Key Points
-
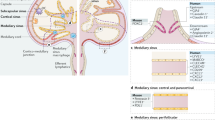
Lymph nodes are essential for the encounter of blood-derived naive lymphocytes with antigens and antigen-presenting cells, such as dendritic cells (DCs), which drain from peripheral tissues through interstitial fluids (lymph). The recirculation of lymphocytes through lymph nodes thus allows the extremely rare populations of naive lymphocytes specific for a given antigen to survey the lymph for the presence of their target antigen in any part of the body, thereby providing an effective immune surveillance for foreign invaders (such as viruses, bacteria and helminths) and for alterations in the body's own cells.
-
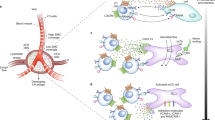
Naive lymphocytes circulating in the blood enter lymph nodes through high endothelial venules (HEVs), which are specialized blood vessels lined by plump endothelial cells. HEVs express high levels of sulphated sialomucins decorated with 6-sulpho sialyl Lewis X, which are ligands for the lymphocyte homing receptor L-selectin. Lymphocytes migrate through HEVs via a multistep adhesion cascade. First, rolling is initiated by L-selectin interactions with HEV sialomucins. Second, sticking (firm arrest) occurs after the activation of lymphocyte integrins by heparan sulphate-bound chemokines. Third, the lymphocytes crawl on the HEV luminal surface. Fourth, the cells rapidly transmigrate across the HEV endothelium via exit ramps.
-
CD11c+ DCs, which are strategically positioned close to HEV walls in vivo, have a crucial role in the regulation of HEV-mediated lymphocyte homing to lymph nodes. It has been shown recently that, in the absence of DCs, the mature adult HEV phenotype reverts to an immature neonatal phenotype, and lymphocyte sticking to HEVs is inhibited.
-
Rather than entering lymph nodes through HEVs (the 'blood' route), some immune cells use the 'lymph' route, particularly DCs that enter terminal lymphatics in the skin, circulate in lymph and then migrate to skin-draining lymph nodes through afferent lymphatics. Lymph nodes are often organized in chains, and naive T cells leaving a peripheral primary lymph node via efferent lymphatics, after entering through HEVs, may also enter downstream secondary lymph nodes through afferent lymphatics.
-
After entering the lymph node through HEVs or lymphatics, lymphocytes and DCs traffic to their respective subcompartments: the paracortical T cell areas for T cells and DCs; and the follicles for B cells. Stromal cell networks formed by fibroblastic reticular cells and follicular dendritic cells, together with stromal cell-derived lymphoid chemokines (namely CCL21, CCL19 and CXCL13), have key roles in guiding immune cells to these lymph node subcompartments.
-
After exploring a given lymph node for several hours, naive lymphocytes that do not encounter their target antigen leave the lymph node through efferent lymphatics. Recent two-photon intravital microscopy analyses indicate that B and T cells exit lymph nodes through cortical sinuses, after sensing sphingosine-1-phosphate (S1P) egress signals from lymphatics via S1P receptor type 1.
Abstract
In search of foreign antigens, lymphocytes recirculate from the blood, through lymph nodes, into lymphatics and back to the blood. Dendritic cells also migrate to lymph nodes for optimal interaction with lymphocytes. This continuous trafficking of immune cells into and out of lymph nodes is essential for immune surveillance of foreign invaders. In this article, we review our current understanding of the functions of high endothelial venules (HEVs), stroma and lymphatics in the entry, positioning and exit of immune cells in lymph nodes during homeostasis, and we highlight the unexpected role of dendritic cells in the control of lymphocyte homing through HEVs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gowans, J. L. The recirculation of lymphocytes from blood to lymph in the rat. J. Physiol. 146, 54–69 (1959).
Gowans, J. L. & Knight, E. J. The route of recirculation of lymphocytes in the rat. Proc. R. Soc. Lond. B 159, 257–282 (1964).
Marchesi, V. T. & Gowans, J. L. The migration of lymphocytes through the endothelium of venules in lymph nodes: an electron microscopic study. Proc. R. Soc. Lond. B 159, 283–290 (1964).
Butcher, E. C. & Picker, L. J. Lymphocyte homing and homeostasis. Science 272, 60–66 (1996).
von Andrian, U. H. & Mempel, T. R. Homing and cellular traffic in lymph nodes. Nature Rev. Immunol. 3, 867–878 (2003).
Girard, J. P. & Springer, T. A. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol. Today 16, 449–457 (1995).
Miyasaka, M. & Tanaka, T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nature Rev. Immunol. 4, 360–370 (2004).
Rosen, S. D. Ligands for L-selectin: homing, inflammation, and beyond. Annu. Rev. Immunol. 22, 129–156 (2004).
Forster, R., Davalos-Misslitz, A. C. & Rot, A. CCR7 and its ligands: balancing immunity and tolerance. Nature Rev. Immunol. 8, 362–371 (2008).
Cyster, J. G. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 23, 127–159 (2005).
Mueller, S. N. & Germain, R. N. Stromal cell contributions to the homeostasis and functionality of the immune system. Nature Rev. Immunol. 9, 618–629 (2009).
Bajenoff, M. et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 25, 989–1001 (2006). This study shows that B and T cells, after entering lymph nodes through HEVs, dynamically crawl along stromal cell networks towards the B cell follicles and T cell areas, respectively.
Cyster, J. G. & Schwab, S. R. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 30, 69–94 (2012).
Tomura, M. et al. Monitoring cellular movement in vivo with photoconvertible fluorescence protein “Kaede” transgenic mice. Proc. Natl Acad. Sci. USA 105, 10871–10876 (2008).
Moussion, C. & Girard, J. P. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature 479, 542–546 (2011). This study demonstrates that CD11c+ DCs are essential for the maintenance of HEVs and that DC-derived lymphotoxin is important for HEV-mediated lymphocyte homing to lymph nodes.
Wendland, M. et al. Lymph node T cell homeostasis relies on steady state homing of dendritic cells. Immunity 35, 945–957 (2011). This reference shows that restoration of CCR7 expression on CD11c+ DCs in CCR7-deficient mice is essential for lymphocyte homing to lymph nodes during homeostasis.
Braun, A. et al. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nature Immunol. 12, 879–887 (2011). This study reveals that naive T cells, like CD11c+ DCs, can enter lymph nodes through afferent lymphatics and use CCR7 for intranodal migration to the T cell areas.
Arnon, T. I. et al. GRK2-dependent S1PR1 desensitization is required for lymphocytes to overcome their attraction to blood. Science 333, 1898–1903 (2011). This reference shows that downregulation of S1PR1 is required for HEV-mediated homing of lymphocytes to lymph nodes.
Alvarez, D., Vollmann, E. H. & von Andrian, U. H. Mechanisms and consequences of dendritic cell migration. Immunity 29, 325–342 (2008).
Forster, R., Braun, A. & Worbs, T. Lymph node homing of T cells and dendritic cells via afferent lymphatics. Trends Immunol. 33, 271–280 (2012).
Thome, R. Endothelien als Phagocyten. Arch. Mikrosk. Anat. 52, 820–842 (1898).
von Schumacher, S. Ueber Phagocytose und die Abfuhrwege de Leucocyten in den Lymphdrusen. Arch. Mikrosk. Anat. 54, 311–328 (1899).
von Andrian, U. H. Intravital microscopy of the peripheral lymph node microcirculation in mice. Microcirculation 3, 287–300 (1996).
Drayton, D. L., Liao, S., Mounzer, R. H. & Ruddle, N. H. Lymphoid organ development: from ontogeny to neogenesis. Nature Immunol. 7, 344–353 (2006).
Martinet, L. et al. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 71, 5678–5687 (2011).
Grigorova, I. L. et al. Cortical sinus probing, S1P1-dependent entry and flow-based capture of egressing T cells. Nature Immunol. 10, 58–65 (2009).
Sinha, R. K., Park, C., Hwang, I. Y., Davis, M. D. & Kehrl, J. H. B lymphocytes exit lymph nodes through cortical lymphatic sinusoids by a mechanism independent of sphingosine-1-phosphate-mediated chemotaxis. Immunity 30, 434–446 (2009).
Pham, T. H., Okada, T., Matloubian, M., Lo, C. G. & Cyster, J. G. S1P1 receptor signaling overrides retention mediated by Gαi-coupled receptors to promote T cell egress. Immunity 28, 122–133 (2008).
Grigorova, I. L., Panteleev, M. & Cyster, J. G. Lymph node cortical sinus organization and relationship to lymphocyte egress dynamics and antigen exposure. Proc. Natl Acad. Sci. USA 107, 20447–20452 (2010). References 26–29 identify cortical sinuses as sites of S1PR1-dependent B and T cell egress from lymph nodes.
Carrasco, Y. R. & Batista, F. D. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity 27, 160–171 (2007).
Junt, T. et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450, 110–114 (2007).
Phan, T. G., Grigorova, I., Okada, T. & Cyster, J. G. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nature Immunol. 8, 992–1000 (2007).
Gretz, J. E., Norbury, C. C., Anderson, A. O., Proudfoot, A. E. & Shaw, S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 192, 1425–1440 (2000).
Sixt, M. et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22, 19–29 (2005).
Roozendaal, R. et al. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity 30, 264–276 (2009). References 33–35 demonstrate that stromal cells in lymph nodes form conduits that deliver small molecules from lymph to the T cell areas and B cell follicles.
Umemoto, E. et al. Nepmucin, a novel HEV sialomucin, mediates L-selectin-dependent lymphocyte rolling and promotes lymphocyte adhesion under flow. J. Exp. Med. 203, 1603–1614 (2006).
Uchimura, K. et al. A major class of L-selectin ligands is eliminated in mice deficient in two sulfotransferases expressed in high endothelial venules. Nature Immunol. 6, 1105–1113 (2005).
Kawashima, H. et al. N-acetylglucosamine-6-O-sulfotransferases 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nature Immunol. 6, 1096–1104 (2005). References 37 and 38 show that two sulphotransferases expressed by HEVs cooperatively control the synthesis of 6-sulpho sialyl Lewis X, the crucial carbohydrate determinant for L-selectin-mediated lymphocyte rolling along HEV walls.
Mitoma, J. et al. Critical functions of N-glycans in L-selectin-mediated lymphocyte homing and recruitment. Nature Immunol. 8, 409–418 (2007). This study reveals that both O -glycans and N -glycans are important for L-selectin-mediated lymphocyte homing to lymph nodes through HEVs.
Yeh, J. C. et al. Novel sulfated lymphocyte homing receptors and their control by a core1 extension β1,3-N-acetylglucosaminyltransferase. Cell 105, 957–969 (2001).
Mitsuoka, C. et al. Identification of a major carbohydrate capping group of the L-selectin ligand on high endothelial venules in human lymph nodes as 6-sulfo sialyl Lewis X. J. Biol. Chem. 273, 11225–11233 (1998).
Arata-Kawai, H. et al. Functional contributions of N- and O-glycans to L-selectin ligands in murine and human lymphoid organs. Am. J. Pathol. 178, 423–433 (2011).
Hirakawa, J. et al. Novel anti-carbohydrate antibodies reveal the cooperative function of sulfated N- and O-glycans in lymphocyte homing. J. Biol. Chem. 285, 40864–40878 (2010).
Streeter, P. R., Rouse, B. T. & Butcher, E. C. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J. Cell Biol. 107, 1853–1862 (1988).
Maly, P. et al. The α(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell 86, 643–653 (1996).
Yang, W. H., Nussbaum, C., Grewal, P. K., Marth, J. D. & Sperandio, M. Coordinated roles of ST3Gal-VI and ST3Gal-IV sialyltransferases in the synthesis of selectin ligands. Blood 120, 1015–1026 (2012).
Girard, J. P. & Springer, T. A. Cloning from purified high endothelial venule cells of hevin, a close relative of the antiadhesive extracellular matrix protein SPARC. Immunity 2, 113–123 (1995).
Girard, J. P., Baekkevold, E. S., Feliu, J., Brandtzaeg, P. & Amalric, F. Molecular cloning and functional analysis of SUT-1, a sulfate transporter from human high endothelial venules. Proc. Natl Acad. Sci. USA 96, 12772–12777 (1999).
Carriere, V. et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc. Natl Acad. Sci. USA 104, 282–287 (2007).
Kanda, H. et al. Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nature Immunol. 9, 415–423 (2008).
Nakasaki, T. et al. Involvement of the lysophosphatidic acid-generating enzyme autotaxin in lymphocyte–endothelial cell interactions. Am. J. Pathol. 173, 1566–1576 (2008).
Bao, X. et al. Endothelial heparan sulfate controls chemokine presentation in recruitment of lymphocytes and dendritic cells to lymph nodes. Immunity 33, 817–829 (2010).
Shamri, R. et al. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nature Immunol. 6, 497–506 (2005).
Shulman, Z. et al. Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity 30, 384–396 (2009).
Park, E. J. et al. Distinct roles for LFA-1 affinity regulation during T-cell adhesion, diapedesis, and interstitial migration in lymph nodes. Blood 115, 1572–1581 (2010).
Boscacci, R. T. et al. Comprehensive analysis of lymph node stroma-expressed Ig superfamily members reveals redundant and nonredundant roles for ICAM-1, ICAM-2, and VCAM-1 in lymphocyte homing. Blood 116, 915–925 (2010).
Park, C. et al. Lymph node B lymphocyte trafficking is constrained by anatomy and highly dependent upon chemoattractant desensitization. Blood 119, 978–989 (2012).
Mionnet, C. et al. High endothelial venules as traffic control points maintaining lymphocyte population homeostasis in lymph nodes. Blood 118, 6115–6122 (2011).
Ueha, S. et al. CCR7 mediates the migration of Foxp3+ regulatory T cells to the paracortical areas of peripheral lymph nodes through high endothelial venules. J. Leukoc. Biol. 82, 1230–1238 (2007).
Seth, S. et al. CCR7 essentially contributes to the homing of plasmacytoid dendritic cells to lymph nodes under steady-state as well as inflammatory conditions. J. Immunol. 186, 3364–3372 (2011).
Liu, K. et al. In vivo analysis of dendritic cell development and homeostasis. Science 324, 392–397 (2009).
Forster, R. et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33 (1999).
Chen, S., Kawashima, H., Lowe, J. B., Lanier, L. L. & Fukuda, M. Suppression of tumor formation in lymph nodes by L-selectin-mediated natural killer cell recruitment. J. Exp. Med. 202, 1679–1689 (2005).
Mebius, R. E., Streeter, P. R., Breve, J., Duijvestijn, A. M. & Kraal, G. The influence of afferent lymphatic vessel interruption on vascular addressin expression. J. Cell Biol. 115, 85–95 (1991).
Mebius, R. E. et al. Expression of GlyCAM-1, an endothelial ligand for L-selectin, is affected by afferent lymphatic flow. J. Immunol. 151, 6769–6776 (1993).
Lacorre, D. A. et al. Plasticity of endothelial cells: rapid dedifferentiation of freshly isolated high endothelial venule endothelial cells outside the lymphoid tissue microenvironment. Blood 103, 4164–4172 (2004).
Browning, J. L. et al. Lymphotoxin-β receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity 23, 539–550 (2005).
Liao, S. & Ruddle, N. H. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J. Immunol. 177, 3369–3379 (2006).
Drayton, D. L., Ying, X., Lee, J., Lesslauer, W. & Ruddle, N. H. Ectopic LTαβ directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J. Exp. Med. 197, 1153–1163 (2003).
Webster, B. et al. Regulation of lymph node vascular growth by dendritic cells. J. Exp. Med. 203, 1903–1913 (2006).
Randolph, G. J., Angeli, V. & Swartz, M. A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nature Rev. Immunol. 5, 617–628 (2005).
Pflicke, H. & Sixt, M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J. Exp. Med. 206, 2925–2935 (2009).
Ohl, L. et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 21, 279–288 (2004).
Tal, O. et al. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J. Exp. Med. 208, 2141–2153 (2011).
Mackay, C. R., Marston, W. L. & Dudler, L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J. Exp. Med. 171, 801–817 (1990).
Schumann, K. et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity 32, 703–713 (2010).
Qu, C. et al. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J. Exp. Med. 200, 1231–1241 (2004).
Lammermann, T. et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453, 51–55 (2008).
Bajenoff, M. et al. Highways, byways and breadcrumbs: directing lymphocyte traffic in the lymph node. Trends Immunol. 28, 346–352 (2007).
Bajenoff, M., Granjeaud, S. & Guerder, S. The strategy of T cell antigen-presenting cell encounter in antigen-draining lymph nodes revealed by imaging of initial T cell activation. J. Exp. Med. 198, 715–724 (2003).
Mempel, T. R., Henrickson, S. E. & Von Andrian, U. H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159 (2004).
Link, A. et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nature Immunol. 8, 1255–1265 (2007).
Worbs, T., Mempel, T. R., Bolter, J., von Andrian, U. H. & Forster, R. CCR7 ligands stimulate the intranodal motility of T lymphocytes in vivo. J. Exp. Med. 204, 489–495 (2007).
Okada, T. & Cyster, J. G. CC chemokine receptor 7 contributes to Gi-dependent T cell motility in the lymph node. J. Immunol. 178, 2973–2978 (2007).
Cahalan, M. D. & Parker, I. Choreography of cell motility and interaction dynamics imaged by two-photon microscopy in lymphoid organs. Annu. Rev. Immunol. 26, 585–626 (2008).
Miller, M. J., Wei, S. H., Parker, I. & Cahalan, M. D. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science 296, 1869–1873 (2002).
Qi, H., Egen, J. G., Huang, A. Y. & Germain, R. N. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science 312, 1672–1676 (2006).
Ansel, K. M. et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 406, 309–314 (2000).
Gatto, D., Paus, D., Basten, A., Mackay, C. R. & Brink, R. Guidance of B cells by the orphan G protein-coupled receptor EBI2 shapes humoral immune responses. Immunity 31, 259–269 (2009).
Pereira, J. P., Kelly, L. M., Xu, Y. & Cyster, J. G. EBI2 mediates B cell segregation between the outer and centre follicle. Nature 460, 1122–1126 (2009).
Katakai, T. et al. Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J. Immunol. 181, 6189–6200 (2008).
Mandala, S. et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296, 346–349 (2002).
Matloubian, M. et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004).
Pham, T. H. et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. 207, 17–27 (2010).
Schwab, S. R. et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 309, 1735–1739 (2005).
Lo, C. G., Xu, Y., Proia, R. L. & Cyster, J. G. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J. Exp. Med. 201, 291–301 (2005).
Pabst, O. et al. Enhanced FTY720-mediated lymphocyte homing requires Gαi signaling and depends on β2 and β7 integrin. J. Immunol. 176, 1474–1480 (2006).
Shiow, L. R. et al. CD69 acts downstream of interferon-α/β to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440, 540–544 (2006).
Wei, S. H. et al. Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nature Immunol. 6, 1228–1235 (2005).
Baluk, P. et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 204, 2349–2362 (2007).
Acknowledgements
We thank M. Sixt and A. Peixoto for helpful comments on the manuscript. Work in the laboratory of J.-P.G. is supported by grants from Fondation ARC pour la Recherche sur le Cancer, Agence Nationale de la Recherche (ANR), Institut National du Cancer (INCA), Fondation RITC and Région Midi-Pyrénées. Research by R.F. is supported by Deutsche Forschungsgemeinschaft (DFG) grants SFB621-A1, SFB738-B5, SFB587-B3, SFB900-B1 and KFO 250-FO 334/2-1. We regret that, owing to space limitations, we could not always quote the work of colleagues who have contributed to the field.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Lymph
-
Interstitial protein-poor aqueous fluid in the extravascular space that is channelled in lymphatic vessels and returned to the circulation via the thoracic duct.
- High endothelial venules
-
(HEVs). Specialized venules (small veins that join capillaries to larger veins) that are lined by plump endothelial cells. HEVs occur in secondary lymphoid organs, except the spleen, and are the main sites of lymphocyte entry from the blood.
- Fibroblastic reticular cells
-
(FRCs). Specialized reticular fibroblasts located in the T cell areas of lymph nodes and other secondary lymphoid organs that produce collagen-rich reticular fibres and form stromal networks and conduits that are important for the trafficking of immune cells.
- Follicular dendritic cells
-
(FDCs). Specialized reticular fibroblasts located in B cell follicles of lymph nodes and other secondary lymphoid organs that present intact antigens to B cells.
- Cortical sinuses
-
Blind-ended lymphatic vessels located in the T cell areas of lymph nodes that mediate the exit of B and T cells from the lymph nodes.
- Two-photon intravital microscopy
-
A fluorescence imaging technique that combines laser-scanning confocal microscopy with long-wavelength multiphoton fluorescence excitation to capture high-resolution three-dimensional images of fluorescent cells or tissues in living animals.
- Haptotactic gradients
-
Gradients of surface-bound ligands that promote directional, receptor-dependent migration of cells towards areas of higher concentrations.
- Pertussis toxin
-
A toxin that blocks Gαi-coupled receptor signalling (including chemokine receptor signalling) by catalysing ADP ribosylation of the Gαi subunit.
Rights and permissions
About this article
Cite this article
Girard, JP., Moussion, C. & Förster, R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol 12, 762–773 (2012). https://doi.org/10.1038/nri3298
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3298