Key Points
-
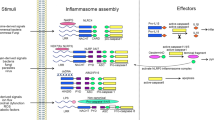
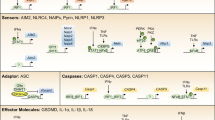
Hosts have evolved strategies to detect and respond rapidly to invading microorganisms and host-cell damage or 'danger signals'. The caspase-1 inflammasome is a dynamic complex in which specific NOD-like receptors (NLRs) and adaptor molecules are brought into play, depending on the nature of the primary trigger.
-
ASC (apoptosis-associated speck-like protein containing a CARD) has a central role in the inflammasome. Through homotypic protein–protein interactions with its own CARD (caspase-recruitment domain) and PYD (pyrin domain), ASC is thought to act as a direct bridge between the sensor NALPs (NACHT-, LRR- and pyrin-domain-containing proteins) and the downstream effector caspase-1.
-
The two-signal activation model is derived from studies of the treatment of cultured macrophages with pathogen-associated molecular patterns, which, in the absence of ATP, are insufficient to activate the inflammasome complex. However, infection with bacterial pathogens both in vitro and in vivo does not require ATP to trigger the inflammasome.
-
NALP3 is involved in sensing toxins, 'danger signals' such as gout crystals, Staphylococcus aureus, Listeria monocytogenes, bacterial RNA and trinitrophenylchloride.
-
IPAF (ICE-protease activating factor) is involved in sensing various intracellular bacterial pathogens, such as Salmonella typhimurium, Shigella flexneri and Legionella pneumophila. Flagellin might be one common pathogen-associated molecule that is recognized by IPAF and/or NAIP5 (neuronal apoptosis inhibitor protein 5).
-
ASC is required for the inflammasome recognition of Francisella tularensis.
Abstract
The NOD-like receptors have important roles in innate immunity as intracellular sensors of microbial components and cell injury. It has been proposed that these cytosolic proteins regulate the cysteine protease caspase-1 within a multiprotein complex known as the 'inflammasome'. Activation of caspase-1 leads to the cleavage and activation of pro-inflammatory cytokines such as interleukin-1β (IL-1β) and IL-18, as well as host-cell death. The analysis of mice that are deficient in various inflammasome components has revealed that the inflammasome is a dynamic entity that is assembled from different adaptors in a stimulus-dependent manner. Here we review recent work on the activation of the inflammasome in response to various bacterial pathogens and tissue damage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Raskin, D. M., Seshadri, R., Pukatzki, S. U. & Mekalanos, J. J. Bacterial genomics and pathogen evolution. Cell 124, 703–714 (2006).
Pizarro-Cerda, J. & Cossart, P. Bacterial adhesion and entry into host cells. Cell 124, 715–727 (2006).
Akira, S. & Takeda, K. Toll-like receptor signalling. Nature Rev. Immunol. 4, 499–511 (2004).
Strober, W., Murray, P. J., Kitani, A. & Watanabe, T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nature Rev. Immunol. 6, 9–20 (2006).
Ting, J. P., Kastner, D. L. & Hoffman, H. M. CATERPILLERs, pyrin and hereditary immunological disorders. Nature Rev. Immunol. 6, 183–195 (2006).
Inohara, N., Chamaillard, M., McDonald, C. & Nunez, G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu. Rev. Biochem. 74, 355–383 (2005).
Kufer, T. A., Fritz, J. H. & Philpott, D. J. NACHT-LRR proteins (NLRs) in bacterial infection and immunity. Trends Microbiol. 13, 381–388 (2005).
Viala, J. et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nature Immunol. 5, 1166–1174 (2004).
Ren, T., Zamboni, D. S., Roy, C. R., Dietrich, W. F. & Vance, R. E. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2, e18 (2006).
Miao, E. A. et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nature Immunol. 7, 569–575 (2006).
Franchi, L. et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nature Immunol. 7, 576–582 (2006). References 10 and 11 provide evidence that caspase-1 is activated in response to flagellin that is secreted by the intracellular pathogen S. typhimurium in an IPAF-dependent manner.
Philpott, D. J. & Girardin, S. E. The role of Toll-like receptors and Nod proteins in bacterial infection. Mol. Immunol. 41, 1099–1108 (2004).
Meylan, E., Tschopp, J. & Karin, M. Intracellular pattern recognition receptors in the host response. Nature 442, 39–44 (2006).
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-1β. Mol. Cell 10, 417–426 (2002). The first description of the caspase-1 inflammasome.
Schmitz, J. et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490 (2005).
Gurcel, L., Abrami, L., Girardin, S., Tschopp, J. & van der Goot, F. G. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 126, 1135–1145 (2006). Describes the role of caspase-1 in activating lipid metabolic pathways in fibroblasts.
Tschopp, J., Irmler, M. & Thome, M. Inhibition of Fas death signals by FLIPs. Curr. Opin. Immunol. 10, 552–558 (1998).
Chu, Z. L. et al. A novel enhancer of the Apaf1 apoptosome involved in cytochrome c-dependent caspase activation and apoptosis. J. Biol. Chem. 276, 9239–9245 (2001).
Martinon, F. & Tschopp, J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 15 September 2006 (doi: 10.1038/sj.cdd.4402038).
Sutterwala, F. S., Ogura, Y., Zamboni, D. S., Roy, C. R. & Flavell, R. A. NALP3: a key player in caspase-1 activation. J. Endotoxin Res. 12, 251–256 (2006).
Ogura, Y., Sutterwala, F. S. & Flavell, R. A. The inflammasome: first line of the immune response to cell stress. Cell 126, 659–662 (2006).
Drenth, J. P. & van der Meer, J. W. The inflammasome — a linebacker of innate defense. N. Engl. J. Med. 355, 730–732 (2006).
Franchi, L., McDonald, C., Kanneganti, T. D., Amer, A. & Nunez, G. Nucleotide-binding oligomerization domain-like receptors: intracellular pattern recognition molecules for pathogen detection and host defense. J. Immunol. 177, 3507–3513 (2006).
Masumoto, J. et al. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J. Biol. Chem. 274, 33835–33838 (1999).
Conway, K. E. et al. TMS1, a novel proapoptotic caspase recruitment domain protein, is a target of methylation-induced gene silencing in human breast cancers. Cancer Res. 60, 6236–6242 (2000).
Martinon, F. & Tschopp, J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell 117, 561–574 (2004).
Agostini, L. et al. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle–Wells autoinflammatory disorder. Immunity 20, 319–325 (2004).
Dowds, T. A., Masumoto, J., Zhu, L., Inohara, N. & Nunez, G. Cryopyrin-induced interleukin 1β secretion in monocytic cells: enhanced activity of disease-associated mutants and requirement for ASC. J. Biol. Chem. 279, 21924–21928 (2004).
Yu, J. W. et al. Cryopyrin and pyrin activate caspase-1, but not NF-κB, via ASC oligomerization. Cell Death Differ. 13, 236–249 (2006).
Hoffman, H. M., Mueller, J. L., Broide, D. H., Wanderer, A. A. & Kolodner, R. D. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle–Wells syndrome. Nature Genet. 29, 301–305 (2001). Describes the first evidence for an association between NALP3/cryopyrin variants and Muckle–Wells syndrome.
McDermott, M. F. & Aksentijevich, I. The autoinflammatory syndromes. Curr. Opin. Allergy Clin. Immunol. 2, 511–516 (2002).
Feldmann, J. et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am. J. Hum. Genet. 71, 198–203 (2002).
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006). Describes NALP3/cyropyrin deficient mice and its requirement for inflammsome activation in response to toxins, ATP, S. aureus , and L. monocytogenes.
Kanneganti, T. D. et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440, 233–236 (2006). Describes NALP3-deficient mice and its requirement for inflammasome activation in response to bacterial RNA and small antiviral compounds.
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006). Describes NALP3 involvement in inflammasome activation in response to danger signals such as uric acid crystals.
Sutterwala, F. S. et al. Critical role for NALP3/CIAS1/cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24, 317–327 (2006).
Geddes, B. J. et al. Human CARD12 is a novel CED4/Apaf-1 family member that induces apoptosis. Biochem. Biophys. Res. Commun. 284, 77–82 (2001).
Poyet, J. L. et al. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J. Biol. Chem. 276, 28309–28313 (2001).
Dinarello, C. A. Biologic basis for interleukin-1 in disease. Blood 87, 2095–2147 (1996).
Messud-Petit, F. et al. Serp2, an inhibitor of the interleukin-1β-converting enzyme, is critical in the pathobiology of myxoma virus. J. Virol. 72, 7830–7839 (1998).
Johnston, J. B. et al. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity 23, 587–598 (2005).
Mariathasan, S. et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 (2004). Describes the role of ASC in response to ATP and of ASC and IPAF in response to the intracellular pathogen S. typhimurium.
Thornberry, N. A. & Molineaux, S. M. Interleukin-1β converting enzyme: a novel cysteine protease required for IL-1β production and implicated in programmed cell death. Protein Sci. 4, 3–12 (1995).
Burns, K., Martinon, F. & Tschopp, J. New insights into the mechanism of IL-1β maturation. Curr. Opin. Immunol. 15, 26–30 (2003).
Wang, S. et al. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92, 501–509 (1998).
Hogquist, K. A., Nett, M. A., Unanue, E. R. & Chaplin, D. D. Interleukin 1 is processed and released during apoptosis. Proc. Natl Acad. Sci. USA 88, 8485–8489 (1991).
Solle, M. et al. Altered cytokine production in mice lacking P2X7 receptors. J. Biol. Chem. 276, 125–132 (2001).
Di Virgilio, F., Baricordi, O. R., Romagnoli, R. & Baraldi, P. G. Leukocyte P2 receptors: a novel target for anti-inflammatory and anti-tumor therapy. Curr. Drug Targets Cardiovasc. Haematol. Disord. 5, 85–99 (2005).
Perregaux, D. & Gabel, C. A. Interleukin-1β maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 269, 15195–15203 (1994).
Walev, I., Reske, K., Palmer, M., Valeva, A. & Bhakdi, S. Potassium-inhibited processing of IL-1β in human monocytes. EMBO J. 14, 1607–1614 (1995).
Walev, I. et al. Potassium regulates IL-1β processing via calcium-independent phospholipase A2 . J. Immunol. 164, 5120–5124 (2000).
Andrei, C. et al. Phospholipases C and A2 control lysosome-mediated IL-1β secretion: Implications for inflammatory processes. Proc. Natl Acad. Sci. USA 101, 9745–9750 (2004).
Yamamoto, M. et al. ASC is essential for LPS-induced activation of procaspase-1 independently of TLR-associated signal adaptor molecules. Genes Cells 9, 1055–1067 (2004).
Kuida, K. et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1β converting enzyme. Science 267, 2000–2003 (1995).
Li, P. et al. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell 80, 401–411 (1995).
Ozoren, N. et al. Distinct roles of TLR2 and the adaptor ASC in IL-1β/IL-18 secretion in response to Listeria monocytogenes. J. Immunol. 176, 4337–4342 (2006).
Chen, Y., Smith, M. R., Thirumalai, K. & Zychlinsky, A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 15, 3853–3860 (1996).
Hersh, D. et al. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl Acad. Sci. USA 96, 2396–2401 (1999).
Hilbi, H. et al. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J. Biol. Chem. 273, 32895–32900 (1998).
Sansonetti, P. J. et al. Caspase-1 activation of IL-1β and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 12, 581–590 (2000).
Lara-Tejero, M. et al. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J. Exp. Med. 203, 1407–1412 (2006).
Raupach, B., Peuschel, S. K., Monack, D. M. & Zychlinsky, A. Caspase-1-mediated activation of interleukin-1β (IL-1β) and IL-18 contributes to innate immune defenses against Salmonella enterica serovar Typhimurium infection. Infect. Immun. 74 (2006).
Galan, J. E. Salmonella interactions with host cells: type III secretion at work. Annu. Rev. Cell Dev. Biol. 17, 53–86 (2001).
Goosney, D. L., Knoechel, D. G. & Finlay, B. B. Enteropathogenic E. coli, Salmonella, and Shigella: masters of host cell cytoskeletal exploitation. Emerg. Infect. Dis. 5, 216–223 (1999).
Cossart, P. & Sansonetti, P. J. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304, 242–248 (2004).
Lee, S. H. & Galan, J. E. Salmonella type III secretion-associated chaperones confer secretion-pathway specificity. Mol. Microbiol. 51, 483–495 (2004).
Zamboni, D. S. et al. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nature Immunol. 7, 318–325 (2006). Describes the requirement of NAIP5/Birc1e and IPAF for recognition of the intracellular pathogen L. pneumophila and activation of the inflammasome.
Yamamoto, Y., Klein, T. W., Newton, C. A., Widen, R. & Friedman, H. Growth of Legionella pneumophila in thioglycolate-elicited peritoneal macrophages from A/J mice. Infect. Immun. 56, 370–375 (1988).
Diez, E. et al. Birc1e is the gene within the Lgn1 locus associated with resistance to Legionella pneumophila. Nature Genet. 33, 55–60 (2003).
Wright, E. K. et al. Naip5 affects host susceptibility to the intracellular pathogen Legionella pneumophila. Curr. Biol. 13, 27–36 (2003).
Miller, L. K. An exegesis of IAPs: salvation and surprises from BIR motifs. Trends Cell Biol. 9, 323–328 (1999).
Damiano, J. S., Oliveira, V., Welsh, K. & Reed, J. C. Heterotypic interactions among NACHT domains: implications for regulation of innate immune responses. Biochem. J. 381, 213–219 (2004).
Molofsky, A. B. et al. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J. Exp. Med. 203, 1093–1104 (2006). References 9 and 73 provide evidence that caspase-1 is activated in response to flagellin that is secreted by the intracellular pathogen L. pneumophila.
Roy, D. et al. A process for controlling intracellular bacterial infections induced by membrane injury. Science 304, 1515–1518 (2004).
Mariathasan, S., Weiss, D. S., Dixit, V. M. & Monack, D. M. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 202, 1043–1049 (2005). Describes the role of ASC in activating the inflammasome in response to the intracellular pathogen F. tularensis.
Gavrilin, M. A. et al. Internalization and phagosome escape required for Francisella to induce human monocyte IL-1β processing and release. Proc. Natl Acad. Sci. USA 103, 141–146 (2006).
Grenier, J. M. et al. Functional screening of five PYPAF family members identifies PYPAF5 as a novel regulator of NF-κB and caspase-1. FEBS Lett. 530, 73–78 (2002).
Wang, L. et al. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-κB and caspase-1-dependent cytokine processing. J. Biol. Chem. 277, 29874–29880 (2002).
Boyden, E. D. & Dietrich, W. F. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nature Genet. 38, 240–244 (2006).
Annand, R. R. et al. Caspase-1 (interleukin-1β-converting enzyme) is inhibited by the human serpin analogue proteinase inhibitor 9. Biochem. J. 342, 655–665 (1999).
Saleh, M. et al. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature 440, 1064–1068 (2006).
Humke, E. W., Shriver, S. K., Starovasnik, M. A., Fairbrother, W. J. & Dixit, V. M. ICEBERG: a novel inhibitor of interleukin-1β generation. Cell 103, 99–111 (2000).
Druilhe, A., Srinivasula, S. M., Razmara, M., Ahmad, M. & Alnemri, E. S. Regulation of IL-1β generation by pseudo-ICE and ICEBERG, two dominant negative caspase recruitment domain proteins. Cell Death Differ. 8, 649–657 (2001).
Lee, S. H., Stehlik, C. & Reed, J. C. COP, a caspase recruitment domain-containing protein and inhibitor of caspase-1 activation processing. J. Biol. Chem. 276, 34495–34500 (2001).
Razmara, M. et al. CARD-8 protein, a new CARD family member that regulates caspase-1 activation and apoptosis. J. Biol. Chem. 277, 13952–13958 (2002).
Lamkanfi, M. et al. INCA, a novel human caspase recruitment domain protein that inhibits interleukin-1β generation. J. Biol. Chem. 279, 51729–51738 (2004).
Aksentijevich, I. et al. Mutation and haplotype studies of familial Mediterranean fever reveal new ancestral relationships and evidence for a high carrier frequency with reduced penetrance in the Ashkenazi Jewish population. Am. J. Hum. Genet. 64, 949–962 (1999).
Chae, J. J. et al. Targeted disruption of pyrin, the FMF protein, causes heightened sensitivity to endotoxin and a defect in macrophage apoptosis. Mol. Cell 11, 591–604 (2003).
Chae, J. J. et al. The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1β production. Proc. Natl Acad. Sci. USA 103, 9982–9987 (2006).
Salvesen, G. S. & Dixit, V. M. Caspase activation: the induced-proximity model. Proc. Natl Acad. Sci. USA 96, 10964–10967 (1999).
Acknowledgements
The author thanks T. Henry, I. Brodsky, S. Falkow and D.S. Weiss for critical reading of the manuscript.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Flagellin
-
A protein that arranges itself in a hollow cylinder to form the filament in bacterial flagellum, and is required for bacterial motility and often for virulence.
- Zymogen
-
An inactive enzyme that requires biochemical change to become an active enzyme.
- Endogenous pyrogen
-
A host molecule that can cause fever and is important for host immune defences.
- Purinergic receptors
-
A family of plasma-membrane molecules that are involved in several known cellular functions, such as vascular reactivity, apoptosis and cytokine secretion.
- Danger signals
-
Molecules that alert the innate immune system and trigger defensive immune responses, which are referred to as danger-associated molecular patterns (DAMPs), that indicate cellular stress.
- Tularaemia
-
A zoonotic infectious disease, also called rabbit fever, that is caused by the bacterium Francisella tularensis and is often transmitted to humans by contact with animal tissues or from tick bites. Francisella tularensis is considered a potential bioterrorism agent owing to the low infectious dose (10 organisms) and its ability to be transmitted by aerosols.
Rights and permissions
About this article
Cite this article
Mariathasan, S., Monack, D. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol 7, 31–40 (2007). https://doi.org/10.1038/nri1997
Issue Date:
DOI: https://doi.org/10.1038/nri1997
This article is cited by
-
Potential of extracellular vesicles for early prediction of severity and potential risk stratification in critical inflammatory diseases
Journal of Cell Communication and Signaling (2023)
-
LncRNA PVT1 upregulates FBN1 by sponging miR-30b-5p to aggravate pulpitis
Molecular & Cellular Toxicology (2023)
-
The Interaction of OTUB1 and TRAF3 Mediates NLRP3 Inflammasome Activity to Regulate TGF-β1-induced BEAS-2B Cell Injury
Applied Biochemistry and Biotechnology (2023)
-
Antiflammatory activity and potential dermatological applications of characterized humic acids from a lignite and a green compost
Scientific Reports (2022)
-
MyD88-dependent BCG immunotherapy reduces tumor and regulates tumor microenvironment in bladder cancer murine model
Scientific Reports (2021)