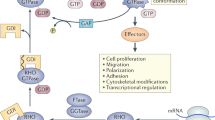
Key Points
-
In mammals, the Rho family of GTPases has 23 members, which are regulated by 79 guanine nucleotide exchange factors (GEFs), 65 GTPase-activating proteins (GAPs) and 3 guanine nucleotide dissociation inhibitors (GDIs). About half of these are expressed by lymphocytes.
-
RAC1 and RAC2 have overlapping and redundant roles in B and T cell development, activation and migration.
-
RHOH has an important role in T cell activation as an adaptor protein, recruiting ζ-chain-associated protein kinase of 70 kDa (ZAP70) to the plasma membrane.
-
The VAV1, VAV2 and VAV3 GEFs have overlapping and redundant roles in B and T cell development and activation but not migration. VAV1 has GEF-independent functions, possibly as an adaptor protein.
-
The GEF DOCK2 (dedicator of cytokinesis2) is a crucial transducer of chemokine receptor signals in both B and T cells, and is also required for T cell activation.
-
The GEF SWAP70 (switch-associated protein 70)is required for polarization of B cells and transmigration across high endothelial venules. The related GEF IBP regulates T helper cell differentiation, in part by binding the transcription factor interferon-regulatory factor 4, a function that might be GEF independent.
Abstract
Rho family GTPases, and the proteins that regulate them, have important roles in many cellular processes, including cell division, survival, migration and adhesion. Although most of our understanding of these proteins has come from studies using cell lines, more recent gene targeting studies in mice are providing insights into the in vivo function of these proteins. Here we review recent progress revealing crucial roles for these proteins in lymphocyte development, activation, differentiation and migration. The emerging picture shows that Rho family GTPases transduce signals from receptors for antigens, chemokines and cytokines, as well as adhesion molecules and pattern recognition receptors, and that they function as focal points for crosstalk between different signalling pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Heasman, S. J. & Ridley, A. J. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nature Rev. Mol. Cell Biol. 9, 690–701 (2008).
Wennerberg, K. & Der, C. J. Rho-family GTPases: it's not only Rac and Rho (and I like it). J. Cell Sci. 117, 1301–1312 (2004).
Bustelo, X. R., Sauzeau, V. & Berenjeno, I. M. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays 29, 356–370 (2007).
Rossman, K. L., Der, C. J. & Sondek, J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nature Rev. Mol. Cell Biol. 6, 167–180 (2005).
Tcherkezian, J. & Lamarche-Vane, N. Current knowledge of the large RhoGAP family of proteins. Biol. Cell 99, 67–86 (2007).
DerMardirossian, C. & Bokoch, G. M. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 15, 356–363 (2005).
Croker, B. A. et al. The Rac2 guanosine triphosphatase regulates B lymphocyte antigen receptor responses and chemotaxis and is required for establishment of B-1a and marginal zone B lymphocytes. J. Immunol. 168, 3376–3386 (2002).
Walmsley, M. J. et al. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science 302, 459–462 (2003).
Gulbranson-Judge, A. et al. Defective immunoglobulin class switching in Vav-deficient mice is attributable to compromised T cell help. Eur. J. Immunol. 29, 477–487 (1999).
Doody, G. M. et al. Signal transduction through Vav-2 participates in humoral immune responses and B cell maturation. Nature Immunol. 2, 542–547 (2001).
Tedford, K. et al. Compensation between Vav-1 and Vav-2 in B cell development and antigen receptor signaling. Nature Immunol. 2, 548–555 (2001).
Fujikawa, K. et al. Vav1/2/3-null mice define an essential role for Vav family proteins in lymphocyte development and activation but a differential requirement in MAPK signaling in T and B cells. J. Exp. Med. 198, 1595–1608 (2003).
Vigorito, E., Gambardella, L., Colucci, F., McAdam, S. & Turner, M. Vav proteins regulate peripheral B-cell survival. Blood 106, 2391–2398 (2005).
Yamada, T., Kurosaki, T. & Hikida, M. Essential roles of mgcRacGAP in multilineage differentiation and survival of murine hematopoietic cells. Biochem. Biophys. Res. Commun. 372, 941–946 (2008).
Kawashima, T. et al. A Rac GTPase-activating protein, MgcRacGAP, is a nuclear localizing signal-containing nuclear chaperone in the activation of STAT transcription factors. Mol. Cell Biol. 29, 1796–1813 (2009).
Saci, A. & Carpenter, C. L. RhoA GTPase regulates B cell receptor signaling. Mol. Cell 17, 205–214 (2005).
Arana, E. et al. Activation of the small GTPase Rac2 via the B cell receptor regulates B cell adhesion and immunological-synapse formation. Immunity 28, 88–99 (2008).
Burbach, B. J., Medeiros, R. B., Mueller, K. L. & Shimizu, Y. T-cell receptor signaling to integrins. Immunol. Rev. 218, 65–81 (2007).
Vigorito, E. et al. Immunological function in mice lacking the Rac-related GTPase RhoG. Mol. Cell Biol. 24, 719–729 (2004).
Bachmann, M. F. et al. The guanine-nucleotide exchange factor Vav is a crucial regulator of B cell receptor activation and B cell responses to nonrepetitive antigens. J. Immunol. 163, 137–142 (1999).
Inabe, K. et al. Vav3 modulates B cell receptor responses by regulating phosphoinositide 3-kinase activation. J. Exp. Med. 195, 189–200 (2002).
Vigorito, E. et al. Vav-dependent and vav-independent phosphatidylinositol 3-kinase activation in murine B cells determined by the nature of the stimulus. J. Immunol. 173, 3209–3214 (2004).
Weber, M. et al. Phospholipase C-γ2 and Vav cooperate within signaling microclusters to propagate B cell spreading in response to membrane-bound antigen. J. Exp. Med. 205, 853–868 (2008).
Hebeis, B., Vigorito, E., Kovesdi, D. & Turner, M. Vav proteins are required for B-lymphocyte responses to LPS. Blood 106, 635–640 (2005).
Stephenson, L. M., Miletic, A. V., Kloeppel, T., Kusin, S. & Swat, W. Vav proteins regulate the plasma cell program and secretory Ig production. J. Immunol. 177, 8620–8625 (2006).
Missy, K. et al. αPIX Rho GTPase guanine nucleotide exchange factor regulates lymphocyte functions and antigen receptor signaling. Mol. Cell. Biol. 28, 3776–3789 (2008).
Girkontaite, I. et al. Lsc is required for marginal zone B cells, regulation of lymphocyte motility and immune responses. Nature Immunol. 2, 855–862 (2001).
Rubtsov, A. et al. Lsc regulates marginal-zone B cell migration and adhesion and is required for the IgM T-dependent antibody response. Immunity 23, 527–538 (2005). References 27 and 28 analyse the role of LSC, which is a RHOA GEF, in B cell development and provide insights into the regulation of marginal zone B cell migration and retention.
Fukui, Y. et al. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature 412, 826–831 (2001). This is the first description of the crucial role of DOCK2 in lymphocyte migration, which has since been analysed further in great detail.
Nombela-Arrieta, C. et al. Differential requirements for DOCK2 and phosphoinositide-3-kinase γ during T and B lymphocyte homing. Immunity 21, 429–441 (2004).
Nombela-Arrieta, C. et al. A central role for DOCK2 during interstitial lymphocyte motility and sphingosine-1-phosphate-mediated egress. J. Exp. Med. 204, 497–510 (2007).
Pearce, G. et al. Signaling protein SWAP-70 is required for efficient B cell homing to lymphoid organs. Nature Immunol. 7, 827–834 (2006).
Quemeneur, L., Angeli, V., Chopin, M. & Jessberger, R. SWAP-70 deficiency causes high-affinity plasma cell generation despite impaired germinal center formation. Blood 111, 2714–2724 (2008).
Hakem, A. et al. RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev. 19, 1974–1979 (2005).
Liu, A. X., Rane, N., Liu, J. P. & Prendergast, G. C. RhoB is dispensable for mouse development, but it modifies susceptibility to tumor formation as well as cell adhesion and growth factor signaling in transformed cells. Mol. Cell Biol. 21, 6906–6912 (2001).
Corre, I., Gomez, M., Vielkind, S. & Cantrell, D. A. Analysis of thymocyte development reveals that the GTPase RhoA is a positive regulator of T cell receptor responses in vivo. J. Exp. Med. 194, 903–914 (2001).
Galandrini, R., Henning, S. W. & Cantrell, D. A. Different functions of the GTPase Rho in prothymocytes and late pre-T cells. Immunity 7, 163–174 (1997).
Henning, S. W., Galandrini, R., Hall, A. & Cantrell, D. A. The GTPase Rho has a critical regulatory role in thymus development. EMBO J. 16, 2397–2407 (1997).
Mullin, M. et al. The RhoA transcriptional program in pre-T cells. FEBS Lett. 581, 4309–4317 (2007).
Gomez, M., Tybulewicz, V. & Cantrell, D. A. Control of pre-T cell proliferation and differentiation by the GTPase Rac-1. Nature Immunol. 1, 348–352 (2000).
Gomez, M., Kioussis, D. & Cantrell, D. A. The GTPase Rac-1 controls cell fate in the thymus by diverting thymocytes from positive to negative selection. Immunity 15, 703–713 (2001).
Lores, P., Morin, L., Luna, R. & Gacon, G. Enhanced apoptosis in the thymus of transgenic mice expressing constitutively activated forms of human Rac2GTPase. Oncogene 15, 601–605 (1997).
Dumont, C. et al. Rac GTPases play critical roles in early T cell development. Blood 113, 3990–3998 (2009).
Guo, F., Cancelas, J. A., Hildeman, D., Williams, D. A. & Zheng, Y. Rac GTPase isoforms Rac1 and Rac2 play a redundant and crucial role in T-cell development. Blood 112, 1767–1775 (2008). Reference 44, together with references 8 and 43, examines the effects of removing RAC1 and RAC2 on B and T cell development.
Radtke, F., Wilson, A., Mancini, S. J. & MacDonald, H. R. Notch regulation of lymphocyte development and function. Nature Immunol. 5, 247–253 (2004).
Fischer, K. D. et al. Defective T-cell receptor signalling and positive selection of Vav-deficient CD4+CD8+ thymocytes. Nature 374, 474–477 (1995).
Tarakhovsky, A. et al. Defective antigen receptor-mediated proliferation of B and T cells in the absence of Vav. Nature 374, 467–470 (1995).
Turner, M. et al. A requirement for the Rho-family GTP exchange factor Vav in positive and negative selection of thymocytes. Immunity 7, 451–460 (1997).
Zhang, R., Alt, F. W., Davidson, L., Orkin, S. H. & Swat, W. Defective signalling through the T- and B-cell antigen receptors in lymphoid cells lacking the vav proto-oncogene. Nature 374, 470–473 (1995). References 46–49 highlight the key role of VAV1 in T cell development and TCR signalling.
Kong, Y. Y. et al. Vav regulates peptide-specific apoptosis in thymocytes. J. Exp. Med. 188, 2099–2111 (1998).
Reynolds, L. F. et al. Vav1 transduces T cell receptor signals to the activation of the Ras/ERK pathway via LAT, Sos and RasGRP1. J. Biol. Chem. 279, 18239–18246 (2004).
Reynolds, L. F. et al. Vav1 transduces T cell receptor signals to the activation of phospholipase C-γ1 via phosphoinositide 3-kinase-dependent and -independent pathways. J. Exp. Med. 195, 1103–1114 (2002).
Swat, W. et al. Essential role for Vav1 in activation, but not development, of γδ T cells. Int. Immunol. 15, 215–221 (2003).
Harenberg, A., Girkontaite, I., Giehl, K. & Fischer, K. D. The Lsc RhoGEF mediates signaling from thromboxane A2 to actin polymerization and apoptosis in thymocytes. Eur. J. Immunol. 35, 1977–1986 (2005).
Klinger, M. B., Guilbault, B. & Kay, R. J. The RhoA- and CDC42-specific exchange factor Dbs promotes expansion of immature thymocytes and deletion of double-positive and single-positive thymocytes. Eur. J. Immunol. 34, 806–816 (2004).
Sanui, T. et al. DOCK2 is essential for antigen-induced translocation of TCR and lipid rafts, but not PKC-θ and LFA-1, in T cells. Immunity 19, 119–129 (2003).
Kunisaki, Y. et al. DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. J. Cell Biol. 174, 647–652 (2006).
Gupta, S. et al. T cell receptor engagement leads to the recruitment of IBP, a novel guanine nucleotide exchange factor, to the immunological synapse. J. Biol. Chem. 278, 43541–43549 (2003).
Gupta, S. et al. Molecular cloning of IBP, a SWAP-70 homologous GEF, which is highly expressed in the immune system. Hum. Immunol. 64, 389–401 (2003).
Tanaka, Y. et al. SWAP-70-like adapter of T cells, an adapter protein that regulates early TCR-initiated signaling in Th2 lineage cells. Immunity 18, 403–414 (2003).
Fanzo, J. C. et al. Loss of IRF-4-binding protein leads to the spontaneous development of systemic autoimmunity. J. Clin. Invest. 116, 703–714 (2006).
Becart, S. et al. SLAT regulates Th1 and Th2 inflammatory responses by controlling Ca2+/NFAT signaling. J. Clin. Invest. 117, 2164–2175 (2007).
Yu, H., Leitenberg, D., Li, B. & Flavell, R. A. Deficiency of small GTPase Rac2 affects T cell activation. J. Exp. Med. 194, 915–926 (2001).
Dorn, T. et al. RhoH is important for positive thymocyte selection and T-cell receptor signaling. Blood 109, 2346–2355 (2007).
Gu, Y. et al. RhoH GTPase recruits and activates Zap70 required for T cell receptor signaling and thymocyte development. Nature Immunol. 7, 1182–1190 (2006). References 64 and 65 reveal an unexpected role for RHOH as an adaptor for ZAP70.
Bustelo, X. R., Ledbetter, J. A. & Barbacid, M. Product of vav proto-oncogene defines a new class of tyrosine protein kinase substrates. Nature 356, 68–71 (1992).
Crespo, P., Schuebel, K. E., Ostrom, A. A., Gutkind, J. S. & Bustelo, X. R. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature 385, 169–172 (1997).
Han, J. et al. Lck regulates Vav activation of members of the Rho family of GTPases. Mol. Cell. Biol. 17, 1346–1353 (1997).
Margolis, B. et al. Tyrosine phosphorylation of vav proto-oncogene product containing SH2 domain and transcription factor motifs. Nature 356, 71–74 (1992).
Fischer, K.-D. et al. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr. Biol. 8, 554–562 (1998).
Holsinger, L. J. et al. Defects in actin cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr. Biol. 8, 563–572 (1998).
Penninger, J. M. et al. The oncogene product Vav is a crucial regulator of primary cytotoxic T cell responses but has no apparent role in CD28-mediated co-stimulation. Eur. J. Immunol. 29, 1709–1718 (1999).
Costello, P. S. et al. The Rho-family GTP exchange factor Vav is a critical transducer of TCR signals to the calcium, ERK and NF-κB pathways. Proc. Natl Acad. Sci. USA 96, 3035–3040 (1999).
Charvet, C., Auberger, P., Tartare-Deckert, S., Bernard, A. & Deckert, M. Vav1 couples T cell receptor to serum response factor-dependent transcription via a MEK-dependent pathway. J. Biol. Chem. 277, 15376–15384 (2002).
Charvet, C. et al. Vav1 promotes T cell cycle progression by linking TCR/CD28 costimulation to FOXO1 and p27kip1 expression. J. Immunol. 177, 5024–5031 (2006).
Cao, Y. et al. Pleiotropic defects in TCR signaling in a Vav-1-null Jurkat T-cell line. EMBO J. 21, 4809–4819 (2002).
Wood, J. E., Schneider, H. & Rudd, C. E. TCR and TCR–CD28 engagement of protein-kinase B (PKB/AKT) and gylcogen synthase kinase-3 (GSK-3) operates independently of guanine nucleotide exchange factor VAV-1. J. Biol. Chem. 281, 32385–32394 (2006).
Villalba, M. et al. Translocation of PKCθ in T cells is mediated by a nonconventional, PI3-K- and Vav-dependent pathway, but does not absolutely require phospholipase C. J. Cell Biol. 157, 253–263 (2002).
Villalba, M. et al. A novel functional interaction between Vav and PKCθ is required for TCR-induced T cell activation. Immunity 12, 151–160 (2000).
Puga, I., Rao, A. & Macian, F. Targeted cleavage of signaling proteins by caspase 3 inhibits T cell receptor signaling in anergic T cells. Immunity 29, 193–204 (2008).
Ardouin, L. et al. Vav1 transduces TCR signals required for LFA-1 function and cell polarization at the immunological synapse. Eur. J. Immunol. 33, 790–797 (2003).
Krawczyk, C. et al. Vav1 controls integrin clustering and MHC/peptide-specific cell adhesion to antigen-presenting cells. Immunity 16, 331–343 (2002).
Villalba, M. et al. Vav1/Rac-dependent actin cytoskeleton reorganization is required for lipid raft clustering in T cells. J. Cell Biol. 155, 331–338 (2001).
Wülfing, C., Bauch, A., Crabtree, G. R. & Davis, M. M. The vav exchange factor is an essential regulator in actin-dependent receptor translocation to the lymphocyte–antigen-presenting cell interface. Proc. Natl Acad. Sci. USA 97, 10150–10155 (2000).
Gomez, T. S. et al. Dynamin 2 regulates T cell activation by controlling actin polymerization at the immunological synapse. Nature Immunol. 6, 261–270 (2005).
Su, I-h . et al. Polycomb group protein Ezh2 controls actin polymerization and cell signaling. Cell 121, 425–436 (2005).
Faure, S. et al. ERM proteins regulate cytoskeleton relaxation promoting T cell–APC conjugation. Nature Immunol. 5, 272–279 (2004).
Tybulewicz, V., Ardouin, L., Prisco, A. & Reynolds, L. F. Vav1: a key signal transducer downstream of the TCR. Immunol. Rev. 192, 42–52 (2003).
Tybulewicz, V. L. J. Vav-family proteins in T-cell signalling. Curr. Opin. Immunol. 17, 267–274 (2005).
Kuhne, M. R., Ku, G. & Weiss, A. A guanine nucleotide exchange factor-independent function of Vav1 in transcriptional activation. J. Biol. Chem. 275, 2185–2190 (2000).
Billadeau, D. D., Mackie, S. M., Schoon, R. A. & Leibson, P. J. Specific subdomains of Vav differentially affect T cell and NK cell activation. J. Immunol. 164, 3971–3981 (2000).
Houlard, M. et al. Vav1 is a component of transcriptionally active complexes. J. Exp. Med. 195, 1115–1127 (2002).
Blanchet, F., Cardona, A., Letimier, F. A., Hershfield, M. S. & Acuto, O. CD28 costimulatory signal induces protein arginine methylation in T cells. J. Exp. Med. 202, 371–377 (2005).
Phee, H., Abraham, R. T. & Weiss, A. Dynamic recruitment of PAK1 to the immunological synapse is mediated by PIX independently of SLP-76 and Vav1. Nature Immunol. 6, 608–617 (2005).
Nishihara, H. et al. DOCK2 associates with CrkL and regulates Rac1 in human leukemia cell lines. Blood 100, 3968–3974 (2002).
Chen, Q. et al. IRF-4-binding protein inhibits interleukin-17 and interleukin-21 production by controlling the activity of IRF-4 transcription factor. Immunity 29, 899–911 (2008). This paper shows that the GEF IBP regulates T H cell development by inhibiting IRF4.
Becart, S. et al. Tyrosine-phosphorylation-dependent translocation of the SLAT protein to the immunological synapse is required for NFAT transcription factor activation. Immunity 29, 704–719 (2008).
Ishizaki, H. et al. Defective chemokine-directed lymphocyte migration and development in the absence of Rho guanosine diphosphate-dissociation inhibitors α and β. J. Immunol. 177, 8512–8521 (2006).
Li, B. et al. Role of the guanosine triphosphatase Rac2 in T helper 1 cell differentiation. Science 288, 2219–2222 (2000).
Croker, B. A. et al. Rac2-deficient mice display perturbed T-cell distribution and chemotaxis, but only minor abnormalities in TH1 responses. Immunol. Cell Biol. 80, 231–240 (2002).
Tanaka, Y., So, T., Lebedeva, S., Croft, M. & Altman, A. Impaired IL-4 and c-Maf expression and enhanced Th1-cell development in Vav1-deficient mice. Blood 106, 1286–1295 (2005).
Korn, T. et al. Vav1-deficient mice are resistant to MOG-induced experimental autoimmune encephalomyelitis due to impaired antigen priming. J. Neuroimmunol. 139, 17–26 (2003).
Weckbecker, G. et al. Strongly reduced alloreactivity and long-term survival times of cardiac allografts in Vav1- and Vav1/Vav2-knockout mice. Transpl. Int. 20, 353–364 (2007).
Tanaka, Y. et al. T helper type 2 differentiation and intracellular trafficking of the interleukin 4 receptor-α subunit controlled by the Rac activator Dock2. Nature Immunol. 8, 1067–1075 (2007). This paper shows that DOCK2 regulates the intracellular trafficking of the IL-4 receptor α-subunit.
Vielkind, S., Gallagher-Gambarelli, M., Gomez, M., Hinton, H. J. & Cantrell, D. A. Integrin regulation by RhoA in thymocytes. J. Immunol. 175, 350–357 (2005).
Bolomini-Vittori, M. et al. Regulation of conformer-specific activation of the integrin LFA-1 by a chemokine-triggered Rho signaling module. Nature Immunol. 10, 185–194 (2009).
Cherry, L. K., Li, X., Schwab, P., Lim, B. & Klickstein, L. B. RhoH is required to maintain the integrin LFA-1 in a nonadhesive state on lymphocytes. Nature Immunol. 5, 961–967 (2004).
Vicente-Manzanares, M. et al. Control of lymphocyte shape and the chemotactic response by the GTP exchange factor Vav. Blood 105, 3026–3034 (2005).
Shulman, Z. et al. DOCK2 regulates chemokine-triggered lateral lymphocyte motility but not transendothelial migration. Blood 108, 2150–2158 (2006).
Krummel, M. F. & Macara, I. Maintenance and modulation of T cell polarity. Nature Immunol. 7, 1143–1149 (2006).
Stowers, L., Yelon, D., Berg, L. J. & Chant, J. Regulation of the polarization of T cells toward antigen-presenting cells by Ras-related GTPase CDC42. Proc. Natl Acad. Sci. USA 92, 5027–5031 (1995).
D'Souza-Schorey, C., Boettner, B. & Van Aelst, L. Rac regulates integrin-mediated spreading and increased adhesion of T lymphocytes. Mol. Cell Biol. 18, 3936–3946 (1998).
del Pozo, M. A., Vicente-Manzanares, M., Tejedor, R., Serrador, J. M. & Sanchez-Madrid, F. Rho GTPases control migration and polarization of adhesion molecules and cytoskeletal ERM components in T lymphocytes. Eur. J. Immunol. 29, 3609–3620 (1999).
Gerard, A., Mertens, A. E., van der Kammen, R. A. & Collard, J. G. The Par polarity complex regulates Rap1- and chemokine-induced T cell polarization. J. Cell Biol. 176, 863–875 (2007).
Volinsky, N., Gantman, A. & Yablonski, D. A Pak- and Pix-dependent branch of the SDF-1α signalling pathway mediates T cell chemotaxis across restrictive barriers. Biochem. J. 397, 213–222 (2006).
Saveliev, A. & Tybulewicz, V. L. Lymphocyte signaling: beyond knockouts. Nature Immunol. 10, 361–364 (2009).
Weisz Hubsman, M., Volinsky, N., Manser, E., Yablonski, D. & Aronheim, A. Autophosphorylation-dependent degradation of Pak1, triggered by the Rho-family GTPase, Chp. Biochem. J. 404, 487–497 (2007).
Smith, A., Bracke, M., Leitinger, B., Porter, J. C. & Hogg, N. LFA-1-induced T cell migration on ICAM-1 involves regulation of MLCK-mediated attachment and ROCK-dependent detachment. J. Cell Sci. 116, 3123–3133 (2003).
Boureux, A., Vignal, E., Faure, S. & Fort, P. Evolution of the Rho family of ras-like GTPases in eukaryotes. Mol. Biol. Evol. 24, 203–216 (2007).
Dudziak, D. et al. Differential antigen processing by dendritic cell subsets in vivo. Science 315, 107–111 (2007).
Acknowledgements
We thank S. Saveliev and E. Schweighoffer for critical reading of the manuscript, A. Ridley for discussions, and S. Shah (Bloomsbury Centre for Bioinformatics, UCL) for analysis of the transcriptome data.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary information S1 (table)
Expression of Rho GTPases and their regulators in lymphocytes (PDF 107 kb)
Related links
Glossary
- Prenyl group
-
An isoprenoid lipid, either farnesyl or geranylgeranyl. Most Rho GTPases are modified by the addition of a prenyl group at their carboxyl terminus. This modification anchors Rho GTPases in membranes.
- Follicular B cell
-
A circulating mature B cell subset that populates the follicles of secondary lymphoid organs such as the spleen, lymph nodes and Peyer's patches.
- Marginal zone B cell
-
A non-circulating B cell subset found mainly in the marginal zone of the spleen, which is located between the non-lymphoid red pulp and the lymphocyte-rich white pulp. These cells are thought to mount early responses to blood-borne antigens.
- B-1 cell
-
A cell belonging to the B cell lineage that populates the peritoneal and pleural cavities and secretes polyspecific, low-affinity IgM.
- Transitional B cell
-
An immature B cell subset found in the spleen having recently arrived from the bone marrow. These cells are short-lived, with half-lives of 24 days, and mature into follicular or marginal zone B cells.
- Immunological synapse
-
A region that can form between two cells of the immune system in close contact. The immunological synapse originally referred to the interaction between a T cell and an antigen-presenting cell. It involves adhesion molecules, as well as antigen receptors and cytokine receptors. The immunological synapse is characterized by polarization of the lymphocyte, resulting, for example, in directed secretion towards the antigen-presenting cell.
- G protein-coupled receptor
-
(GPCR). One of a large group of receptors that bind a diverse set of molecules, including chemokines, complement components, biologically active amines and neurotransmitters. GPCRs are seven-transmembrane-spanning receptors and are coupled to heterotrimeric, GTP-regulated signalling proteins.
- Sphingosine 1 -phosphate
-
(S1P). A sphingolipid involved in signalling. In the immune system, S1P induces the egress of lymphocytes from lymphoid organs by binding to SIP receptors on the cells.
- Intravital microscopy
-
This is used to examine biological processes, such as leukocyte–endothelial-cell interactions, in living tissue. In general, translucent tissues are used, such as the mesentery or cremaster muscle, or lymph nodes, which can be exposed and mounted for microscopic observation.
- High endothelial venule
-
A specialized venule with a cuboidal endothelial lining. They are found in peripheral lymph nodes and Peyers patches. High endothelial venules are the sites of entry of lymphocytes into lymphoid tissues from the blood.
- Lamellipodium
-
A flattened, sheet-like protrusion that is supported by a meshwork of Factin and extends at the leading edge of crawling cells.
- Uropod
-
A slender protrusion that forms at the trailing end of migrating leukocytes.
- Positive selection
-
A process in the thymus that leads to the survival of immature thymocytes that express T cell receptors that bind with an appropriate affinity to self peptide–MHC complexes.
- Negative selection
-
A process in the thymus that eliminates thymocytes that express T cell receptors that bind with high affinity to self peptide-MHC complexes.
- γδ T cells
-
T cells that express the γδ T cell receptor. These T cells are present in the skin, vagina and intestinal epithelium as intraepithelial lymphocytes (IELs). Although the exact function of γδ T cells is unknown, it has been suggested that mucosal γδ T cells are involved in innate immune responses.
- Ezrin, radixin and moesin (ERM) proteins
-
ERM proteins function as general cytolinkers between the cortical actin-filament network and the plasma membrane, and are thought to function mainly through their ability to bind both F-actin and the cytoplasmic regions of integral membrane proteins.
- Experimental autoimmune encephalomyelitis
-
(EAE). An experimental model of the human disease multiple sclerosis. EAE is an autoimmune disease mediated by CD4+ T helper 1 (TH1) cells and interleukin–17-producing TH17 cells reactive to components of the myelin sheath that infiltrate the nervous parenchyma, release pro-inflammatory cytokines and chemokines, promote leukocyte infiltration and contribute to demyelination.
Rights and permissions
About this article
Cite this article
Tybulewicz, V., Henderson, R. Rho family GTPases and their regulators in lymphocytes. Nat Rev Immunol 9, 630–644 (2009). https://doi.org/10.1038/nri2606
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri2606
This article is cited by
-
ROCK1/2 signaling contributes to corticosteroid-refractory acute graft-versus-host disease
Nature Communications (2024)
-
Role of Rho GTPases in inflammatory bowel disease
Cell Death Discovery (2023)
-
The phosphatidylinositol-transfer protein Nir3 promotes PI(4,5)P2 replenishment in response to TCR signaling during T cell development and survival
Nature Immunology (2023)
-
Exploring the therapeutic promise of targeting Rho kinase in rheumatoid arthritis
Inflammopharmacology (2021)
-
Secretome of Cultured Human Endothelial Cells in Simulated Microgravity
Bulletin of Experimental Biology and Medicine (2019)