Key Points
-
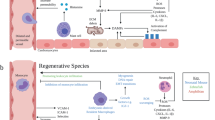
Cardiac injury can lead to cardiomyocyte death, intense inflammation, scar formation and, over time, adverse cardiac remodelling.
-
Following injury, cardiac inflammation is triggered by the release of conserved endogenous molecules and the production of pro-inflammatory cytokines and chemokines that lead to cellular infiltration.
-
Early activation of mast cells leads to neutrophil recruitment, a robust inflammatory response and tissue damage.
-
Recruited monocytes and resident macrophages modulate both tissue injury and tissue healing.
-
Macrophage origin may dictate function in the heart. Primitive embryonically derived macrophages mediate cardiac tissue repair, whereas bone marrow-derived monocytes contribute to inflammation following cardiac injury.
-
Lymphocytes and macrophages are involved in the complex transition from initial cardiac tissue inflammation to wound healing.
Abstract
Despite the advances that have been made in developing new therapeutics, cardiovascular disease remains the leading cause of worldwide mortality. Therefore, understanding the mechanisms underlying cardiovascular tissue injury and repair is of prime importance. Following cardiac tissue injury, the immune system has an important and complex role in driving both the acute inflammatory response and the regenerative response. This Review summarizes the role of the immune system in cardiovascular disease — focusing on the idea that the immune system evolved to promote tissue homeostasis following injury and/or infection, and that the inherent cost of this evolutionary development is unwanted inflammatory damage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ma, X., Cong, P., Hou, X., Edgecombe, G. D. & Strausfeld, N. J. An exceptionally preserved arthropod cardiovascular system from the early Cambrian. Nature Commun. 5, 3560 (2014).
Bier, E. & Bodmer, R. Drosophila, an emerging model for cardiac disease. Gene 342, 1–11 (2004).
Lozano, R. et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128 (2012).
Pinto, A. R. et al. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS ONE. 7, e36814 (2012).
Epelman, S. et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40, 91–104 (2014). This is the first study to show that cardiac macrophages are not a single population but are composed of distinct subsets, with different origins and functions.
Nahrendorf, M. et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 (2007). This report shows the initial characterization of cardiac macrophages in the resting heart and after ischaemic injury.
Swirski, F. K. & Nahrendorf, M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339, 161–166 (2013).
Choi, J. H. et al. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J. Exp. Med. 206, 497–505 (2009).
Frangogiannis, N. G. et al. Resident cardiac mast cells degranulate and release preformed TNF-α, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation 98, 699–710 (1998).
Zouggari, Y. et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nature Med. 19, 1273–1280 (2013).
Saxena, A. et al. Regulatory T cells are recruited in the infarcted mouse myocardium and may modulate fibroblast phenotype and function. Am. J. Physiol. Heart Circ. Physiol. 307, H1233–H1242 (2014).
Roger, V. L. et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 125, e2–e220 (2012).
Jacoby, D. & McKenna, W. J. Genetics of inherited cardiomyopathy. Eur. Heart J. 33, 296–304 (2012).
Sangiuliano, B., Perez, N. M., Moreira, D. F. & Belizario, J. E. Cell death-associated molecular-pattern molecules: inflammatory signaling and control. Mediators Inflamm. 2014, 821043 (2014).
Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12, 991–1045 (1994).
Janeway, C. A. Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54 Pt. 1, 1–13 (1989).
Mann, D. L., Topkara, V. K., Evans, S. & Barger, P. M. Innate immunity in the adult mammalian heart: for whom the cell tolls. Trans. Am. Clin. Climatol. Assoc. 121, 34–50 (2010).
Mezzaroma, E. et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc. Natl Acad. Sci. USA 108, 19725–19730 (2011).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Rathinam, V. A. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nature Immunol. 11, 395–402 (2010).
Frantz, S. et al. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J. Clin. Invest. 104, 271–280 (1999).
Birks, E. J. et al. Increased toll-like receptor 4 in the myocardium of patients requiring left ventricular assist devices. J. Heart Lung Transplant. 23, 228–235 (2004).
Kashiwagi, M. et al. Differential expression of Toll-like receptor 4 and human monocyte subsets in acute myocardial infarction. Atherosclerosis 221, 249–253 (2012).
Arslan, F. et al. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation 121, 80–90 (2010).
Fallach, R. et al. Cardiomyocyte Toll-like receptor 4 is involved in heart dysfunction following septic shock or myocardial ischemia. J. Mol. Cell Cardiol. 48, 1236–1244 (2010).
Binck, B. W. et al. Bone marrow-derived cells contribute to contractile dysfunction in endotoxic shock. Am. J. Physiol. Heart Circ. Physiol. 288, H577–H583 (2005).
Oyama, J. et al. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation 109, 784–789 (2004).
Tavener, S. A. et al. Immune cell Toll-like receptor 4 is required for cardiac myocyte impairment during endotoxemia. Circ. Res. 95, 700–707 (2004).
Oka, T. et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 485, 251–255 (2012). This is the first study to show that endogenous DAMPs are released from the myocardium during haemodynamic strain, a process that impairs cardiac function.
Geddes, K., Magalhaes, J. G. & Girardin, S. E. Unleashing the therapeutic potential of NOD-like receptors. Nature Rev. Drug Discov. 8, 465–479 (2009).
Kawaguchi, M. et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 123, 594–604 (2011).
McCartney, S. A. et al. RNA sensor-induced type I IFN prevents diabetes caused by a β cell-tropic virus in mice. J. Clin. Invest. 121, 1497–1507 (2011).
Philip, J., Xu, Z., Bowles, N. E. & Vallejo, J. G. Cardiac-specific overexpression of melanoma differentiation-associated gene-5 protects mice from lethal viral myocarditis. Circ. Heart Fail. 6, 326–334 (2013).
Lech, M. et al. Quantitative expression of C-type lectin receptors in humans and mice. Int. J. Mol. Sci. 13, 10113–10131 (2012).
Frangogiannis, N. G. & Entman, M. L. Chemokines in myocardial ischemia. Trends Cardiovasc. Med. 15, 163–169 (2005).
Frangogiannis, N. G. The mechanistic basis of infarct healing. Antioxid. Redox. Signal. 8, 1907–1939 (2006).
Chakraborti, T., Mandal, A., Mandal, M., Das, S. & Chakraborti, S. Complement activation in heart diseases. Role of oxidants. Cell Signal. 12, 607–617 (2000).
Foreman, K. E., Glovsky, M. M., Warner, R. L., Horvath, S. J. & Ward, P. A. Comparative effect of C3a and C5a on adhesion molecule expression on neutrophils and endothelial cells. Inflammation 20, 1–9 (1996).
Bhattacharya, K. et al. Mast cell deficient W/Wv mice have lower serum IL-6 and less cardiac tissue necrosis than their normal littermates following myocardial ischemia-reperfusion. Int. J. Immunopathol. Pharmacol. 20, 69–74 (2007).
Ayach, B. B. et al. Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction. Proc. Natl Acad. Sci. USA 103, 2304–2309 (2006).
Waskow, C., Paul, S., Haller, C., Gassmann, M. & Rodewald, H. R. Viable c-Kit(W/W) mutants reveal pivotal role for c-kit in the maintenance of lymphopoiesis. Immunity 17, 277–288 (2002).
Dreyer, W. J. et al. Kinetics of C5a release in cardiac lymph of dogs experiencing coronary artery ischemia-reperfusion injury. Circ. Res. 71, 1518–1524 (1992).
Newburger, P. E. & Dale, D. C. Evaluation and management of patients with isolated neutropenia. Semin. Hematol. 50, 198–206 (2013).
Singh, M. & Saini, H. K. Resident cardiac mast cells and ischemia-reperfusion injury. J. Cardiovasc. Pharmacol. Ther. 8, 135–148 (2003).
McDonald, B. et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330, 362–366 (2010).
Li, W. et al. Intravital 2-photon imaging of leukocyte trafficking in beating heart. J. Clin. Invest. 122, 2499–2508 (2012).
Gwechenberger, M. et al. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation 99, 546–551 (1999).
Youker, K. et al. Neutrophil adherence to isolated adult cardiac myocytes. Induction by cardiac lymph collected during ischemia and reperfusion. J. Clin. Invest. 89, 602–609 (1992). This important early study demonstrated the role of neutrophils in cardiomyocyte injury.
Entman, M. L. et al. Neutrophil induced oxidative injury of cardiac myocytes. A compartmented system requiring CD11b/CD18–ICAM-1 adherence. J. Clin. Invest. 90, 1335–1345 (1992).
Entman, M. L. et al. Neutrophil adherence to isolated adult canine myocytes. Evidence for a CD18-dependent mechanism. J. Clin. Invest. 85, 1497–1506 (1990).
Tyagi, S., Klickstein, L. B. & Nicholson-Weller, A. C5a-stimulated human neutrophils use a subset of β2 integrins to support the adhesion-dependent phase of superoxide production. J. Leukoc. Biol. 68, 679–686 (2000).
Kawakami, R. et al. Overexpression of brain natriuretic peptide facilitates neutrophil infiltration and cardiac matrix metalloproteinase-9 expression after acute myocardial infarction. Circulation 110, 3306–3312 (2004).
Romson, J. L. et al. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation 67, 1016–1023 (1983).
Jolly, S. R. et al. Reduction of myocardial infarct size by neutrophil depletion: effect of duration of occlusion. Am. Heart J. 112, 682–690 (1986).
Van Furth, R. & Cohn, Z. A. The origin and kinetics of mononuclear phagocytes. J. Exp. Med. 128, 415–435 (1968).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010).
Schulz, C. et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 (2012).
Yona, S. et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 38, 79–91 (2013).
Hashimoto, D. et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 38, 792–804 (2013).
Guilliams, M. et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 210, 1977–1992 (2013).
Jakubzick, C. et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 39, 599–610 (2013).
Hanna, R. N. et al. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nature Immunol. 12, 778–785 (2011).
Hettinger, J. et al. Origin of monocytes and macrophages in a committed progenitor. Nature Immunol. 14, 821–830 (2013).
Ingersoll, M. A. et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood 115, e10–e19 (2010).
Auffray, C. et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317, 666–670 (2007).
Carlin, L. M. et al. Nr4a1-dependent Ly6Clow monocytes monitor endothelial cells and orchestrate their disposal. Cell 153, 362–375 (2013).
Frangogiannis, N. G. et al. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation 115, 584–592 (2007). An important early study showing that CCR2 deficiency limits cardiac injury. Later studies would suggest this was due to a lack of blood monocytes.
Hilgendorf, I. et al. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ. Res. 114, 1611–1622 (2014).
Leuschner, F. et al. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ. Res. 107, 1364–1373 (2010).
Swirski, F. K. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 (2009). This is the first study to show that the spleen can be a source of monocytes following cardiac injury.
Leuschner, F. et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J. Exp. Med. 209, 123–137 (2012).
Molawi, K. et al. Progressive replacement of embryo-derived cardiac macrophages with age. J. Exp. Med. 211, 2151–2158 (2014).
Bracey, N. A. et al. Mitochondrial NLRP3 protein induces reactive oxygen species to promote Smad protein signaling and fibrosis independent from the inflammasome. J. Biol. Chem. 289, 19571–19584 (2014).
Bracey, N. A. et al. The Nlrp3 inflammasome promotes myocardial dysfunction in structural cardiomyopathy through interleukin-1β. Exp. Physiol. 98, 462–472 (2013).
Dunay, I. R. et al. Gr1+ inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity 29, 306–317 (2008).
Kim, Y. G. et al. The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes. Immunity 34, 769–780 (2011).
van Amerongen, M. J., Harmsen, M. C., van Rooijen, N., Petersen, A. H. & van Luyn, M. J. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am. J. Pathol. 170, 818–829 (2007).
Panizzi, P. et al. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J. Am. Coll. Cardiol. 55, 1629–1638 (2010).
Dewald, O. et al. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ. Res. 96, 881–889 (2005).
Leuschner, F. et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nature Biotech. 29, 1005–1010 (2011).
Serbina, N. V. & Pamer, E. G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nature Immunol. 7, 311–317 (2006).
Zhou, L. et al. Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ. Res. 98, 1177–1185 (2006).
Aurora, A. B. et al. Macrophages are required for neonatal heart regeneration. J. Clin. Invest. 124, 1382–1392 (2014).
Porrello, E. R. et al. Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080 (2011). This is the first study to show that the mammalian neonatal heart can regenerate fully, similarly to what is observed in more primitive organisms.
Lavine, K. et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl Acad. Sci. 111, 16029–16034 (2014). This is the first study to show that resident neonatal heart macrophages, not recruited monocytes, have a key role in neonatal heart regeneration. Similar findings were seen in reference 83.
Marodi, L. Neonatal innate immunity to infectious agents. Infect. Immun. 74, 1999–2006 (2006).
Munoz-Espin, D. et al. Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118 (2013).
Storer, M. et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155, 1119–1130 (2013).
Wan, E. et al. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ. Res. 113, 1004–1012 (2013).
Gautier, E. L. et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nature Immunol. 13, 1118–1128 (2012).
Mounier, R. et al. AMPKα1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell. Metab. 18, 251–264 (2013).
Arnold, L. et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069 (2007).
Hofmann, U. et al. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation 125, 1652–1663 (2012).
Matsumoto, K. et al. Regulatory T lymphocytes attenuate myocardial infarction-induced ventricular remodeling in mice. Int. Heart J. 52, 382–387 (2011).
Dobaczewski, M., Xia, Y., Bujak, M., Gonzalez-Quesada, C. & Frangogiannis, N. G. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am. J. Pathol. 176, 2177–2187 (2010).
Yang, Z. et al. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation 114, 2056–2064 (2006).
Clynes, R. Protective mechanisms of IVIG. Curr. Opin. Immunol. 19, 646–651 (2007).
Curato, C. et al. Identification of noncytotoxic and IL-10-producing CD8+AT2R+ T cell population in response to ischemic heart injury. J. Immunol. 185, 6286–6293 (2010).
Kaschina, E. et al. Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction? Circulation 118, 2523–2532 (2008).
Cohn, J. N. & Tognoni, G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N. Engl. J. Med. 345, 1667–1675 (2001).
Dickstein, K. & Kjekshus, J. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan. Lancet 360, 752–760 (2002).
Mehta, P. K. & Griendling, K. K. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 292, C82–C97 (2007).
Bouchentouf, M. et al. Induction of cardiac angiogenesis requires killer cell lectin-like receptor 1 and α4β7 integrin expression by NK cells. J. Immunol. 185, 7014–7025 (2010).
Fadok, V. A. et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Invest. 101, 890–898 (1998).
Voll, R. E. et al. Immunosuppressive effects of apoptotic cells. Nature 390, 350–351 (1997).
Xue, J. et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40, 274–288 (2014).
Stark, M. A. et al. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 22, 285–294 (2005).
Tan, W. et al. IL-17F/IL-17R interaction stimulates granulopoiesis in mice. Exp. Hematol. 36, 1417–1427 (2008).
Schwarzenberger, P. et al. Requirement of endogenous stem cell factor and granulocyte-colony-stimulating factor for IL-17-mediated granulopoiesis. J. Immunol. 164, 4783–4789 (2000).
Yan, X. et al. Deleterious effect of the IL-23/IL-17A axis and γδT cells on left ventricular remodeling after myocardial infarction. J. Am. Heart Assoc. 1, e004408 (2012).
Liao, Y. H. et al. Interleukin-17A contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil infiltration. J. Am. Coll. Cardiol. 59, 420–429 (2012).
Feng, W. et al. IL-17 induces myocardial fibrosis and enhances RANKL/OPG and MMP/TIMP signaling in isoproterenol-induced heart failure. Exp. Mol. Pathol. 87, 212–218 (2009).
Savvatis, K. et al. Interleukin-23 deficiency leads to impaired wound healing and adverse prognosis after myocardial infarction. Circ. Heart Fail. 7, 161–171 (2014).
Haudek, S. B. et al. Rho kinase-1 mediates cardiac fibrosis by regulating fibroblast precursor cell differentiation. Cardiovasc. Res. 83, 511–518 (2009).
Lim, D. S. et al. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation 103, 789–791 (2001).
Xu, J. et al. CCR2 mediates the uptake of bone marrow-derived fibroblast precursors in angiotensin II-induced cardiac fibrosis. Am. J. Physiol. Heart Circ. Physiol. 301, H538–H547 (2011).
Haudek, S. B. et al. Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J. Mol. Cell Cardiol. 49, 499–507 (2010).
Sadoshima, J. & Izumo, S. Molecular characterization of angiotensin II-induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ. Res. 73, 413–423 (1993).
Marko, L. et al. Interferon-γ signaling inhibition ameliorates angiotensin II-induced cardiac damage. Hypertension 60, 1430–1436 (2012).
Han, Y. L. et al. Reciprocal interaction between macrophages and T cells stimulates IFN-γ and MCP-1 production in Ang II-induced cardiac inflammation and fibrosis. PLoS ONE. 7, e35506 (2012).
Crawford, J. R., Haudek, S. B., Cieslik, K. A., Trial, J. & Entman, M. L. Origin of developmental precursors dictates the pathophysiologic role of cardiac fibroblasts. J. Cardiovasc. Transl. Res. 5, 749–759 (2012).
Coura, J. R. & Borges-Pereira, J. Chagas disease: 100 years after its discovery. A systemic review. Acta Trop. 115, 5–13 (2010).
Kindermann, I. et al. Update on myocarditis. J. Am. Coll. Cardiol. 59, 779–792 (2012).
Neu, N. et al. Cardiac myosin induces myocarditis in genetically predisposed mice. J. Immunol. 139, 3630–3636 (1987).
Sagar, S., Liu, P. P. & Cooper, L. T. Jr. Myocarditis. Lancet 379, 738–747 (2012).
Kuhl, U. et al. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation 111, 887–893 (2005).
Martino, T. A. et al. The coxsackie-adenovirus receptor (CAR) is used by reference strains and clinical isolates representing all six serotypes of coxsackievirus group B and by swine vesicular disease virus. Virology 271, 99–108 (2000).
Coyne, C. B. & Bergelson, J. M. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell 124, 119–131 (2006). This was the first study to show how cardiotropic viruses penetrate host epithelial barriers.
Liu, P. P. & Opavsky, M. A. Viral myocarditis: receptors that bridge the cardiovascular with the immune system? Circ. Res. 86, 253–254 (2000).
Kallewaard, N. L. et al. Tissue-specific deletion of the coxsackievirus and adenovirus receptor protects mice from virus-induced pancreatitis and myocarditis. Cell Host Microbe 6, 91–98 (2009).
Liu, P. et al. The tyrosine kinase p56lck is essential in coxsackievirus B3-mediated heart disease. Nature Med. 6, 429–434 (2000). The first study to detail the mechanisms through which cardiotropic viruses mediate intracellular signalling events and cell injury.
Irie-Sasaki, J. et al. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature 409, 349–354 (2001).
Valaperti, A. et al. Innate immune interleukin-1 receptor-associated kinase 4 exacerbates viral myocarditis by reducing CCR5+ CD11b+ monocyte migration and impairing interferon production. Circulation 128, 1542–1554 (2013).
Riad, A. et al. Myeloid differentiation factor-88 contributes to TLR9-mediated modulation of acute coxsackievirus B3-induced myocarditis in vivo. Am. J. Physiol. Heart Circ. Physiol. 298, H2024–H2031 (2010).
Fuse, K. et al. Myeloid differentiation factor-88 plays a crucial role in the pathogenesis of Coxsackievirus B3-induced myocarditis and influences type I interferon production. Circulation 112, 2276–2285 (2005).
Holm, G. H. et al. Interferon regulatory factor 3 attenuates reovirus myocarditis and contributes to viral clearance. J. Virol. 84, 6900–6908 (2010).
Opavsky, M. A. et al. Susceptibility to myocarditis is dependent on the response of αβ T lymphocytes to coxsackieviral infection. Circ. Res. 85, 551–558 (1999).
Shi, Y. et al. Regulatory T cells protect mice against coxsackievirus-induced myocarditis through the transforming growth factor β-coxsackie-adenovirus receptor pathway. Circulation 121, 2624–2634 (2010).
Mason, J. W. et al. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N. Engl. J. Med. 333, 269–275 (1995).
Kuhl, U. et al. Interferon-β treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation 107, 2793–2798 (2003).
Poss, K. D., Wilson, L. G. & Keating, M. T. Heart regeneration in zebrafish. Science 298, 2188–2190 (2002).
Laube, F., Heister, M., Scholz, C., Borchardt, T. & Braun, T. Re-programming of newt cardiomyocytes is induced by tissue regeneration. J. Cell Sci. 119, 4719–4729 (2006).
Godwin, J. W., Pinto, A. R. & Rosenthal, N. A. Macrophages are required for adult salamander limb regeneration. Proc. Natl Acad. Sci. USA 110, 9415–9420 (2013).
Buchmann, K. Evolution of innate immunity: clues from invertebrates via fish to mammals. Front. Immunol. 5, 459 (2014).
Epelman, S., Lavine, K. J. & Randolph, G. J. Origin and functions of tissue macrophages. Immunity 41, 21–35 (2014).
Dai, X. M. et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 99, 111–120 (2002).
McKercher, S. R. et al. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J. 15, 5647–5658 (1996).
Wiktor-Jedrzejczak, W. et al. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc. Natl Acad. Sci. USA 87, 4828–4832 (1990).
Nucera, S., Biziato, D. & De, P. M. The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. Int. J. Dev. Biol. 55, 495–503 (2011).
Fantin, A. et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116, 829–840 (2010).
Arnold, T. & Betsholtz, C. The importance of microglia in the development of the vasculature in the central nervous system. Vasc. Cell 5, 4 (2013).
Lobov, I. B. et al. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature 437, 417–421 (2005).
Naqvi, N. et al. A proliferative burst during preadolescence establishes the final cardiomyocyte number. Cell 157, 795–807 (2014).
Bergmann, O. et al. Evidence for cardiomyocyte renewal in humans. Science 324, 98–102 (2009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Granulation tissue
-
This is tissue that arises after cardiac tissue injury, when the replacement of cardiomyocytes with collagen and extracellular matrix occurs in order to maintain the integrity of the myocardial wall.
- Innate B cells
-
These cells are thought to become rapidly activated in the absence of classical T cell-dependent antigen presentation mechanisms. They are mobilized by other cell types and/or inflammatory triggers.
- ApoE-deficient mice
-
These mice are used to model atherosclerosis. They have increased total plasma cholesterol levels and increased bone marrow production of monocytes and heightened inflammatory responses.
Rights and permissions
About this article
Cite this article
Epelman, S., Liu, P. & Mann, D. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol 15, 117–129 (2015). https://doi.org/10.1038/nri3800
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3800
This article is cited by
-
The role of cardiac microenvironment in cardiovascular diseases: implications for therapy
Human Cell (2024)
-
MiR-206 may regulate mitochondrial ROS contribute to the progression of Myocardial infarction via TREM1
BMC Cardiovascular Disorders (2023)
-
Control of the post-infarct immune microenvironment through biotherapeutic and biomaterial-based approaches
Drug Delivery and Translational Research (2023)
-
Food-grade titanium dioxide and zinc oxide nanoparticles induce toxicity and cardiac damage after oral exposure in rats
Particle and Fibre Toxicology (2023)
-
Inflammation in myocardial infarction: roles of mesenchymal stem cells and their secretome
Cell Death Discovery (2022)