Key Points
-
Hypoxia and inflammation are frequently co-incidental microenvironmental features of sites of concentrated physiological or pathological immune activity.
-
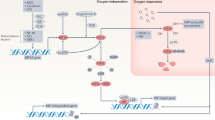
Hypoxia activates hypoxia-inducible factor, which is a major regulator of multiple aspects of immune cell function. Consequently, hypoxia plays a key role in the regulation of immunity and inflammation.
-
The impact of hypoxia on immunity and inflammation is site-specific and cell type-specific.
-
Pharmacological hydroxylase inhibition, which activates hypoxia-sensitive pathways, is profoundly protective in multiple models of inflammation.
Abstract
Immunological niches are focal sites of immune activity that can have varying microenvironmental features. Hypoxia is a feature of physiological and pathological immunological niches. The impact of hypoxia on immunity and inflammation can vary depending on the microenvironment and immune processes occurring in a given niche. In physiological immunological niches, such as the bone marrow, lymphoid tissue, placenta and intestinal mucosa, physiological hypoxia controls innate and adaptive immunity by modulating immune cell proliferation, development and effector function, largely via transcriptional changes driven by hypoxia-inducible factor (HIF). By contrast, in pathological immunological niches, such as tumours and chronically inflamed, infected or ischaemic tissues, pathological hypoxia can drive tissue dysfunction and disease development through immune cell dysregulation. Here, we differentiate between the effects of physiological and pathological hypoxia on immune cells and the consequences for immunity and inflammation in different immunological niches. Furthermore, we discuss the possibility of targeting hypoxia-sensitive pathways in immune cells for the treatment of inflammatory disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Beerman, I., Luis, T. C., Singbrant, S., Lo Celso, C. & Méndez-Ferrer, S. The evolving view of the hematopoietic stem cell niche. Exp. Hematol. 50, 22–26 (2017).
Shah, D. K. & Zúñiga-Pflücker, J. C. An overview of the intrathymic intricacies of T cell development. J. Immunol. 192, 4017–4023 (2014).
Campbell, E. L., Kao, D. J. & Colgan, S. P. Neutrophils and the inflammatory tissue microenvironment in the mucosa. Immunol. Rev. 273, 112–120 (2016).
Lin, E. W., Karakasheva, T. A., Hicks, P. D., Bass, A. J. & Rustgi, A. K. The tumor microenvironment in esophageal cancer. Oncogene 35, 5337–5349 (2016).
Maru, Y. The lung metastatic niche. J. Mol. Med. (Berl.) 93, 1185–1192 (2015).
Biswas, S. et al. Microenvironmental control of stem cell fate in intestinal homeostasis and disease. J. Pathol. 237, 135–145 (2015).
Hallenbeck, J. M., Hansson, G. K. & Becker, K. J. Immunology of ischemic vascular disease: plaque to attack. Trends Immunol. 26, 550–556 (2005).
Gobert, A. P. & Wilson, K. T. The immune battle against Helicobacter pylori infection: NO offense. Trends Microbiol. 24, 366–376 (2016).
Quante, M. & Wang, T. C. Inflammation and stem cells in gastrointestinal carcinogenesis. Physiology (Bethesda) 23, 350–359 (2008).
Multhoff, G., Molls, M. & Radons, J. Chronic inflammation in cancer development. Front. Immunol. 2, 98 (2012).
Taylor, C. T., Doherty. G., Fallon, P. G. & Cummins, E. P. Hypoxia-dependent regulation of inflammatory pathways in immune cells. J. Clin. Invest. 126, 3716–3724 (2016).
Cummins, E. P., Keogh, C. E., Crean, D. & Taylor, C. T. The role of HIF in immunity and inflammation. Mol. Aspects Med. 47–48, 24–34 (2016).
Scholz, C. C. & Taylor, C. T. Targeting the HIF pathway in inflammation and immunity. Curr. Opin. Pharmacol. 13, 646–653 (2013).
Taylor, C. T. & McElwain, J. C. Ancient atmospheres and the evolution of oxygen sensing via the hypoxia-inducible factor in metazoans. Physiology (Bethesda) 25, 272–279 (2010).
Colgan, S. P. & Taylor, C. T. Hypoxia: an alarm signal during intestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 7, 281–287 (2010).
Zheng, L., Kelly, C. J. & Colgan, S. P. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A review in the theme: cellular responses to hypoxia. Am. J. Physiol. Cell Physiol. 309, C350–C360 (2015).
Semenza, G. L. The hypoxic tumor microenvironment: a driving force for breast cancer progression. Biochim. Biophys. Acta 1863, 382–391 (2016).
Semenza, G. L. Molecular mechanisms mediating metastasis of hypoxic breast cancer cells. Trends Mol. Med. 18, 534–543 (2012).
Colgan, S. P., Campbell, E. L. & Kominsky, D. J. Hypoxia and mucosal inflammation. Annu. Rev. Pathol. 11, 77–100 (2016).
Eltzschig, H. K. & Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 364, 656–665 (2011).
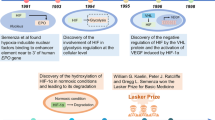
Loenarz, C. et al. The hypoxia-inducible transcription factor pathway regulates oxygen sensing in the simplest animal, Trichoplax adhaerens. EMBO Rep. 12, 63–70 (2011). This paper identifies the highly evolutionarily conserved nature of the oxygen-sensing hydroxylase–HIF-dependent pathway in all metazoans.
Cummins, E. P. & Taylor, C. T. Hypoxia-responsive transcription factors. Pflugers Arch. 450, 363–371 (2005).
Semenza, G. L. Hypoxia-inducible factors in physiology and medicine. Cell 148, 399–408 (2012).
Ratcliffe, P. J. Oxygen sensing and hypoxia signalling pathways in animals: the implications of physiology for cancer. J. Physiol. 591, 2027–2042 (2013).
Bunn, H. F. & Poyton, R. O. Oxygen sensing and molecular adaptation to hypoxia. Physiol. Rev. 76, 839–885 (1996).
Taylor, C. T. & Moncada, S. Nitric oxide, cytochrome C oxidase, and the cellular response to hypoxia. Arterioscler. Thromb. Vasc. Biol. 30, 643–647 (2010).
Kaelin, W. G. Jr. The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat. Rev. Cancer 8, 865–873 (2008).
Kaelin, W. G. Jr & Ratcliffe, P. J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 (2008).
Selfridge, A. C. et al. Hypercapnia suppresses the HIF-dependent adaptive response to hypoxia. J. Biol. Chem. 291, 11800–11808 (2016).
Hubbi, M. E. & Semenza, G. L. An essential role for chaperone-mediated autophagy in cell cycle progression. Autophagy 11, 850–851 (2015).
Hubbi, M. E., Gilkes, D. M., Hu, H., Kshitiz, Ahmed, I. & Semenza, G. L. Cyclin-dependent kinases regulate lysosomal degradation of hypoxia-inducible factor 1α to promote cell-cycle progression. Proc. Natl Acad. Sci. USA 111, E3325–E3334 (2014).
Intlekofer, A. M. et al. L-2-Hydroxyglutarate production arises from noncanonical enzyme function at acidic pH. Nat. Chem. Biol. 13, 494–500 (2017).
Tyrakis, P. A. et al. S-2-hydroxyglutarate regulates CD8+ T-lymphocyte fate. Nature 540, 236–241 (2016). This study demonstrates that the HIF pathway plays a key role in regulating antitumour activity in cytotoxic T lymphocytes.
Koivunen, P. et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature 483, 484–488 (2012).
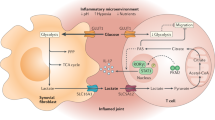
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013). This study demonstrates that succinate is an immunometabolite that regulates innate immunity through the HIF pathway.
Chandel, N. S. Mitochondrial regulation of oxygen sensing. Adv. Exp. Med. Biol. 661, 339–354 (2010).
Schödel, J., Mole, D. R. & Ratcliffe, P. J. Pan-genomic binding of hypoxia-inducible transcription factors. Biol. Chem. 394, 507–517 (2013).
Mole, D. R. et al. Genome-wide association of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha DNA binding with expression profiling of hypoxia-inducible transcripts. J. Biol. Chem. 284, 16767–16775 (2009).
Duan, C. Hypoxia-inducible factor 3 biology: complexities and emerging themes. Am. J. Physiol. Cell Physiol. 310, C260–269 (2016).
Palazon, A., Goldrath, A. W., Nizet, V. & Johnson, R. S. HIF transcription factors, inflammation, and immunity. Immunity 41, 518–528 (2014).
Lenihan, C. R. & Taylor, C. T. The impact of hypoxia on cell death pathways. Biochem. Soc. Trans. 41, 657–663 (2013).
Haase, V. H. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 27, 41–53 (2013).
Cummins, E. P. & Crean, D. Hypoxia and inflammatory bowel disease. Microbes Infect. 19, 210–221 (2017).
Glover, L. E. Lee, J. S. & Colgan, S. P. Oxygen metabolism and barrier regulation in the intestinal mucosa. J. Clin. Invest. 126, 3680–3688 (2016).
Ramakrishnan, S. K. & Shah, Y. M. Role of intestinal HIF-2α in health and disease. Annu. Rev. Physiol. 78, 301–325 (2016).
Ivan, M. & Kaelin, W. G. Jr. The EGLN-HIF O2-sensing system: multiple inputs and feedbacks. Mol. Cell 66, 772–779 (2017).
Harris, A. J., Thompson, A. R., Whyte, M. K. & Walmsley, S. R. HIF-mediated innate immune responses: cell signaling and therapeutic implications. Hypoxia (Auckl.) 2, 47–58 (2014).
Lin, N. & Simon, M. C. Hypoxia-inducible factors: key regulators of myeloid cells during inflammation. J. Clin. Invest. 126, 3661–3671 (2016).
Mills, E. & O'Neill, L. A. Succinate: a metabolic signal in inflammation. Trends Cell Biol. 24, 313–320 (2014).
Hammami, A., Charpentier, T., Smans, M. & Stäger, S. IRF-5-mediated inflammation limits CD8+ T Cell expansion by inducing HIF-1α and impairing dendritic cell functions during Leishmania infection. PLoS Pathog. 11, e1004938 (2015).
Wobben, R. et al. Role of hypoxia inducible factor-1α for interferon synthesis in mouse dendritic cells. Biol. Chem. 394, 495–505 (2013).
Cho, S. H. et al. Germinal centre hypoxia and regulation of antibody qualities by a hypoxia response system. Nature 537, 234–238 (2016).
Ward, J. B. et al. Hydroxylase inhibition attenuates colonic epithelial secretory function and ameliorates experimental diarrhea. FASEB J. 25, 535–543 (2011).
Kelly, C. J. et al. Fundamental role for HIF-1α in constitutive expression of human β defensin-1. Mucosal Immunol. 6, 1110–1118 (2013).
Du, J. et al. pVHL negatively regulates antiviral signaling by targeting MAVS for proteasomal degradation. J. Immunol. 195, 1782–1790 (2015).
Stehle, F. et al. VHL-dependent alterations in the secretome of renal cell carcinoma: association with immune cell response? Oncotarget 6, 43420–43437 (2015).
Scholz, C. C. & Taylor, C. T. Hydroxylase-dependent regulation of the NF-κB pathway. Biol. Chem. 394, 479–493 (2013).
Corcoran, S. E. & O'Neill, L. A. HIF1α and metabolic reprogramming in inflammation. J. Clin. Invest. 126, 3699–3707 (2016).
Mills, E. L. et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167, 457–470 (2016).
O'Neill, L. A., Kishton, R. J. & Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 16553–16565 (2016).
Semenza, G. L., Roth, P. H., Fang, H. M. & Wang, G. L. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 269, 23757–23763 (1994). This study identifies the control of glycolytic metabolism by the HIF pathway.
Halligan, D. N., Murphy, S. J. & Taylor, C. T. The hypoxia-inducible factor (HIF) couples immunity with metabolism. Semin. Immunol. 28, 469–477 (2016).
Michiels, C., Tellier, C. & Feron, O. Cycling hypoxia: a key feature of the tumor microenvironment. Biochim. Biophys. Acta 1866, 76–86 (2016).
Toffoli, S. & Michiels, C. Intermittent hypoxia is a key regulator of cancer cell and endothelial cell interplay in tumours. FEBS J. 275, 2991–3002 (2008).
Ryan, S., Taylor, C. T. & McNicholas, W. T. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 112, 2660–2667 (2005).
Taylor, C. T., Kent, B. D., Crinion, S. J., McNicholas, W. T. & Ryan, S. Human adipocytes are highly sensitive to intermittent hypoxia induced NF-kappaB activity and subsequent inflammatory gene expression. Biochem. Biophys. Res. Commun. 447, 660–665 (2014).
D'Ignazio, L., Bandarra, D. & Rocha, S. NF-κB and HIF crosstalk in immune responses. FEBS J. 283, 413–424 (2016).
Taylor, C. T. Interdependent roles for hypoxia inducible factor and nuclear factor-kappaB in hypoxic inflammation. J. Physiol. 586, 4055–4059 (2008).
Zhong, Z., Sanchez-Lopez, E. & Karin, M. Autophagy, inflammation, and immunity: a troika governing cancer and its treatment. Cell 166, 288–298 (2016).
Shalapour, S. & Karin, M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J. Clin. Invest. 125, 3347–3355 (2015).
Frede, S., Stockmann, C., Freitag, P. & Fandrey, J. Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-kappaB. Biochem. J. 396, 517–527 (2006).
Pérez, S., Taléns-Visconti, R., Rius-Pérez, S., Finamor, I. & Sastre, J. Redox signaling in the gastrointestinal tract. Free Radic. Biol. Med. 104, 75–103 (2017).
Chandel, N. S. et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J. Biol. Chem. 275, 25130–25138 (2000).
Masson, N. et al. The FIH hydroxylase is a cellular peroxide sensor that modulates HIF transcriptional activity. EMBO Rep. 13, 251–257 (2012).
Hagen, T., Taylor, C. T., Lam, F. & Moncada, S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science 302, 1975–1978 (2003). This study demonstrates that nitric oxide, a key mediator of inflammation, can regulate HIF stability through the control of intracellular oxygen availability.
Lohninger, L. et al. Hydrogen sulphide induces HIF-1α and Nrf2 in THP-1 macrophages. Biochimie 112, 187–195 (2015).
Flannigan, K. L. et al. Proresolution effects of hydrogen sulfide during colitis are mediated through hypoxia-inducible factor-1α. FASEB J. 29, 1591–1602 (2015).
Wu, B., Teng, H., Yang, G., Wu, L. & Wang, R. Hydrogen sulfide inhibits the translational expression of hypoxia-inducible factor-1α. Br. J. Pharmacol. 167, 1492–1505 (2012).
Eliasson, P. & Jönsson, J. I. The hematopoietic stem cell niche: low in oxygen but a nice place to be. J. Cell. Physiol. 222, 17–22 (2010).
Parmar, K., Mauch, P., Vergilio, J. A., Sackstein, R. & Down, J. D. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc. Natl Acad. Sci. USA 104, 5431–5436 (2007).
Mortensen, B. T. et al. Changing bone marrow micro-environment during development of acute myeloid leukaemia in rats. Br. J. Haematol. 102, 458–464 (1998).
Morikawa, T. & Takubo, K. Hypoxia regulates the hematopoietic stem cell niche. Pflugers Arch. 468, 13–22 (2015).
Forristal, C. E. et al. Pharmacologic stabilization of HIF-1α increases hematopoietic stem cell quiescence in vivo and accelerates blood recovery after severe irradiation. Blood 121, 759–769 (2013).
Takubo, K. et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 7, 391–402 (2010).
Forristal, C. E. & Levesque, J. P. Targeting the hypoxia-sensing pathway in clinical hematology. Stem Cells Transl Med. 3, 135–140 (2014).
Gezer, D., Vukovic, M., Soga, T., Pollard, P. J. & Kranc, K. R. Concise review: genetic dissection of hypoxia signaling pathways in normal and leukemic stem cells. Stem Cells 32, 1390–1397 (2014).
Annese, V., Navarro-Guerrero, E., Rodríguez-Prieto, I. & Pardal, R. Physiological plasticity of neural-crest-derived stem cells in the adult mammalian carotid body. Cell Rep. 19, 471–478 (2017).
Mazumdar, J. et al. O2 regulates stem cells through Wnt/β-catenin signalling. Nat. Cell Biol. 12, 1007–1013 (2010).
Simsek, T. et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 7, 380–390 (2010).
Krock, B. L. et al. The aryl hydrocarbon receptor nuclear translocator is an essential regulator of murine hematopoietic stem cell viability. Blood 125, 3263–3272 (2015).
Guitart, A. V. et al. Hif-2α is not essential for cell-autonomous hematopoietic stem cell maintenance. Blood 122, 1741–1745 (2013).
Abbott, R. K. et al. Germinal center hypoxia potentiates immunoglobulin class switch recombination. J. Immunol. 197, 4014–4020 (2016). References 52 and 92 demonstrate that physiological hypoxia in GCs plays a key role in B cell development.
Kumpel, B. M. & Manoussaka, M. S. Placental immunology and maternal alloimmune responses. Vox Sang. 102, 2–12 (2012).
Fryer, B. H. & Simon, M. C. Hypoxia, HIF and the placenta. Cell Cycle 5, 495–498 (2006).
Macklin, P. S., McAuliffe, J., Pugh, C. W. & Yamamoto, A. Hypoxia and HIF pathway in cancer and the placenta. Placenta 56, 8–13 (2017).
Yaghi, L. et al. Hypoxia inducible factor-1 mediates the expression of the immune checkpoint HLA-G in glioma cells through hypoxia response element located in exon 2. Oncotarget 7, 63690–63707 (2016).
Barsoum, I. B., Smallwood, C. A., Siemens, D. R. & Graham, C. H. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 74, 665–674 (2014).
Webster, W. S. & Abela, D. The effect of hypoxia in development. Birth Defects Res. C Embryo Today 81, 215–228 (2007).
Filiano, A. J., Gadani, S. P. & Kipnis, J. Interactions of innate and adaptive immunity in brain development and function. Brain Res. 1617, 18–27 (2015).
Dowling, D. J. & Levy, O. Ontogeny of early life immunity. Trends Immunol. 35, 299–310 (2014).
Shepherd, A. P. Metabolic control of intestinal oxygenation and blood flow. Fed. Proc. 41, 2084–2089 (1982).
Karhausen, J. et al. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J. Clin. Invest. 114, 1098–1106 (2004). This study identifies a protective role for the HIF pathway in intestinal inflammation.
Evans, S. M. et al. Detection of hypoxia in human squamous cell carcinoma by EF5 binding. Cancer Res. 60, 2018–2024 (2000).
Albenberg, L. et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology 147, 1055–1063 (2014).
Goda, F. et al. In vivo oximetry using EPR and India ink. Magn. Reson. Med. 33, 237–245 (1995).
He, G. et al. Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proc. Natl Acad. Sci. USA 96, 4586–4591 (1999).
Labiano, S., Palazon, A. & Melero, I. Immune response regulation in the tumor microenvironment by hypoxia. Semin. Oncol. 42, 378–386 (2015).
Unwith, S., Zhao, H., Hennah, L. & Ma, D. The potential role of HIF on tumour progression and dissemination. Int. J. Cancer. 136, 2491–2503 (2015).
Courtnay, R. et al. Cancer metabolism and the Warburg effect: the role of HIF-1 and PI3K. Mol. Biol. Rep. 42, 841–851 (2015).
Miller, J. F. & Sadelain, M. The journey from discoveries in fundamental immunology to cancer immunotherapy. Cancer Cell 27, 439–449 (2015).
Maus, M. V. et al. Adoptive immunotherapy for cancer or viruses. Annu. Rev. Immunol. 32, 189–225 (2014).
Corzo, C. A. et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 207, 2439–2453 (2010).
Williams, A. E. & Chambers, R. C. The mercurial nature of neutrophils: still an enigma in ARDS? Am. J. Physiol. Lung Cell. Mol. Physiol. 306, L217–L230 (2014).
Liu, Z. et al. AMP-activated protein kinase and Glycogen Synthase Kinase 3β modulate the severity of sepsis-induced lung injury. Mol. Med. 21, 937–950 (2015).
Campbell, E. L. et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 40, 66–77 (2014). This study identifies that the neutrophil oxidative burst is a key cause of mucosal hypoxia in inflammatory disease of the intestine.
Huang, J. S. et al. Chronic granulomatous disease caused by a deficiency in p47(phox) mimicking Crohn's disease. Clin. Gastroenterol. Hepatol. 2, 690–695 (2004).
Manresa, M. C., Godson, C. & Taylor, C. T. Hypoxia-sensitive pathways in inflammation-driven fibrosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R1369–R1380 (2014).
Devraj, G., Beerlage, C., Brüne, B. & Kempf, V. A. Hypoxia and HIF-1 activation in bacterial infections. Microbes Infect. 19, 144–156 (2017).
Werth, N. et al. Activation of hypoxia inducible factor 1 is a general phenomenon in infections with human pathogens. PLoS ONE. 5, e11576 (2010).
Schaffer, K. & Taylor, C. T. The impact of hypoxia on bacterial infection. FEBS J. 282, 2260–2266 (2015).
Worlitzsch, D. et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Invest. 109, 317–325 (2002).
Schaible, B., Schaffer, K. & Taylor, C. T. Hypoxia, innate immunity and infection in the lung. Respir. Physiol. Neurobiol. 174, 235–243 (2010).
Schaible, B. et al. Hypoxia modulates infection of epithelial cells by Pseudomonas aeruginosa. PLoS ONE. 8, e56491 (2013).
Schaible, B., Taylor, C. T. & Schaffer, K. Hypoxia increases antibiotic resistance in Pseudomonas aeruginosa through altering the composition of multidrug efflux pumps. Antimicrob. Agents Chemother. 56, 2114–2118 (2012).
Schaible, B. et al. Hypoxia reduces the pathogenicity of Pseudomonas aeruginosa by decreasing the expression of multiple virulence factors. J. Infect. Dis. 215, 1459–1467 (2017).
Koh, H. S. et al. The HIF-1/glial TIM-3 axis controls inflammation-associated brain damage under hypoxia. Nat. Commun. 6, 6340 (2015).
Zhang, J. et al. Hypoxia-Inducible Factor-2α limits natural killer T cell cytotoxicity in renal ischemia/reperfusion injury. J. Am. Soc. Nephrol. 27, 92–106 (2016).
Luo, L. et al. The role of HIF-1 in up-regulating MICA expression on human renal proximal tubular epithelial cells during hypoxia/reoxygenation. BMC Cell Biol. 11, 91 (2010).
Chan, M. C., Holt-Martyn, J. P., Schofield, C. J. & Ratcliffe, P. J. Pharmacological targeting of the HIF hydroxylases—a new field in medicine 1development. Mol. Aspects Med. 47–48, 54–75 (2016).
Muchnik, E. & Kaplan, J. HIF prolyl hydroxylase inhibitors for anemia. Expert Opin. Investig. Drugs 20, 645–656 (2011).
Maxwell, P. H. & Eckardt, K. U. HIF prolyl hydroxylase inhibitors for the treatment of renal anaemia and beyond. Nat. Rev. Nephrol. 12, 157–168 (2016).
Cho, H. et al. On-target efficacy of a HIF-2α antagonist in preclinical kidney cancer models. Nature. 539, 107–111 (2016).
Chen, W. et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 539, 112–117 (2016). References 132 and 133 demonstrate that selective targeting of the HIF2 pathway may be of clinical benefit in kidney cancer.
Cummins, E. P., Doherty, G. A. & Taylor, C. T. Hydroxylases as therapeutic targets in inflammatory bowel disease. Lab. Invest. 93, 378–383 (2013).
Hams, E. et al. The hydroxylase inhibitor dimethyloxallyl glycine attenuates endotoxic shock via alternative activation of macrophages and IL-10 production by B1 cells. Shock 36, 295–302 (2011).
Provenzano, R. et al. Oral hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) for the treatment of anemia in patients with CKD. Clin. J. Am. Soc. Nephrol. 11, 982–991 (2016).
Brigandi, R. A. et al. A novel hypoxia-inducible factor-prolyl hydroxylase inhibitor (GSK1278863) for anemia in CKD: 28 day, phase 2A randomized trial. Am. J. Kidney Dis. 67, 861–871 (2016).
Pergola, P. E., Spinowitz, B. S., Hartman, C. S., Maroni, B. J. & Haase, V. H. Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int. 90, 1115–1122 (2016).
Tambuwala, M. M. et al. Targeted delivery of the hydroxylase inhibitor DMOG provides enhanced efficacy with reduced systemic exposure in a murine model of colitis. J. Control. Release 217, 221–227 (2015).
Maxwell, P. H. et al. Sites of erythropoietin production. Kidney Int. 51, 393–401 (1997).
Spencer, J. A. et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508, 269–273 (2014).
Grimm, C. & Willmann, G. Hypoxia in the eye: a two-sided coin. High Alt. Med. Biol. 13169–13175 (2012).
Cummins, E. P. et al. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc. Natl Acad. Sci. USA 103, 18154–18159 (2006).
Cockman, M. E. et al. Posttranslational hydroxylation of ankyrin repeats in IkappaB proteins by the hypoxia-inducible factor (HIF) asparaginyl hydroxylase, factor inhibiting HIF (FIH). Proc. Natl Acad. Sci. USA; 103, 14767–1 4772. References 143 and 144 demonstrate that components of the NF-κB pathway are targets for hydroxylation.
Ghosh, S., Paul, A. & Sen, E. Tumor necrosis factor α-induced hypoxia-inducible factor 1α-β-catenin axis regulates major histocompatibility complex class I gene activation through chromatin remodeling. Mol. Cell. Biol. 33, 2718–2731 (2013).
Dang, E. V. et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell 146, 772–784 (2011).
Clambey, E. T. et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc. Natl Acad. Sci. USA 109, E2784–E2793 (2012).
Cummins, E. P. et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 134, 156–165 (2008).
Hindryckx, P. et al. Longitudinal quantification of inflammation in the murine dextran sodium sulfate-induced colitis model using muPET/CT. Inflamm. Bowel Dis. 17, 2058–2064 (2011).
Cosin-Roger, J. et al. Hypoxia ameliorates intestinal inflammation through NLRP3/mTOR downregulation and autophagy activation. Nat. Commun. 8, 98 (2017).
Robinson, A. et al. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 134, 145–155 (2008). References 148 and 151 demonstrate that pharmacological hydroxylase inhibition is protective in colitis.
Hindryckx, P. et al. Hydroxylase inhibition abrogates TNF-alpha-induced intestinal epithelial damage by hypoxia-inducible factor-1-dependent repression of FADD. J. Immunol. 185, 6306–6316 (2010).
Hirota, S. A. et al. Hypoxia-inducible factor signaling provides protection in Clostridium difficile-induced intestinal injury. Gastroenterology 139, 259–269 (2010).
Hart, M. L. et al. Hypoxia-inducible factor-1 alpha dependent protection from intestinal ischemia/reperfusion injury involves ecto-5′-nucleotidase (CD73) and the A2B adenosine receptor. J. Immunol. 186, 4367–4374 (2011).
Marchbank, T., Mahmood, A., Harten, S., Maxwell, P. H. & Playford, R. J. Dimethyloxalyglycine stimulates the early stages of gastrointestinal repair processes through VEGF-dependent mechanisms. Lab. Invest. 91, 1684–1694 (2011).
Keely, S. et al. Contribution of epithelial innate immunity to systemic protection afforded by prolyl hydroxylase inhibition in murine colitis. Mucosal Immunol. 7, 114–123 (2014).
Marks, E. et al. Oral delivery of prolyl hydroxylase inhibitor: AKB-4924 promotes localized mucosal healing in a mouse model of colitis. Inflamm. Bowel Dis. 21, 267–275 (2015).
Taniguchi, C. M. et al. PHD inhibition mitigates and protects against radiation-induced gastrointestinal toxicity via HIF2. Sci. Transl Med. 6, 236ra64 (2014).
Jeong, S. et al. Lipophilic modification enhances anti-colitic properties of rosmarinic acid by potentiating its HIF-prolyl hydroxylases inhibitory activity. Eur. J. Pharmacol. 747, 114–122 (2015).
Gupta, R. et al. Therapeutic treatment with a novel hypoxia-inducible factor hydroxylase inhibitor (TRC160334) ameliorates murine colitis. Clin. Exp. Gastroenterol. 7, 13–23 (2014).
Jamadarkhana, P. et al. Treatment with a novel hypoxia-inducible factor hydroxylase inhibitor (TRC160334) ameliorates ischemic acute kidney injury. Am. J. Nephrol. 36, 208–218 (2012).
Bernhardt, W. M. et al. Donor treatment with a PHD-inhibitor activating HIFs prevents graft injury and prolongs survival in an allogenic kidney transplant model. Proc. Natl Acad. Sci. USA 106, 21276–21281 (2009).
Manresa, M. C. et al. Hydroxylase inhibition regulates inflammation-induced intestinal fibrosis through the suppression of ERK-mediated TGF-β1 signaling [corrected]. Am. J. Physiol. Gastrointest. Liver Physiol. 311, G1076–G1090 (2016).
Acknowledgements
Work from the authors' laboratories is funded through research grants from Science Foundation Ireland, the European Union and the US National Institutes of Health.
Author information
Authors and Affiliations
Contributions
C.T.T. and S.P.C. both contributed to discussions of the content and the writing, review and editing of this manuscript
Corresponding author
Ethics declarations
Competing interests
C.T.T. and S.P.C. are members of the Scientific Advisory Board of Akebia Therapeutics.
Glossary
- Microenvironmental features
-
Physiochemical conditions found within a specific niche or tissue.
- Hypoxia
-
The condition that arises when cellular oxygen demand exceeds supply.
- Electron transport chain
-
(ETC). Primary eukaryotic system for the reduction of molecular oxygen and the generation of ATP. Located within mitochondria.
- Oxidative phosphorylation
-
(OXPHOS). The generation of cellular ATP using energy derived from electron transport during aerobic respiration.
- Lysosomal degradation pathway
-
A mechanism of intracellular protein degradation that involves proteolysis in lysosomal compartments.
- Glycolysis
-
The utilization of glucose to generate ATP.
- Physiological angiogenesis
-
The normal growth of blood vessels in healthy tissues.
- Carotid body
-
Small organelle situated at the bifurcation of the carotid artery responsible for sensing blood oxygen levels and regulating the respiratory rate.
- Semi-allogeneic trophoblasts
-
Fetal cells that express both maternal and paternal surface antigens.
- Crypt–villus axis
-
Structure at the mucosal surface of the small intestine.
- Erythropoiesis
-
The process by which red blood cell production is controlled. Involves the release of erythropoietin from cells of the kidney and liver.
Rights and permissions
About this article
Cite this article
Taylor, C., Colgan, S. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol 17, 774–785 (2017). https://doi.org/10.1038/nri.2017.103
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri.2017.103
This article is cited by
-
Attenuation of inflammatory bowel disease by oral administration of mucoadhesive polydopamine-coated yeast β-glucan via ROS scavenging and gut microbiota regulation
Journal of Nanobiotechnology (2024)
-
Nanoparticles in tumor microenvironment remodeling and cancer immunotherapy
Journal of Hematology & Oncology (2024)
-
Efficacy of quercetin in ameliorating hypoxia-induced hematological and histopathological alterations in rohu Labeo rohita
Fish Physiology and Biochemistry (2024)
-
Predicting the risk of pulmonary infection in patients with chronic kidney failure: A-C2GH2S risk score—a retrospective study
International Urology and Nephrology (2024)
-
PHD1-3 oxygen sensors in vivo—lessons learned from gene deletions
Pflügers Archiv - European Journal of Physiology (2024)