Key Points
-
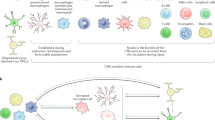
Microglial cells are highly ramified cells and their cell processes are active and plastic even during normal conditions. Similar to macrophages, microglial cells express Toll-like receptors (TLRs) and can respond to TLR ligands by the production of pro-inflammatory mediators. They are the main innate immune cells of the central nervous system (CNS).
-
These cells can be activated during systemic infections without the integrity of the blood–brain barrier (BBB) being compromised. Some regions of the brain have no BBB, known as the circumventricular organs, and the response to circulating pathogens at these sites is similar to that in most systemic organs.
-
A robust inflammatory response occurs in the cerebral tissue of mouse models of herpes simplex virus (HSV) encephalitis. The inflammatory response is initially required to restrict the first stages of viral replication, but this response must be subsequently suppressed to avoid severe neuronal damage. By contrast, infiltration of leukocytes into the CNS is a crucial process in both the vulnerability to (through TLR3 and the chemokine CCL2) and defence against (through TLR7) West Nile virus encephalitis in mice.
-
The activation of innate immune signalling pathways in microglial cells not only occurs in response to infectious organisms, but also during brain injury and chronic disease. It remains highly debated whether such a response has neuroprotective or neurodestructive effects.
-
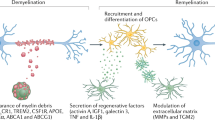
Acute administration of lipopolysaccharide (LPS) modulates the expression of key genes involved in the recruitment and differentiation of oligodendrocyte progenitor cells and in oligodendrocyte-mediated remyelination. In contrast to these beneficial effects of LPS in the adult brain, the activation of TLRs is toxic to oligodendrocytes in developing brains.
-
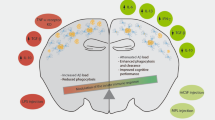
Receptors of the innate immune system are involved in the removal of amyloid-β (Aβ) in the brain. Indeed, the Aβ load is modulated in part by TLR2 and TLR4, and activation of TLR2, TLR4 and TLR9 increases the uptake of Aβ by microglial cells in the brains of mouse models of Alzheimer's disease.
-
Bone marrow-derived microglial cells have been shown to be closely associated with Aβ plaques and to slow the progression of the disease by removing Aβ from the CNS. Moreover, blocking the transforming growth factor-β-induced signalling pathway in peripheral macrophages and systemic administration of macrophage colony-stimulating factor improved Alzheimer's disease-like pathology.
-
A better understanding of the innate immune response in the cerebral tissue will help us to develop innovative strategies to activate microglial cells more effectively whilst avoiding detrimental effects on the neuronal elements. In this regard, the development of new synthetic and pure TLR agonists is required to stimulate these innate immune cells specifically towards neuroprotective functions.
Abstract
Microglial cells are the main innate immune cells of the complex cellular structure of the brain. These cells respond quickly to pathogens and injury, accumulate in regions of degeneration and produce a wide variety of pro-inflammatory molecules. These observations have resulted in active debate regarding the exact role of microglial cells in the brain and whether they have beneficial or detrimental functions. Careful targeting of these cells could have therapeutic benefits for several types of trauma and disease specific to the central nervous system. This Review discusses the molecular details underlying the innate immune response in the brain during infection, injury and disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005). This reference shows that microglial cells are highly dynamic in the resting state in vivo and continuously sample their microenvironment.
Rivest, S. Molecular insights on the cerebral innate immune system. Brain Behav. Immun. 17, 13–19 (2003).
Rivest, S. Cannabinoids in microglia: a new trick for immune surveillance and neuroprotection. Neuron 49, 4–8 (2006).
Nadeau, S. & Rivest, S. Role of microglial-derived tumor necrosis factor in mediating CD14 transcription and NF-κB activity in the brain during endotoxemia. J. Neurosci. 20, 3456–3468 (2000). This study provides evidence that microglial cell-derived TNF mediates an autocrine and paracrine stimulatory loop that activates the innate immune response in the brain during endotoxaemia.
Nguyen, M. D., Julien, J. P. & Rivest, S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nature Rev. Neurosci. 3, 216–227 (2002).
Hanisch, U. K. & Kettenmann, H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nature Neurosci. 10, 1387–1394 (2007).
Soulet, D. & Rivest, S. Bone-marrow-derived microglia: myth or reality? Curr. Opin. Pharmacol. 8, 508–518 (2008).
Chakravarty, S. & Herkenham, M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J. Neurosci. 25, 1788–1796 (2005).
Lacroix, S., Feinstein, D. & Rivest, S. The bacterial endotoxin lipopolysaccharide has the ability to target the brain in upregulating its membrane CD14 receptor within specific cellular populations. Brain Pathol. 8, 625–640 (1998). This is the first evidence that the expression of an innate immune receptor by microglial cells is regulated by circulating LPS in vivo.
Quan, N., Whiteside, M. & Herkenham, M. Time course and localization patterns of interleukin-1β mRNA expression in the brain and pituitary after peripheral administration of lipopolysaccharide. Neuroscience 83, 281–293 (1997).
Quan, N., Whiteside, M., Kim, L. & Herkenham, M. Induction of inhibitory factor κBα mRNA in the central nervous system after peripheral lipopolysaccharide administration: an in situ hybridization histochemistry study in the rat. Proc. Natl Acad. Sci. USA 94, 10985–10990 (1997).
Lalancette-Hebert, M., Phaneuf, D., Soucy, G., Weng, Y. C. & Kriz, J. Live imaging of Toll-like receptor 2 response in cerebral ischaemia reveals a role of olfactory bulb microglia as modulators of inflammation. Brain 132, 940–954 (2009).
Laflamme, N., Soucy, G. & Rivest, S. Circulating cell wall components derived from Gram-negative and not Gram-positive bacteria cause a profound transcriptional activation of the gene encoding toll-like receptor 2 in the CNS. J. Neurochem. 70, 648–657 (2001).
Nadeau, S. & Rivest, S. Regulation of the gene encoding tumor necrosis factor alpha in the rat brain and pituitary in response to different models of systemic immune challenge. J. Neuropathol. Exp. Neurol. 58, 61–77 (1999).
Bouchard, C., Page, J., Bedard, A., Tremblay, P. & Vallieres, L. G protein-coupled receptor 84, a microglia-associated protein expressed in neuroinflammatory conditions. Glia 55, 790–800 (2007).
Glezer, I., Chernomoretz, A., David, S., Plante, M. M. & Rivest, S. Genes involved in the balance between neuronal survival and death during inflammation. PLoS ONE 2, e310 (2007).
Glezer, I., Simard, A. R. & Rivest, S. Neuroprotective role of the innate immune system by microglia. Neuroscience 147, 867–883 (2007).
Glezer, I., Zekki, H., Scavone, C. & Rivest, S. Modulation of the innate immune response by NMDA receptors has neuropathological consequences. J. Neurosci. 23, 11094–11103 (2003).
Bowman, C. C., Rasley, A., Tranguch, S. L. & Marriott, I. Cultured astrocytes express toll-like receptors for bacterial products. Glia 43, 281–291 (2003).
Zhou, H., Lapointe, B. M., Clark, S. R., Zbytnuik, L. & Kubes, P. A requirement for microglial TLR4 in leukocyte recruitment into brain in response to lipopolysaccharide. J. Immunol. 177, 8103–8110 (2006).
Rolls, A. et al. Toll-like receptors modulate adult hippocampal neurogenesis. Nature Cell Biol. 9, 1081–1088 (2007).
Shechter, R. et al. Toll-like receptor 4 restricts retinal progenitor cell proliferation. J. Cell Biol. 183, 393–400 (2008). References 21 and 22 show that TLR2 and TLR4 modulate post-natal neurogenesis and that these receptors have roles in the CNS in addition to their classical innate immune functions.
Glezer, I., Lapointe, A. & Rivest, S. Innate immunity triggers oligodendrocyte progenitor reactivity and confines damages to brain injuries. FASEB J. 20, 750–752 (2006). This study provides data supporting the beneficial effects of LPS in modulating important genes involved in the remyelination process in the mouse brain.
Laflamme, N., Echchannaoui, H., Landmann, R. & Rivest, S. Cooperation between toll-like receptor 2 and 4 in the brain of mice challenged with cell wall components derived from Gram-negative and Gram-positive bacteria. Eur. J. Immunol. 33, 1127–1138 (2003).
Chen, K. et al. Activation of Toll-like receptor 2 on microglia promotes cell uptake of Alzheimer disease-associated amyloid β peptide. J. Biol. Chem. 281, 3651–3659 (2006).
Jack, C. S. et al. TLR signaling tailors innate immune responses in human microglia and astrocytes. J. Immunol. 175, 4320–4330 (2005).
Bsibsi, M. et al. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia 53, 688–695 (2006).
Dalpke, A. H. et al. Immunostimulatory CpG-DNA activates murine microglia. J. Immunol. 168, 4854–4863 (2002).
Stevens, S. L. et al. Toll-like receptor 9: a new target of ischemic preconditioning in the brain. J. Cereb. Blood Flow Metab. 28, 1040–1047 (2008).
McMaster, A. & Ray, D. W. Drug insight: selective agonists and antagonists of the glucocorticoid receptor. Nature Clin. Pract. Endocrinol. Metab. 4, 91–101 (2008).
O'Neill, L. A. When signaling pathways collide: positive and negative regulation of toll-like receptor signal transduction. Immunity 29, 12–20 (2008).
Ogawa, S. et al. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell 122, 707–721 (2005). This paper elegantly shows that the glucocorticoid receptor represses the expression of a large set of functionally related inflammatory response genes by disrupting p65–IRF signalling complexes that are required for TLR4- or TLR9-dependent, but not TLR3-dependent, transcriptional activation in macrophages.
Glezer, I. & Rivest, S. Glucocorticoids: protectors of the brain during innate immune responses. Neuroscientist 10, 538–552 (2004).
Nadeau, S. & Rivest, S. Glucocorticoids play a fundamental role in protecting the brain during innate immune response. J. Neurosci. 23, 5536–5544 (2003).
Soulet, D. & Rivest, S. Polyamines play a critical role in the control of the innate immune response in the mouse CNS. J. Cell Biol. 162, 257–268 (2003). References 33–35 provide evidence that glucocorticoid feedback on the innate immune response by microglial cells is a crucial neuroprotective mechanism when the cerebral tissue is exposed to LPS. Without such negative feedback, LPS becomes highly toxic to neuronal elements.
Castano, A., Herrera, A. J., Cano, J. & Machado, A. Lipopolysaccharide intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J. Neurochem. 70, 1584–1592 (1998).
Kim, W. G. et al. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J. Neurosci. 20, 6309–6316 (2000).
Nadeau, S. & Rivest, S. Endotoxemia prevents the cerebral inflammatory wave induced by intraparenchymal lipopolysaccharide injection: role of glucocorticoids and CD14. J. Immunol. 169, 3370–3381 (2002).
Rowitch, D. H. Glial specification in the vertebrate neural tube. Nature Rev. Neurosci. 5, 409–419 (2004).
Takebayashi, H. et al. The basic helix-loop-helix factor Olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr. Biol. 12, 1157–1163 (2002).
Zhou, Q., Choi, G. & Anderson, D. J. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron 31, 791–807 (2001).
Arnett, H. A. et al. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science 306, 2111–2115 (2004).
Xin, M. et al. Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice. J. Neurosci. 25, 1354–1365 (2005).
Lu, Q. R. et al. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 109, 75–86 (2002).
Zhou, Q. & Anderson, D. J. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell 109, 61–73 (2002).
Pringle, N. P., Mudhar, H. S., Collarini, E. J. & Richardson, W. D. PDGF receptors in the rat CNS: during late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development 115, 535–551 (1992).
Setzu, A. et al. Inflammation stimulates myelination by transplanted oligodendrocyte precursor cells. Glia 54, 297–303 (2006).
Lehnardt, S. et al. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J. Neurosci. 22, 2478–2486 (2002).
Lehnardt, S. et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc. Natl Acad. Sci. USA 100, 8514–8519 (2003). References 48 and 49 show that LPS is toxic to oligodendrocytes when they are co-cultured with microglial cells and that stereotactic intracerebral injection of LPS into the developing pericallosal white matter of immature rodents resulted in loss of oligodendrocytes and hypomyelination.
Nau, R. & Bruck, W. Neuronal injury in bacterial meningitis: mechanisms and implications for therapy. Trends Neurosci. 25, 38–45 (2002).
Lehnardt, S. et al. A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J. Immunol. 177, 583–592 (2006).
Cameron, J. S. et al. Toll-like receptor 3 is a potent negative regulator of axonal growth in mammals. J. Neurosci. 27, 13033–13041 (2007).
Lathia, J. D. et al. Toll-like receptor 3 is a negative regulator of embryonic neural progenitor cell proliferation. J. Neurosci. 28, 13978–13984 (2008).
Ma, Y. et al. Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis. J. Cell Biol. 175, 209–215 (2006).
Sigurdardottir, B., Bjornsson, O. M., Jonsdottir, K. E., Erlendsdottir, H. & Gudmundsson, S. Acute bacterial meningitis in adults. A 20-year overview. Arch. Intern. Med. 157, 425–430 (1997).
Klein, M. et al. Innate immunity to pneumococcal infection of the central nervous system depends on toll-like receptor (TLR)2 and TLR4. J. Infect. Dis. 198, 1028–1036 (2008).
Letiembre, M., Echchannaoui, H., Ferracin, F., Rivest, S. & Landmann, R. Toll-like receptor-2 deficiency is associated with enhanced brain TNF gene expression during pneumococcal meningitis. J. Neuroimmunol. 168, 21–33 (2005).
Lewandowski, G. & Hobbs, M. V. Evidence for deficiencies in intracerebral cytokine production, adhesion molecule induction, and T cell recruitment in herpes simplex virus type-2 infected mice. J. Neuroimmunol. 81, 58–65 (1998).
Boivin, G., Coulombe, Z. & Rivest, S. Intranasal herpes simplex virus type 2 inoculation causes a profound thymidine kinase dependent cerebral inflammatory response in the mouse hindbrain. Eur. J. Neurosci. 16, 29–43 (2002).
Sergerie, Y., Boivin, G., Gosselin, D. & Rivest, S. Delayed but not early glucocorticoid treatment protects the host during experimental herpes simplex virus encephalitis in mice. J. Infect. Dis. 195, 817–825 (2007).
Sergerie, Y., Rivest, S. & Boivin, G. Tumor necrosis factor-α and interleukin-1β play a critical role in the resistance against lethal herpes simplex virus encephalitis. J. Infect. Dis. 196, 853–860 (2007).
Sorensen, L. N. et al. TLR2 and TLR9 synergistically control herpes simplex virus infection in the brain. J. Immunol. 181, 8604–8612 (2008).
Zhang, S. Y. et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science 317, 1522–1527 (2007). This work indicates that TLR3 in humans selectively protects against severe infection of the CNS by HSV1.
Wang, T. et al. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nature Med. 10, 1366–1373 (2004).
Town, T. et al. Toll-like receptor 7 mitigates lethal West Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity 30, 242–253 (2009).
Getts, D. R. et al. Ly6c+ “inflammatory monocytes” are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J. Exp. Med. 205, 2319–2337 (2008). References 64–66 show that the infiltration of leukocytes into the CNS is a crucial process in both the vulnerability to (through TLR3 and CCL2) and defence against (through TLR7) West Nile virus encephalitis in mice.
Mildner, A. et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nature Neurosci. 10, 1544–1553 (2007).
Allan, S. M. & Rothwell, N. J. Cytokines and acute neurodegeneration. Nature Rev. Neurosci. 2, 734–744 (2001).
Allan, S. M., Tyrrell, P. J. & Rothwell, N. J. Interleukin-1 and neuronal injury. Nature Rev. Immunol. 5, 629–640 (2005).
Wyss-Coray, T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nature Med. 12, 1005–1015 (2006).
Wyss-Coray, T. & Mucke, L. Inflammation in neurodegenerative disease — a double-edged sword. Neuron 35, 419–432 (2002).
Arnett, H. A. et al. TNFα promotes proliferation of oligodendrocyte progenitors and remyelination. Nature Neurosci. 4, 1116–1122 (2001).
Herx, L. M., Rivest, S. & Yong, V. W. Central nervous system-initiated inflammation and neurotrophism in trauma: IL-1β is required for the production of ciliary neurotrophic factor. J. Immunol. 165, 2232–2239 (2000).
Mason, J. L., Suzuki, K., Chaplin, D. D. & Matsushima, G. K. Interleukin-1β promotes repair of the CNS. J. Neurosci. 21, 7046–7052 (2001).
Turrin, N. P. & Rivest, S. Tumor necrosis factor α but not interleukin 1β mediates neuroprotection in response to acute nitric oxide excitotoxicity. J. Neurosci. 26, 143–151 (2006). References 72–75 show a neuroprotective role of IL-1β and TNF in the CNS. IL-1β mediates its beneficial effects by stimulating the production of neurotrophic factors and by promoting remyelination, whereas TNF activates microglial cells and favours remyelination.
Boivin, A. et al. Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. J. Neurosci. 27, 12396–12406 (2007).
Kigerl, K. A. et al. Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J. Neurochem. 102, 37–50 (2007).
Kilic, U., Kilic, E., Matter, C. M., Bassetti, C. L. & Hermann, D. M. TLR-4 deficiency protects against focal cerebral ischemia and axotomy-induced neurodegeneration. Neurobiol. Dis. 31, 33–40 (2008).
Tang, S. C. et al. Toll-like receptor-4 mediates neuronal apoptosis induced by amyloid β-peptide and the membrane lipid peroxidation product 4-hydroxynonenal. Exp. Neurol. 213, 114–121 (2008).
Wang, Q., Tang, X. N. & Yenari, M. A. The inflammatory response in stroke. J. Neuroimmunol. 184, 53–68 (2007).
Liesz, A. et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nature Med. 15, 192–199 (2009).
Priller, J. et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nature Med. 7, 1356–1361 (2001).
Priller, J. et al. Neogenesis of cerebellar Purkinje neurons from gene-marked bone marrow cells in vivo. J. Cell Biol. 155, 733–738 (2001).
Gowing, G., Vallieres, L. & Julien, J. P. Mouse model for ablation of proliferating microglia in acute CNS injuries. Glia 53, 331–337 (2006).
Barrette, B. et al. Requirement of myeloid cells for axon regeneration. J. Neurosci. 28, 9363–9376 (2008).
Carmen, J., Gowing, G., Julien, J. P. & Kerr, D. Altered immune response to CNS viral infection in mice with a conditional knock-down of macrophage-lineage cells. Glia 54, 71–80 (2006).
Galarneau, H., Villeneuve, J., Gowing, G., Julien, J. P. & Vallieres, L. Increased glioma growth in mice depleted of macrophages. Cancer Res. 67, 8874–8881 (2007).
Lalancette-Hebert, M., Gowing, G., Simard, A., Weng, Y. C. & Kriz, J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J. Neurosci. 27, 2596–2605 (2007).
Rolls, A. et al. Two faces of chondroitin sulfate proteoglycan in spinal cord repair: a role in microglia/macrophage activation. PLoS Med. 5, e171 (2008).
Yin, Y. et al. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nature Neurosci. 9, 843–852 (2006).
Cui, Q., Yin, Y. & Benowitz, L. I. The role of macrophages in optic nerve regeneration. Neuroscience 158, 1039–1048 (2008).
Popovich, P. G. et al. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp. Neurol. 158, 351–365 (1999).
Gensel, J. C. et al. Macrophages promote axon regeneration with concurrent neurotoxicity. J. Neurosci. 29, 3956–3968 (2009).
Horn, K. P., Busch, S. A., Hawthorne, A. L., van Rooijen, N. & Silver, J. Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J. Neurosci. 28, 9330–9341 (2008).
Pineau, I. & Lacroix, S. Endogenous signals initiating inflammation in the injured nervous system. Glia 57, 351–361 (2008).
Sisodia, S. S. & St George-Hyslop, P. H. γ-Secretase, Notch, Aβ and Alzheimer's disease: where do the presenilins fit in? Nature Rev. Neurosci. 3, 281–290 (2002).
Lesne, S. et al. A specific amyloid-β protein assembly in the brain impairs memory. Nature 440, 352–357 (2006). This study found that memory deficits in middle-aged transgenic mice are caused by the extracellular accumulation of self-aggregates of a 56 kDa soluble amyloid-β peptide, which was named Aβ*56.Aβ*56 purified from the brains of the transgenic mice disrupts memory when administered to young rats and could have a crucial role in Alzheimer's disease.
Combarros, O. et al. CD14 receptor polymorphism and Alzheimer's disease risk. Neurosci. Lett. 380, 193–196 (2005).
Liu, Y. et al. LPS receptor (CD14): a receptor for phagocytosis of Alzheimer's amyloid peptide. Brain 128, 1778–1789 (2005).
Richard, K. L., Filali, M., Prefontaine, P. & Rivest, S. Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid β1–42 and delay the cognitive decline in a mouse model of Alzheimer's disease. J. Neurosci. 28, 5784–5793 (2008).
Tahara, K. et al. Role of toll-like receptor signalling in Aβ uptake and clearance. Brain 129, 3006–3019 (2006).
Scholtzova, H. et al. Induction of toll-like receptor 9 signaling as a method for ameliorating Alzheimer's disease-related pathology. J. Neurosci. 29, 1846–1854 (2009).
Fiala, M. et al. Innate immunity and transcription of MGAT-III and Toll-like receptors in Alzheimer's disease patients are improved by bisdemethoxycurcumin. Proc. Natl Acad. Sci. USA 104, 12849–12854 (2007). References 100–103 provide evidence that TLR2, TLR4 and TLR9 function as a natural defence mechanism to clear Aβ from the CNS and that activation of these receptors could be used as a new therapeutic approach for Alzheimer's disease.
Willingham, S. B. & Ting, J. P. NLRs and the dangers of pollution and aging. Nature Immunol. 9, 831–833 (2008).
Halle, A. et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nature Immunol. 9, 857–865 (2008).
Simard, A. R., Soulet, D., Gowing, G., Julien, J. P. & Rivest, S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron 49, 489–502 (2006).
Town, T. et al. Blocking TGF-β–Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nature Med. 14, 681–687 (2008).
Ray, S. et al. Classification and prediction of clinical Alzheimer's diagnosis based on plasma signaling proteins. Nature Med. 13, 1359–1362 (2007).
Majumdar, A. et al. Activation of microglia acidifies lysosomes and leads to degradation of Alzheimer amyloid fibrils. Mol. Biol. Cell 18, 1490–1496 (2007).
Boissonneault, V. et al. Powerful beneficial effects of macrophage colony-stimulating factor on β-amyloid deposition and cognitive impairment in Alzheimer's disease. Brain 132, 1078–1092 (2009). References 106, 107 and 110 show that bone marrow-derived microglial cell precursors can clear senile plaques, but this process is decreased in mouse models of Alzheimer's disease. Increased recruitment of these cells to the brain by the blockade of TGFβ signalling or systemic M-CSF administration ameliorates the clearance of Aβ and improves cognitive function in mice that develop the disease.
Martin, B. K. et al. Cognitive function over time in the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch. Neurol. 65, 896–905 (2008).
Acknowledgements
The Canadian Institutes in Health Research (CIHR) and Neuroscience Canada (Brain Repair Program) support this research. S.R. holds a Canadian Research Chair in Neuroimmunology. The author is grateful to D. Soulet, I. Glezer and M.M. Plante for the original graphical illustrations.
Author information
Authors and Affiliations
Related links
Glossary
- Microglial cell
-
A phagocytic cell of myeloid origin that is involved in the innate immune response in the central nervous system. Microglial cells are thought to be brain-resident macrophages.
- Toll-like receptor
-
(TLR). A type of pattern recognition receptor that recognizes unique structures derived from microorganisms. Signalling through TLRs promotes inflammatory immune responses, cytokine production and cell activation in response to microorganisms.
- Blood–brain barrier
-
(BBB). A barrier formed by tight junctions between endothelial cells that markedly limits the entry of leukocytes and all large molecules — including to a large extent immunoglobulins, cytokines and complement proteins — to the central nervous system.
- Choroid plexus
-
A capillary bed that is covered by transporting ependymal cells and that protrudes into the cerebral ventricles. The ependymal cells produce cerebrospinal fluid.
- Leptomeninges
-
The pia mater and the arachnoid mater considered together.
- Astrocyte
-
A star-shaped glial cell of the central nervous system that forms a structural and functional interface between non-nervous tissues and neurons.
- Multiple sclerosis
-
A chronic inflammatory and demyelinating disease of the central nervous system. Multiple sclerosis involves an autoimmune response against components of myelin, which is thought to contribute to disease pathogenesis.
- Alzheimer's disease
-
The most common type of neurodegenerative dementia. Patients often have impairments in learning and memory. The neuropathology of the disease includes neuron loss in the cerebral cortex and in some subcortical regions and the presence of aggregates in the form of plaques (containing amyloid-β) and neurofibrillary tangles (containing hyperphosphorylated tau).
- Ischaemic injury
-
Damage to neurons that results from a deficiency in the blood supply to that region of the brain, owing to functional constriction or physical obstruction of a blood vessel. Ischaemic stroke is an episode of acute regional ischaemia in the brain, usually caused by thrombi or emboli from atherosclerotic plaques, that leads to the death of nerve cells.
- Cross-tolerance
-
A transient state of hyporesponsiveness of host antigen-presenting cells to other TLR ligands after previous exposure to a TLR ligand.
- Glucocorticoids
-
A group of compounds that belongs to the corticosteroid family. These compounds can be naturally produced (hormones) or synthetic. They affect metabolism and have anti-inflammatory and immunosuppressive effects. Some synthetic glucocorticoids (for example, dexamethasone) are used as chemotherapeutic drugs.
- Substantia nigra
-
A part of the midbrain that contains dopamine-producing neurons.
- Oligodendrocyte progenitor cell
-
The progenitor of oligodendrocytes in the CNS. The progenitor cells are generated in restricted, stem cell-containing regions of the CNS, from where they migrate to the axon tracts that become myelinated.
- Oligodendrocyte
-
A type of glial cell that produces the myelin sheath that insulates axons and improves the speed and reliability of signal transmission by neurons.
- Myelination
-
The formation of an insulating layer (that is, a myelin sheath) around a nerve fibre or axon to increase the speed at which action potentials are conducted.
- Cerebrospinal fluid
-
A liquid that is produced by ependymal cells of the choroid plexus, which thereafter circulates in the cerebroventricular system.
- Senile plaque
-
A site of amyloid-β accumulation and damaged neurons in the brains of mouse models and patients with Alzheimer's disease.
- Inflammasome
-
A molecular complex of several proteins that upon assembly cleaves pro-IL-1β, thereby producing active IL-1β.
Rights and permissions
About this article
Cite this article
Rivest, S. Regulation of innate immune responses in the brain. Nat Rev Immunol 9, 429–439 (2009). https://doi.org/10.1038/nri2565
Issue Date:
DOI: https://doi.org/10.1038/nri2565
This article is cited by
-
Regulation of Inflammation by IRAK-M Pathway Can Be Associated with nAchRalpha7 Activation and COVID-19
Molecular Neurobiology (2024)
-
Regulation of Microglial Activation by Wnt/β-Catenin Signaling After Global Cerebral Ischemia in Mice
Molecular Neurobiology (2024)
-
Roles and regulation of microglia activity in multiple sclerosis: insights from animal models
Nature Reviews Neuroscience (2023)
-
Species-specific metabolic reprogramming in human and mouse microglia during inflammatory pathway induction
Nature Communications (2023)
-
Roles and mechanisms of exosomal microRNAs in viral infections
Archives of Virology (2023)