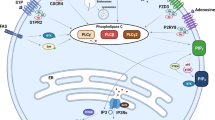
Key Points
-
Transformation of early B cells supplants the role of interleukin-7 receptor (IL-7R) and pre-B cell receptor (BCR) signalling.
-
Continual BCR signalling is required for the propagation of many B cell malignancies.
-
The transcription factor forkhead box protein O1 (FOXO1) and the B cell linker protein BLNK have distinct roles in early B cells versus peripheral B cells.
-
The crucial BCR signalling pathways regulated by phosphoinsoitide 3-kinase (PI3K), spleen tyrosine kinase (SYK) and Bruton tyrosine kinase (BTK) are valid therapeutic targets for B cell chronic lymphocytic leukaemia (B-CLL) and B cell non-Hodgkin's lymphoma (B-NHL).
-
Signalling via the BCR is modulated by additional receptors responding to microenvironmental cues.
Abstract
The B cell receptor (BCR) and its precursor (pre-BCR) control B cell homeostasis, differentiation and function. Moreover, aberrant pre-BCR and BCR signalling have a central role in B cell neoplasia; for example, enhanced positive signalling or disrupted negative signalling downstream of the pre-BCR promotes B cell acute lymphocytic leukaemia. The emerging distinctions between tonic and chronic active BCR signalling have contributed to the identification of oncogenic targets downstream of BCR signalling in mature B cell neoplasms. Indeed, the encouraging results of several ongoing clinical trials that target the activity of phosphoinositide 3-kinase δ-isoform (PI3Kδ), Bruton tyrosine kinase (BTK) or spleen tyrosine kinase (SYK) downstream of the BCR highlight the therapeutic potential of inhibiting BCR signalling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
23 August 2013
In the original version of this article, in the section under the subheading “NF-κB activity in B cell survival and neoplasia” on page 587, reference 97 should also have been included in the following sentence “In ABC-DLBCL, oncogenic amino acid substitutions in the coiled-coiled region of CARMA1 disrupt the intramolecular inhibition that is otherwise mediated by the linker region, which results in the enhanced association of CARMA1 with BCL-10 and in sustained NF-κB activation96 (Fig. 4).” The author apologizes for this error.
References
Corfe, S. A. & Paige, C. J. The many roles of IL-7 in B cell development; mediator of survival, proliferation and differentiation. Semin. Immunol. 24, 198–208 (2012).
Iacobucci, I. et al. Cytogenetic and molecular predictors of outcome in acute lymphocytic leukemia: recent developments. Curr. Hematol. Malig. Rep. 7, 133–143 (2012).
Zhang, J. et al. Key pathways are frequently mutated in high-risk childhood acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood 118, 3080–3087 (2011).
Kraus, M., Alimzhanov, M. B., Rajewsky, N. & Rajewsky, K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igα/β heterodimer. Cell 117, 787–800 (2004).
Lam, K. P., Kuhn, R. & Rajewsky, K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell 90, 1073–1083 (1997).
Kuppers, R. Mechanisms of B-cell lymphoma pathogenesis. Nature Rev. Cancer 5, 251–262 (2005).
Jiang, Y., Soong, T. D., Wang, L., Melnick, A. M. & Elemento, O. Genome-wide detection of genes targeted by non-Ig somatic hypermutation in lymphoma. PLoS ONE 7, e40332 (2012).
Robbiani, D. F. et al. AID is required for the chromosomal breaks in c-myc that lead to c-myc/IgH translocations. Cell 135, 1028–1038 (2008).
Takizawa, M. et al. AID expression levels determine the extent of cMyc oncogenic translocations and the incidence of B cell tumor development. J. Exp. Med. 205, 1949–1957 (2008).
Dominguez-Sola, D. et al. The proto-oncogene MYC is required for selection in the germinal center and cyclic reentry. Nature Immunol. 13, 1083–1091 (2012).
Calado, D. P. et al. The cell-cycle regulator c-Myc is essential for the formation and maintenance of germinal centers. Nature Immunol. 13, 1092–1100 (2012).
Bende, R. J., van Maldegem, F. & van Noesel, C. J. Chronic inflammatory disease, lymphoid tissue neogenesis and extranodal marginal zone B-cell lymphomas. Haematologica 94, 1109–1123 (2009).
Pierce, S. K. & Liu, W. The tipping points in the initiation of B cell signalling: how small changes make big differences. Nature Rev. Immunol. 10, 767–777 (2010).
Rawlings, D. J., Schwartz, M. A., Jackson, S. W. & Meyer-Bahlburg, A. Integration of B cell responses through Toll-like receptors and antigen receptors. Nature Rev. Immunol. 12, 282–294 (2012).
Batista, F. D., Treanor, B. & Harwood, N. E. Visualizing a role for the actin cytoskeleton in the regulation of B-cell activation. Immunol. Rev. 237, 191–204 (2010).
Herzog, S., Reth, M. & Jumaa, H. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling. Nature Rev. Immunol. 9, 195–205 (2009).
Ohnishi, K. & Melchers, F. The nonimmunoglobulin portion of lambda5 mediates cell-autonomous pre-B cell receptor signaling. Nature Immunol (2003).
Imamura, Y. et al. BLNK binds active H-Ras to promote B cell receptor-mediated capping and ERK activation. J. Biol. Chem. 284, 9804–9813 (2009).
Wossning, T. et al. Deregulated Syk inhibits differentiation and induces growth factor–independent proliferation of pre–B cells. J. Exp. Med. 203, 2829–2840 (2006).
Jumaa, H. et al. Deficiency of the adaptor SLP-65 in pre-B-cell acute lymphoblastic leukaemia. Nature 423, 452–456 (2003).
Flemming, A., Brummer, T., Reth, M. & Jumaa, H. The adaptor protein SLP-65 acts as a tumor suppressor that limits pre-B cell expansion. Nature Immunol. 4, 38–43 (2003). References 21and 22 establish the role of BLNK as a tumour suppressor in B-ALL.
Nakayama, J. et al. BLNK suppresses pre-B-cell leukemogenesis through inhibition of JAK3. Blood 113, 1483–1492 (2009).
Ochiai, K. et al. A self-reinforcing regulatory network triggered by limiting IL-7 activates pre-BCR signaling and differentiation. Nature Immunol. 13, 300–307 (2012).
Herzog, S. et al. SLP-65 regulates immunoglobulin light chain gene recombination through the PI(3)K-PKB-Foxo pathway. Nature Immunol. 9, 623–631 (2008).
Amin, R. H. & Schlissel, M. S. Foxo1 directly regulates the transcription of recombination-activating genes during B cell development. Nature Immunol. 9, 613–622 (2008).
Dengler, H. S. et al. Distinct functions for the transcription factor Foxo1 at various stages of B cell differentiation. Nature Immunol. 9, 1388–1398 (2008).
Fernandez de Mattos, S. et al. FoxO3a and BCR-ABL regulate cyclin D2 transcription through a STAT5/BCL6-dependent mechanism. Mol. Cell. Biol. 24, 10058–10071 (2004).
Nahar, R. et al. Pre-B cell receptor–mediated activation of BCL6 induces pre-B cell quiescence through transcriptional repression of MYC. Blood 118, 4174–4178 (2011).
Kersseboom, R. et al. Bruton's tyrosine kinase cooperates with the B cell linker protein SLP-65 as a tumor suppressor in Pre-B cells. J. Exp. Med. 198, 91–98 (2003).
Yasuda, T. et al. Erk kinases link pre-B cell receptor signaling to transcriptional events required for early B cell expansion. Immunity 28, 499–508 (2008).
Iritani, B. M., Forbush, K. A., Farrar, M. A. & Perlmutter, R. M. Control of B cell development by Ras-mediated activation of Raf. EMBO J. 16, 7019–7031 (1997).
Shaw, A. C., Swat, W., Ferrini, R., Davidson, L. & Alt, F. W. Activated Ras signals developmental progression of recombinase-activating gene (RAG)-deficient pro-B lymphocytes. J. Exp. Med. 189, 123–129 (1999).
Tartaglia, M. et al. Genetic evidence for lineage-related and differentiation stage-related contribution of somatic PTPN11 mutations to leukemogenesis in childhood acute leukemia. Blood 104, 307–313 (2004).
Xu, D. et al. Non–lineage/stage-restricted effects of a gain-of-function mutation in tyrosine phosphatase Ptpn11 (Shp2) on malignant transformation of hematopoietic cells. J. Exp. Med. 208, 1977–1988 (2011).
Klein, F. et al. The BCR–ABL1 kinase bypasses selection for the expression of a Pre–B cell receptor in Pre–B acute lymphoblastic leukemia cells. J. Exp. Med. 199, 673–685 (2004).
Duy, C. et al. BCL6 enables Ph+ acute lymphoblastic leukaemia cells to survive BCR-ABL1 kinase inhibition. Nature 473, 384–388 (2011). This paper provides insight into the molecular countermechanisms that arise in leukaemic cells exposed to cytotoxic tyrosine kinase inhibitors.
Duy, C. et al. BCL6 is critical for the development of a diverse primary B cell repertoire. J. Exp. Med. 207, 1209–1221 (2010).
Tang, T. T.-L. et al. The forkhead transcription factor AFX activates apoptosis by induction of the BCL-6 transcriptional repressor. J. Biol. Chem. 277, 14255–14265 (2002).
Liu, W., Meckel, T., Tolar, P., Won Sohn, H. & Pierce, S. K. Intrinsic properties of immunoglobulin IgG1 isotype-switched B cell receptors promote microclustering and the initiation of signaling. Immunity 32, 778–789 (2010).
Horikawa, K. et al. Enhancement and suppression of signaling by the conserved tail of IgG memory-type B cell antigen receptors. J. Exp. Med. 204, 759–769 (2007).
Waisman, A. et al. IgG1 B cell receptor signaling is inhibited by CD22 and promotes the development of B cells whose survival is less dependent on Igα/β. J. Exp. Med. 204, 747–758 (2007).
Roulland, S. et al. in Advances in Immunology (ed. Frederick, W. A.) 1–46 (Academic, 2011).
Ruminy, P. et al. The isotype of the BCR as a surrogate for the GCB and ABC molecular subtypes in diffuse large B-cell lymphoma. Leukemia 25, 681–688 (2011).
Sebastián, E. et al. Molecular characterization of immunoglobulin gene rearrangements in diffuse large B-cell lymphoma: antigen-driven origin and IGHV4-34 as a particular subgroup of the non-GCB subtype. Am. J. Pathol. 181, 1879–1888 (2012).
Stevenson, F. K., Krysov, S., Davies, A. J., Steele, A. J. & Packham, G. B-cell receptor signaling in chronic lymphocytic leukemia. Blood 118, 4313–4320 (2011).
Lossos, I. S. et al. Molecular analysis of immunoglobulin genes in diffuse large B-cell lymphomas. Blood 95, 1797–1803 (2000).
Bahler, D. W. & Levy, R. Clonal evolution of a follicular lymphoma: evidence for antigen selection. Proc. Natl Acad. Sci. 89, 6770–6774 (1992).
Refaeli, Y. et al. The B cell antigen receptor and overexpression of MYC can cooperate in the genesis of B cell lymphomas. PLoS Biol. 6, e152 (2008).
Krysov, S. et al. Surface IgM of CLL cells displays unusual glycans indicative of engagement of antigen in vivo. Blood 115, 4198–4205 (2010).
Radcliffe, C. M. et al. Human follicular lymphoma cells contain oligomannose glycans in the antigen-binding site of the B-cell receptor. J. Biol. Chem. 282, 7405–7415 (2007).
Minden, M. D.-v. et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature 489, 309–312 (2012).
Davis, R. E. et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 463, 88–92 (2010). This paper shows the essential role of BTK and CARMA11 in ABC–DLBCL. It also reports the occurrence of gain-of-function mutations in CD79B.
Xu, Y., Harder, K. W., Huntington, N. D., Hibbs, M. L. & Tarlinton, D. M. Lyn tyrosine kinase: accentuating the positive and the negative. Immunity 22, 9–18 (2005).
Chen, L. et al. Expression of ZAP-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia. Blood 100, 4609–4614 (2002).
Contri, A. et al. Chronic lymphocytic leukemia B cells contain anomalous Lyn tyrosine kinase, a putative contribution to defective apoptosis. J. Clin. Invest. 115, 369–378 (2005).
Gobessi, S. et al. Inhibition of constitutive and BCR-induced Syk activation downregulates Mcl-1 and induces apoptosis in chronic lymphocytic leukemia B cells. Leukemia 23, 686–697 (2008).
Buchner, M. et al. Spleen tyrosine kinase is overexpressed and represents a potential therapeutic target in chronic lymphocytic leukemia. Cancer Res. 69, 5424–5432 (2009).
Chen, L. et al. SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood 111, 2230–2237 (2008).
Pao, L. I. et al. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity 27, 35–48 (2007).
Oka, T. et al. Gene silencing of the tyrosine phosphatase SHP1 gene by aberrant methylation in leukemias/lymphomas. Cancer Res. 62, 6390–6394 (2002).
Grossmann, K. S., Rosário, M., Birchmeier, C. & Birchmeier, W. in Advances in Cancer Research (eds George, F. V. W. & George, K.) 53–89 (Academic, 2010).
Monroe, J. G. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nature Rev. Immunol. 6, 283–294 (2006).
Gururajan, M., Jennings, C. D. & Bondada, S. Cutting edge: constitutive B cell receptor signaling is critical for basal growth of B lymphoma. J. Immunol. 176, 5715–5719 (2006).
Ngo, V. N. et al. A loss-of-function RNA interference screen for molecular targets in cancer. Nature 441, 106–110 (2006).
Gazumyan, A., Reichlin, A. & Nussenzweig, M. C. Ig-β tyrosine residues contribute to the control of B cell receptor signaling by regulating receptor internalization. J. Exp. Med. 203, 1785–1794 (2006).
O'Neill, Shannon, K. et al. Monophosphorylation of CD79a and CD79b ITAM motifs initiates a SHIP-1 phosphatase-mediated inhibitory signaling cascade required for B cell anergy. Immunity 35, 746–756 (2011).
Alfarano, A. et al. An alternatively spliced form of CD79b gene may account for altered B-cell receptor expression in B-chronic lymphocytic leukemia. Blood 93, 2327–2335 (1999).
Cragg, M. S. et al. The alternative transcript of CD79b is overexpressed in B-CLL and inhibits signaling for apoptosis. Blood 100, 3068–3076 (2002).
Okkenhaug, K. et al. Impaired B and T cell antigen receptor signaling in p110δ PI 3-kinase mutant mice. Science 297, 1031–1034 (2002).
Clayton, E. et al. A crucial role for the p110δ subunit of phosphatidylinositol 3-kinase in B cell development and activation. J. Exp. Med. 196, 753–763 (2002).
Srinivasan, L. et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell 139, 573–586 (2009).
Kang, S., Denley, A., Vanhaesebroeck, B. & Vogt, P. K. Oncogenic transformation induced by the p110β, -γ, and -δ isoforms of class I phosphoinositide 3-kinase. Proc. Natl Acad. Sci. USA 103, 1289–1294 (2006).
Schmitz, R. et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature 490, 116–120 (2012).
Sander, S. et al. Synergy between PI3K signaling and MYC in Burkitt lymphomagenesis. Cancer Cell 22, 167–179 (2012). References 74 and 75 report the importance of the PI3K pathway and cyclin D3 in Burkitt's lymphoma.
Cabodi, S., del Pilar Camacho-Leal, M., Di Stefano, P. & Defilippi, P. Integrin signalling adaptors: not only figurants in the cancer story. Nature Rev. Cancer 10, 858–870 (2010).
Kloo, B. et al. Critical role of PI3K signaling for NF-κB-dependent survival in a subset of activated B-cell-like diffuse large B-cell lymphoma cells. Proc. Natl Acad. Sci. USA 108, 272–277 (2011).
Lenz, G. et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc. Natl Acad. Sci. USA 105, 13520–13525 (2008).
Rudelius, M. et al. Constitutive activation of Akt contributes to the pathogenesis and survival of mantle cell lymphoma. Blood 108, 1668–1676 (2006).
Sakai, A., Thieblemont, C., Wellmann, A., Jaffe, E. S. & Raffeld, M. PTEN gene alterations in lymphoid neoplasms. Blood 92, 3410–3415 (1998).
Miletic, A. V. et al. Coordinate suppression of B cell lymphoma by PTEN and SHIP phosphatases. J. Exp. Med. 207, 2407–2420 (2010). This paper establishes the importance of SHIP1 and PTEN as tumour suppressors in B cell lymphoma.
Baracho, G. V., Miletic, A. V., Omori, S. A., Cato, M. H. & Rickert, R. C. Emergence of the PI3-kinase pathway as a central modulator of normal and aberrant B cell differentiation. Curr. Opin. Immunol. 23, 178–183 (2011).
Cato, M. H., Chintalapati, S. K., Yau, I. W., Omori, S. A. & Rickert, R. C. Cyclin D3 is selectively required for proliferative expansion of germinal center B cells. Mol. Cell. Biol. 31, 127–137 (2011).
Peled, J. U. et al. Requirement for cyclin D3 in germinal center formation and function. Cell Res. 20, 631–646 (2010).
Poe, J. C., Minard-Colin, V., Kountikov, E. I., Haas, K. M. & Tedder, T. F. A. c-Myc and surface CD19 signaling amplification loop promotes B cell lymphoma development and progression in mice. J. Immunol. 189, 2318–2325 (2012).
Chung, E. Y. et al. CD19 is a major B cell receptor–independent activator of MYC-driven B-lymphomagenesis. J. Clin. Invest. 122, 2257–2266 (2012).
Xie, L. et al. FOXO1 is a tumor suppressor in classical Hodgkin lymphoma. Blood 119, 3503–3511 (2012).
Bouchard, C. et al. FoxO transcription factors suppress Myc-driven lymphomagenesis via direct activation of Arf. Genes Dev. 21, 2775–2787 (2007).
Ozenne, P., Eymin, B., Brambilla, E. & Gazzeri, S. The ARF tumor suppressor: structure, functions and status in cancer. Int. J. Cancer 127, 2239–2247 (2010).
Morin, R. D. et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature 476, 298–303 (2011).
Kingeter, L. M. & Schaefer, B. C. Malt1 and cIAP2-Malt1 as effectors of NF-κB activation: kissing cousins or distant relatives? Cell. Signal. 22, 9–22 (2010).
Schmitz, R. et al. TNFAIP3 (A20) is a tumor suppressor gene in Hodgkin lymphoma and primary mediastinal B cell lymphoma. J. Exp. Med. 206, 981–989 (2009).
Kato, M. et al. Frequent inactivation of A20 in B-cell lymphomas. Nature 459, 712–716 (2009).
Novak, U. et al. The NF-κB negative regulator TNFAIP3 (A20) is inactivated by somatic mutations and genomic deletions in marginal zone lymphomas. Blood 113, 4918–4921 (2009).
Tavares, R. M. et al. The ubiquitin modifying enzyme A20 restricts B cell survival and prevents autoimmunity. Immunity 33, 181–191 (2010).
Bende, R. J. et al. Among B cell non-Hodgkin's lymphomas, MALT lymphomas express a unique antibody repertoire with frequent rheumatoid factor reactivity. J. Exp. Med. 201, 1229–1241 (2005).
Lenz, G. et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science 319, 1676–1679 (2008).
Lamason, R. L., McCully, R. R., Lew, S. M. & Pomerantz, J. L. Oncogenic CARD11 mutations induce hyperactive signaling by disrupting autoinhibition by the PKC-responsive inhibitory domain. Biochemistry 49, 8240–8250 (2010).
Sommer, K. et al. Phosphorylation of the CARMA1 linker controls NF-κB activation. Immunity 23, 561–574 (2005).
Hailfinger, S. et al. Essential role of MALT1 protease activity in activated B cell-like diffuse large B-cell lymphoma. Proc. Natl Acad. Sci. USA 106, 19946–19951 (2009).
Ferch, U. et al. Inhibition of MALT1 protease activity is selectively toxic for activated B cell-like diffuse large B cell lymphoma cells. J. Exp. Med. 206, 2313–2320 (2009).
Yan, Q. et al. BCR and TLR signaling pathways are recurrently targeted by genetic changes in splenic marginal zone lymphomas. Haematologica 97, 595–598 (2012).
Rossi, D. et al. The coding genome of splenic marginal zone lymphoma: activation of NOTCH2 and other pathways regulating marginal zone development. J. Exp. Med. 209, 1537–1551 (2012).
Sagaert, X., Van Cutsem, E., De Hertogh, G., Geboes, K. & Tousseyn, T. Gastric MALT lymphoma: a model of chronic inflammation-induced tumor development. Nature Rev. Gastroenterol. Hepatol. 7, 336–346 (2010).
Pasparakis, M., Schmidt-Supprian, M. & Rajewsky, K. IκB kinase signaling is essential for maintenance of mature B cells. J. Exp. Med. 196, 743–752 (2002).
Li, Z. W., Omori, S. A., Labuda, T., Karin, M. & Rickert, R. C. IKKβ is required for peripheral B cell survival and proliferation. J. Immunol. 170, 4630–4637 (2003).
Leitges, M. et al. Immunodeficiency in protein kinase cβ-deficient mice. Science 273, 788–791 (1996).
Ruefli-Brasse, A. A., French, D. M. & Dixit, V. M. Regulation of NF-κB-dependent lymphocyte activation and development by paracaspase. Science 302, 1581–1584 (2003).
Ferch, U. et al. MALT1 directs B cell receptor-induced canonical nuclear factor-κB signaling selectively to the c-Rel subunit. Nature Immunol. 8, 984–991 (2007).
Jun, J. E. et al. Identifying the MAGUK protein Carma-1 as a central regulator of humoral immune responses and atopy by genome-wide mouse mutagenesis. Immunity 18, 751–762 (2003).
Kaileh, M. & Sen, R. NF-κB function in B lymphocytes. Immunol. Rev. 246, 254–271 (2012).
Newton, K. & Dixit, V. M. Mice lacking the CARD of CARMA1 exhibit defective B lymphocyte development and impaired proliferation of their B and T lymphocytes. Curr. Biol. 13, 1247–1251 (2003).
Su, T. T. et al. PKC-β controls IκB kinase lipid raft recruitment and activation in response to BCR signaling. Nature Immunol. 3, 780–786 (2002).
Rui, L., Schmitz, R., Ceribelli, M. & Staudt, L. M. Malignant pirates of the immune system. Nature Immunol. 12, 933–940 (2011).
Oh-hora, M., Johmura, S., Hashimoto, A., Hikida, M. & Kurosaki, T. Requirement for Ras guanine nucleotide releasing protein 3 in coupling phospholipase C-γ2 to Ras in B cell receptor signaling. J. Exp. Med. 198, 1841–1851 (2003).
Teixeira, C., Stang, S. L., Zheng, Y., Beswick, N. S. & Stone, J. C. Integration of DAG signaling systems mediated by PKC-dependent phosphorylation of RasGRP3. Blood 102, 1414–1420 (2003).
Coughlin, J. J., Stang, S. L., Dower, N. A. & Stone, J. C. RasGRP1 and RasGRP3 regulate B cell proliferation by facilitating B cell receptor-Ras signaling. J. Immunol. 175, 7179–7184 (2005).
Sanchez, A. et al. Epigenetic inactivation of the ERK inhibitor Spry2 in B-cell diffuse lymphomas. Oncogene 27, 4969–4972 (2008).
Frank, M. J. et al. Expression of sprouty2 inhibits B-cell proliferation and is epigenetically silenced in mouse and human B-cell lymphomas. Blood 113, 2478–2487 (2009).
Richards, J. D., Davé, S. H., Chou, C.-H. G., Mamchak, A. A. & DeFranco, A. L. Inhibition of the MEK/ERK signaling pathway blocks a subset of B cell responses to antigen. J. Immunol. 166, 3855–3864 (2001).
Petlickovski, A. et al. Sustained signaling through the B-cell receptor induces Mcl-1 and promotes survival of chronic lymphocytic leukemia B cells. Blood 105, 4820–4827 (2005).
Krysov, S. et al. Surface IgM stimulation induces MEK1/2-dependent MYC expression in chronic lymphocytic leukemia cells. Blood 119, 170–179 (2012).
Schrader, A. et al. Global gene expression changes of in vitro stimulated human transformed germinal centre B cells as surrogate for oncogenic pathway activation in individual aggressive B cell lymphomas. Cell Commun. Signal. 10, 43 (2012).
Platanias, L. C. Map kinase signaling pathways and hematologic malignancies. Blood 101, 4667–4679 (2003).
Messmer, B. T. et al. In vivo measurements document the dynamic cellular kinetics of chronic lymphocytic leukemia B cells. J. Clin. Invest. 115, 755–764 (2005).
O'Reilly, L. A. et al. MEK/ERK-mediated phosphorylation of Bim is required to ensure survival of T and B lymphocytes during mitogenic stimulation. J. Immunol. 183, 261–269 (2009).
Paterson, A. et al. Mechanisms and clinical significance of BIM phosphorylation in chronic lymphocytic leukemia. Blood 119, 1726–1736 (2012).
Bhalla, S. et al. The novel anti-MEK small molecule AZD6244 induces BIM-dependent and AKT-independent apoptosis in diffuse large B-cell lymphoma. Blood 118, 1052–1061 (2011).
Irish, J. M., Czerwinski, D. K., Nolan, G. P. & Levy, R. Altered B-cell receptor signaling kinetics distinguish human follicular lymphoma B cells from tumor-infiltrating nonmalignant B cells. Blood 108, 3135–3142 (2006).
Pham, L. V. & Ford, R. J. The role of BAFF-R dysregulation in B-lymphoid lineage malignancies. Cell Cycle 10, 191–190 (2011).
Rickert, R. C., Jellusova, J. & Miletic, A. V. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol. Rev. 244, 115–133 (2011).
Schweighoffer, E. et al. The BAFF receptor transduces survival signals by co-opting the B cell receptor signaling pathway. Immunity 38, 475–488 (2013). This paper indicates that maintenance of B cell homeostasis through BAFFR signalling might involve SYK activation downstream of the BCR.
Dye, J. R., Palvanov, A., Guo, B. & Rothstein, T. L. B cell receptor cross-talk: exposure to lipopolysaccharide induces an alternate pathway for B cell receptor-induced ERK phosphorylation and NF-κB activation. J. Immunol. 179, 229–235 (2007).
Mizuno, T. & Rothstein, T. L. B cell receptor (BCR) cross-talk: CD40 engagement creates an alternate pathway for BCR signaling that activates IκB kinase/IκBα/NF-κB without the need for PI3K and phospholipase Cγ. J. Immunol. 174, 6062–6070 (2005).
Guo, B. & Rothstein, T. L. B cell receptor (BCR) cross-talk: IL-4 creates an alternate pathway for BCR-induced ERK activation that is phosphatidylinositol 3-kinase independent. J. Immunol. 174, 5375–5381 (2005).
Ngo, V. N. et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 470, 115–119 (2011).
Arana, E., Harwood, N. E. & Batista, F. D. Regulation of integrin activation through the B-cell receptor. J. Cell Sci. 121, 2279–2286 (2008).
Lenz, G. et al. Stromal gene signatures in large-B-cell lymphomas. N. Engl. J. Med. 359, 2313–2323 (2008).
Spaargaren, M. et al. The B cell antigen receptor controls integrin activity through Btk and PLCγ2. J. Exp. Med. 198, 1539–1550 (2003).
Arana, E. et al. Activation of the small GTPase Rac2 via the B cell receptor regulates B cell adhesion and immunological-synapse formation. Immunity 28, 88–99 (2008).
Fruman, D. A. & Rommel, C. PI3Kδ inhibitors in cancer: rationale and serendipity merge in the clinic. Cancer Discov. 1, 562–572 (2011).
Burger, J. A. Targeting the microenvironment in chronic lymphocytic leukemia is changing the therapeutic landscape. Curr. Opin. Oncol. 24, 643–649 (2012).
Friedberg, J. W. et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 115, 2578–2585 (2010).
Chen, L. et al. ZAP-70 enhances IgM signaling independent of its kinase activity in chronic lymphocytic leukemia. Blood 111, 2685–2692 (2008).
Hoellenriegel, J. et al. Selective, novel spleen tyrosine kinase (Syk) inhibitors suppress chronic lymphocytic leukemia B-cell activation and migration. Leukemia 26, 1576–1583 (2012).
Burger, J. A. & Buggy, J. J. Emerging drug profiles: Bruton tyrosine kinase (BTK) inhibitor ibrutinib (PCI-32765). Leuk. Lymphoma 21 Feb 2013 [epub ahead of print].
Hendriks, R. W. Drug discovery: new Btk inhibitor holds promise. Nature Chem. Biol. 7, 4–5 (2011).
Seifert, M. et al. Cellular origin and pathophysiology of chronic lymphocytic leukemia. J. Exp. Med. 209, 2183–2198 (2012).
Acknowledgements
The work in the R.C.R.'s laboratory has been supported by US National Institutes of Health (NIH) grants AI041649, HL088686 and AI084883.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Glossary
- pro-B cells
-
Cells of the earliest committed stage of B cell development, which is characterized by ongoing immunoglobulin heavy-chain gene rearrangements and a dependence on interleukin-7 receptor signalling.
- Large pre-B cells
-
Cycling (also known as blasting) early B cells that express a functional pre-B cell receptor.
- B cell-derived acute lymphocytic leukaemia
-
(B-ALL). A leukaemia subtype that accounts for the majority of ALL cases and that is derived from the proliferative pro-B cell or pre-B cell compartment in the bone marrow. The genetic basis of B-ALL is usually attributed to the breakpoint cluster region (BCR)–ABL1 translocation or to mutations affecting one or more of the runt-related transcription factor 1 (RUNX1), pre-B cell leukaemia homeobox 1 (PBX1), mixed-lineage leukaemia (MLL), protein tyrosine phosphatase non-receptor type 11 (PTPN11) and RAS genes.
- Small pre-B cell stage
-
A resting stage in which the pre-B cell receptor is down-regulated and recombination activating gene (RAG)-mediated rearrangement of immunoglobulin light-chain genes occurs.
- Marginal zone B cells
-
A mature B cell subset that localizes to the splenic marginal zone and to the area proximal to the marginal sinus.
- Germinal centre
-
A microenvironment of the secondary lymphoid tissues (for example, the spleen, Peyer's patches and lymph nodes) composed of proliferating B cells (which are induced to mutate rearranged variable regions of their heavy-chain and light-chain genes after contact with antigens) and T helper cells. B cells with modified B cell receptors that cannot bind to antigens die by apoptosis, whereas those that bind to antigens are positively selected to exit the germinal centre as memory cells or plasmablasts.
- Activation-induced cytidine deaminase
-
(AID). A B cell-restricted deaminase that can induce double-strand DNA breaks. It is responsible for somatic hypermutation and class-switch recombination.
- Class-switch recombination
-
(CSR). A region-specific recombination process that occurs in activated B cells and results in a change in the class of antibody that is produced. Although the antigen specificity remains the same, the switch in the constant portion of the antibody confers distinct effector properties.
- B cell non-Hodgkin's lymphomas
-
(B-NHLs). A group of B cell malignancies that includes both aggressive (rapidly growing) and indolent (slow-growing) types, such as Burkitt's lymphoma, B cell chronic lymphocytic leukaemia (B-CLL), diffuse large B cell lymphoma, follicular lymphoma, precursor B cell-derived lymphocytic lymphoma and mantle cell lymphoma.
- Follicular lymphoma
-
The most common type of indolent B cell non-Hodgkin's lymphoma. It is characterized by the overexpression of the pro-survival factor B cell lymphoma 2 (BCL-2), which is caused by the translocation of BCL2 into the immunoglobulin heavy-chain locus.
- Burkitt's lymphoma
-
An aggressive type of B cell non-Hodgkin's lymphoma that is characterized by the translocation of MYC into the immunoglobulin heavy-chain locus.
- Diffuse large B cell lymphoma
-
(DLBCL). The most common type of B cell non-Hodgkin's lymphoma in adults, which is composed of the activated B cell (ABC)-derived and the germinal centre B cell (GCB)-derived subtypes. GCB-DLBCL is frequently associated with the constitutive expression of B cell lymphoma 6, whereas ABC-DLBCL is more aggressive and is characterized by chronic active B cell receptor signalling.
- B cell chronic lymphocytic leukaemia
-
(B-CLL). An adult form of B cell leukaemia derived from CD5+ mature or memory B cells. It consists of two subtypes — unmutated and mutated — that are defined by the presence of immunoglobulin mutations, which indicates passage through the germinal centre response. The unmutated subtype is associated with a worse clinical prognosis.
- MYC
-
The oncogene encoding a transcription factor that promotes proliferation, growth and global transcription. Translocation of MYC into the immunoglobulin locus is a hallmark of Burkitt's lymphoma.
- Marginal zone lymphoma
-
(MZL). A group of indolent marginal zone B cell-derived lymphomas that includes splenic marginal zone lymphoma, nodal marginal zone lymphoma and extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (also known as MALT lymphoma). MALT lymphoma can arise as a result of the overexpression of MALT lymphoma translocation protein 1 (MALT1) or the inhibitor of apoptosis protein 2 (IAP2)–MALT1 fusion protein. It can also arise from gastric inflammation caused by chronic Helicobacter pylori infection.
- JAK3–STAT5 signalling
-
The cytokine receptor-associated Janus kinases (JAKs) phosphorylate the signal transducer and activator of transcription (STAT) proteins, which facilitates their dimerization and transport to the nucleus to drive gene transcription.
- Forkhead box protein O1
-
(FOXO1). A member of the FOXO transcription factor family that is negatively regulated by phosphorylation by AKT or serine/threonine protein kinases.
- Philadelphia chromosome-expressing B-ALL
-
(Ph+ B cell acute lymphocytic leukaemia). Leukaemic cells that have the Philadelphia chromosome caused by a translocation and a juxtaposition of the breakpoint cluster region (BCR) and ABL1 genes.
- B cell co-receptor molecules
-
Numerous cell surface molecules that can positively or negatively regulate B cell activation. A subset of these are involved in regulating B cell receptor signalling in the resting state (CD19 and CD22), or following co-recognition of complement-bearing antigens (CD19 and CD21) or immune complexes (CD32).
- Class IA PI3K isoforms
-
Heterodimeric molecules consisting of a 110 kDa catalytic subunit and a smaller regulatory subunit. The catalytic subunits phosphoinositide 3-kinase α-isoform (PI3Kα; also known as p110α) and PI3Kβ (also known as p110β) are ubiquitously expressed, whereas PI3Kδ (also known as p110δ) is expressed primarily in haematopoietic cells. The regulatory subunits prevent the degradation of the catalytic subunit and inhibit its kinase activity, as well as promoting the SH2 domain-dependent recruitment of the holoenzyme to tyrosine-phosphorylated adaptor proteins such as CD19 and B cell adapter for PI3K (BCAP).
- B1 cells
-
A self-renewing subset of mature B cells that predominates in the pleural cavities and that is primarily responsible for the production of natural serum IgM.
- MicroRNA
-
(miRNA). A small RNA molecule that regulates the expression of genes through several mechanisms, including binding to the 3′ untranslated region (3′ UTR) of a target mRNA.
- CBM complex
-
A multienzyme complex consisting of CARD-containing MAGUK protein 1(CARMA1), B cell lymphoma 10 (BCL-10) and mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1) that activates nuclear factor-κB following the multimerization and the upstream activation by protein kinase Cβ in B cells.
- Inside-out signalling
-
The process through which intracellular signalling mechanisms result in the activation of cell surface receptors, such as integrins. By contrast, outside-in signalling is the process through which the ligation of cell surface receptors activates signalling pathways inside the cell.
Rights and permissions
About this article
Cite this article
Rickert, R. New insights into pre-BCR and BCR signalling with relevance to B cell malignancies. Nat Rev Immunol 13, 578–591 (2013). https://doi.org/10.1038/nri3487
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3487
This article is cited by
-
Dasatinib overcomes glucocorticoid resistance in B-cell acute lymphoblastic leukemia
Nature Communications (2023)
-
Idelalisib activates AKT via increased recruitment of PI3Kδ/PI3Kβ to BCR signalosome while reducing PDK1 in post-therapy CLL cells
Leukemia (2022)
-
Supplying the trip to antibody production—nutrients, signaling, and the programming of cellular metabolism in the mature B lineage
Cellular & Molecular Immunology (2022)
-
Characterization of the mechanism of action of lanraplenib, a novel spleen tyrosine kinase inhibitor, in models of lupus nephritis
BMC Rheumatology (2021)
-
Targeted PI3K/AKT-hyperactivation induces cell death in chronic lymphocytic leukemia
Nature Communications (2021)