitis). 10–15% of patients with chronic HCV infection develop systemic cryoglobulinaemic vasculitis. Cryoglobulins are cold-insoluble immune complexes containing rheumatoid factor and polyclonal IgG that deposit on vascular endothelium, causing vasculitis in organs such as the skin and kidneys, and in peripheral nerves.
- Adverse events
-
Medical occurrences that are temporally (but not necessarily causally) associated with the use of a medicinal product. The severity of adverse events is graded from 1 to 4, with the grades representing mild, moderate, severe and potentially life-threatening events, respectively. Any adverse event that causes death, is life threatening, requires hospitalization, or results in persistent or significant disability or incapacity is considered a serious adverse event.
- Alopecia areata
-
A prevalent autoimmune disease (affecting 1.7% of the general population) that leads to hair loss on the scalp and other areas of the body. Infiltration of CD4+ and CD8+ T cells around hair follicles is associated with the condition.
- TRANSREG clinical trial
-
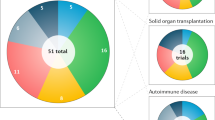
An open-label Phase II clinical trial investigating the stimulation of regulatory T cells by low-dose interleukin-2 in 11 autoimmune diseases: rheumatoid arthritis, ankylosing spondylitis, systemic lupus erythematosus, psoriasis, Behcet disease, Wegener granulomatosis, Takayasu disease, Crohn disease, ulcerative colitis, autoimmune hepatitis and sclerosing cholangitis (ClinicalTrials.gov identifier: NCT01988506).
Rights and permissions
About this article
Cite this article
Klatzmann, D., Abbas, A. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol 15, 283–294 (2015). https://doi.org/10.1038/nri3823
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3823
This article is cited by
-
Identifying the sensor elements of regulatory T cells in atherosclerosis
Nature Cardiovascular Research (2024)
-
Efficacy, Safety and the Lymphocyte Subset Changes of Low-Dose IL-2 in Patients with Autoimmune Rheumatic Diseases: A Systematic Review and Meta-Analysis
Rheumatology and Therapy (2024)
-
Molecular Engineering of Interleukin-2 for Enhanced Therapeutic Activity in Autoimmune Diseases
BioDrugs (2024)
-
Reigniting hope in cancer treatment: the promise and pitfalls of IL-2 and IL-2R targeting strategies
Molecular Cancer (2023)
-
Regulatory T cells in autoimmune kidney diseases and transplantation
Nature Reviews Nephrology (2023)