Key Points
-
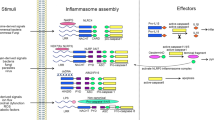
Inflammasomes are multiprotein complexes that are assembled by pattern-recognition receptors following the detection of pathogenic microorganisms and danger signals in the cytosol of host cells.
-
Inflammasomes activate inflammatory caspases, cysteine-dependent aspartate-directed proteases, which promote the maturation of the cytokines interleukin-1β (IL-1β) and IL-18, and induce a lytic type of cell death that is known as pyroptosis.
-
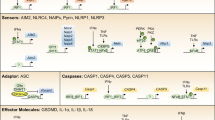
Canonical inflammasomes activate caspase 1 and are assembled by the protein pyrin or by members of the NOD-like receptor (NLR) and pyrin and HIN domain-containing (PYHIN) protein families, which detect diverse pathogen- and host-derived danger signals. The bacterial cell wall component lipopolysaccharide (LPS), one of the strongest immune system activators, leads to the assembly of non-canonical inflammasomes and the activation of caspases 4, 5 and 11.
-
Upon activation, inflammasome-forming receptors oligomerize in multi-subunit wheel-shaped assemblies, as exemplified by the cryo-electron microscopy structure of the NAIP–NLRC4 complex. These receptor complexes initiate the oligomerization of filaments formed by the inflammasome adaptor protein ASC, which aggregate to form the macromolecular ASC speck and serve as activation points for caspase 1.
-
Pyroptosis induction requires the protein gasdermin D, which is processed into an amino-terminal and carboxy-terminal fragment by inflammatory caspases. This releases the N-terminal domain of gasdermin D from an intramolecular inhibitory interaction with its C-terminal domain, thereby allowing the N-terminal domain to initiate pyroptosis.
-
Dysregulated inflammasome activation is linked to acquired and hereditary inflammatory disorders. Recent progress has shed light on the diverse mechanisms that regulate inflammasome assembly and activation on both a translational and a post-translational level.
Abstract
Inflammasomes are multiprotein signalling platforms that control the inflammatory response and coordinate antimicrobial host defences. They are assembled by pattern-recognition receptors following the detection of pathogenic microorganisms and danger signals in the cytosol of host cells, and they activate inflammatory caspases to produce cytokines and to induce pyroptotic cell death. The clinical importance of inflammasomes reaches beyond infectious disease, as dysregulated inflammasome activity is associated with numerous hereditary and acquired inflammatory disorders. In this Review, we discuss the recent developments in inflammasome research with a focus on the molecular mechanisms that govern inflammasome assembly, signalling and regulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-1β. Mol. Cell 10, 417–426 (2002).
von Moltke, J., Ayres, J. S., Kofoed, E. M., Chavarria-Smith, J. & Vance, R. E. Recognition of bacteria by inflammasomes. Annu. Rev. Immunol. 31, 73–106 (2013).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015). References 3 and 4 show that inflammatory caspases cleave gasdermin D, resulting in the generation of an N-terminal gasdermin D fragment that drives pyroptosis.
Broz, P. & Monack, D. M. Newly described pattern recognition receptors team up against intracellular pathogens. Nat. Rev. Immunol. 13, 551–565 (2013).
Boyden, E. D. & Dietrich, W. F. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 38, 240–244 (2006).
Levinsohn, J. L. et al. Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome. PLoS Pathog. 8, e1002638 (2012).
Hellmich, K. A. et al. Anthrax lethal factor cleaves mouse Nlrp1b in both toxin-sensitive and toxin-resistant macrophages. PLoS ONE 7, e49741 (2012).
Chavarria-Smith, J. & Vance, R. E. Direct proteolytic cleavage of NLRP1B is necessary and sufficient for inflammasome activation by anthrax lethal factor. PLoS Pathog. 9, e1003452 (2013).
Frew, B. C., Joag, V. R. & Mogridge, J. Proteolytic processing of Nlrp1b is required for inflammasome activity. PLoS Pathog. 8, e1002659 (2012).
Ewald, S. E., Chavarria-Smith, J. & Boothroyd, J. C. NLRP1 is an inflammasome sensor for Toxoplasma gondii. Infect. Immun. 82, 460–468 (2014).
Latz, E., Xiao, T. S. & Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13, 397–411 (2013).
Munoz-Planillo, R. et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 (2013). This study establishes that potassium efflux is the common denominator of a wide array of chemically and physically distinct NLRP3 stimuli.
He, Y., Zeng, M. Y., Yang, D., Motro, B. & Nunez, G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530, 354–357 (2016).
Shi, H. et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat. Immunol. 17, 250–258 (2016).
Schmid-Burgk, J. L. et al. A genome-wide CRISPR (clustered regularly interspaced short palindromic repeats) screen identifies NEK7 as an essential component of NLRP3 inflammasome activation. J. Biol. Chem. 291, 103–109 (2016). References 14–16 report that NEK7, a kinase that coordinates microtubule dynamics for spindle formation, is an essential component of the NLRP3 inflammasome.
Mariathasan, S. et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 (2004).
Miao, E. A. et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat. Immunol. 7, 569–575 (2006).
Franchi, L. et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nat. Immunol. 7, 576–582 (2006).
Miao, E. A. et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc. Natl Acad. Sci. USA 107, 3076–3308 (2010).
Zhao, Y. et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477, 596–600 (2011).
Kofoed, E. M. & Vance, R. E. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477, 592–595 (2011).
Tenthorey, J. L., Kofoed, E. M., Daugherty, M. D., Malik, H. S. & Vance, R. E. Molecular basis for specific recognition of bacterial ligands by NAIP/NLRC4 inflammasomes. Mol. Cell 54, 17–29 (2014).
Kortmann, J., Brubaker, S. W. & Monack, D. M. Cutting edge: inflammasome activation in primary human macrophages is dependent on flagellin. J. Immunol. 195, 815–819 (2015).
Muruve, D. A. et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452, 103–107 (2008).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Fernandes-Alnemri, T., Yu, J. W., Datta, P., Wu, J. & Alnemri, E. S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 (2009).
Roberts, T. L. et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323, 1057–1060 (2009).
Rathinam, V. A. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402 (2010).
Fernandes-Alnemri, T. et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat. Immunol. 11, 385–393 (2010).
Jones, J. W. et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc. Natl Acad. Sci. USA 107, 9771–9776 (2010).
Sauer, J. D. et al. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe 7, 412–419 (2010).
Dihlmann, S. et al. Increased expression and activation of absent in melanoma 2 inflammasome components in lymphocytic infiltrates of abdominal aortic aneurysms. Mol. Med. 20, 230–237 (2014).
Dombrowski, Y. et al. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci. Transl Med. 3, 82ra38 (2011).
Javierre, B. M. et al. Changes in the pattern of DNA methylation associate with twin discordance in systemic lupus erythematosus. Genome Res. 20, 170–179 (2010).
Dihlmann, S. et al. Lack of absent in melanoma 2 (AIM2) expression in tumor cells is closely associated with poor survival in colorectal cancer patients. Int. J. Cancer 135, 2387–2396 (2014).
Ponomareva, L. et al. AIM2, an IFN-inducible cytosolic DNA sensor, in the development of benign prostate hyperplasia and prostate cancer. Mol. Cancer Res. 11, 1193–1202 (2013).
Man, S. M. et al. Critical role for the DNA sensor AIM2 in stem cell proliferation and cancer. Cell 162, 45–58 (2015).
Wilson, J. E. et al. Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nat. Med. 21, 906–913 (2015).
Chae, J. J. et al. The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1β production. Proc. Natl Acad. Sci. USA 103, 9982–9987 (2006).
Hesker, P. R., Nguyen, M., Kovarova, M., Ting, J. P. & Koller, B. H. Genetic loss of murine pyrin, the Familial Mediterranean Fever protein, increases interleukin-1β levels. PLoS ONE 7, e51105 (2012).
Chae, J. J. et al. Gain-of-function pyrin mutations induce NLRP3 protein-independent interleukin-1β activation and severe autoinflammation in mice. Immunity 34, 755–768 (2011).
Masters, S. L. et al. Familial autoinflammation with neutrophilic dermatosis reveals a regulatory mechanism of pyrin activation. Sci. Transl Med. 8, 332ra45 (2016). This study describes a new mutation in pyrin that causes an autoinflammatory disorder known as pyrin-associated autoinflammation with neutrophilic dermatosis.
Xu, H. et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature 513, 237–241 (2014). This study demonstrates that pyrin assembles an inflammasome complex after detecting the inactivation of RHOA by bacterial pathogens.
Waite, A. L. et al. Pyrin and ASC co-localize to cellular sites that are rich in polymerizing actin. Exp. Biol. Med. (Maywood) 234, 40–52 (2009).
Kim, M. L. et al. Aberrant actin depolymerization triggers the pyrin inflammasome and autoinflammatory disease that is dependent on IL-18, not IL-1β. J. Exp. Med. 212, 927–938 (2015).
Hasegawa, M. et al. Protective role of commensals against Clostridium difficile infection via an IL-1β-mediated positive-feedback loop. J. Immunol. 189, 3085–3091 (2012).
Kimura, T. et al. TRIM-mediated precision autophagy targets cytoplasmic regulators of innate immunity. J. Cell Biol. 210, 973–989 (2015).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011). This paper reports the identification of a new non-canonical inflammasome pathway that results in caspase 11 activation in response to Gram-negative bacterial infections.
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014). This study shows that activation of the non-canonical inflammasome pathway involves the direct binding of LPS to mouse caspase 11 or human caspase 4 and caspase 5.
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Hagar, J. A., Powell, D. A., Aachoui, Y., Ernst, R. K. & Miao, E. A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013).
Broz, P. et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490, 288–291 (2012).
Baker, P. J. et al. NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur. J. Immunol. 45, 2918–2926 (2015).
Schmid-Burgk, J. L. et al. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur. J. Immunol. 45, 2911–2917 (2015).
Ruhl, S. & Broz, P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K efflux. Eur. J. Immunol. 45, 2927–2936 (2015).
Elinav, E. et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757 (2011).
Levy, M. et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell 163, 1428–1443 (2015).
Anand, P. K. et al. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature 488, 389–393 (2012).
Wlodarska, M. et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 156, 1045–1059 (2014).
Wang, P. et al. Nlrp6 regulates intestinal antiviral innate immunity. Science 350, 826–830 (2015).
Unterholzner, L. et al. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 11, 997–1004 (2010).
Storek, K. M., Gertsvolf, N. A., Ohlson, M. B. & Monack, D. M. cGAS and Ifi204 cooperate to produce type I IFNs in response to Francisella infection. J. Immunol. 194, 3236–3245 (2015).
Kerur, N. et al. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe 9, 363–375 (2011).
Doitsh, G. et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505, 509–514 (2014).
Monroe, K. M. et al. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science 343, 428–432 (2014).
Halff, E. F. et al. Formation and structure of a NAIP5–NLRC4 inflammasome induced by direct interactions with conserved N- and C-terminal regions of flagellin. J. Biol. Chem. 287, 38460–38472 (2012).
Hu, Z. et al. Structural and biochemical basis for induced self-propagation of NLRC4. Science 350, 399–404 (2015).
Zhang, L. et al. Cryo-EM structure of the activated NAIP2–NLRC4 inflammasome reveals nucleated polymerization. Science 350, 404–409 (2015). References 68 and 69 determine the structure of the NAIP–NLRC4 complex and characterize the assembly mechanism.
Diebolder, C. A., Halff, E. F., Koster, A. J., Huizinga, E. G. & Koning, R. I. Cryoelectron tomography of the NAIP5/NLRC4 inflammasome: implications for NLR activation. Structure 23, 2349–2357 (2015).
Hu, Z. et al. Crystal structure of NLRC4 reveals its autoinhibition mechanism. Science 341, 172–175 (2013).
Romberg, N. et al. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat. Genet. 46, 1135–1139 (2014).
Kitamura, A., Sasaki, Y., Abe, T., Kano, H. & Yasutomo, K. An inherited mutation in NLRC4 causes autoinflammation in human and mice. J. Exp. Med. 211, 2385–2396 (2014).
Canna, S. W. et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat. Genet. 46, 1140–1146 (2014). References 72–74 describe gain-of-function mutations in NLRC4 that cause autoinflammation with recurrent macrophage activation syndrome (MAS).
Jin, T. et al. Structures of the HIN domain:DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity 36, 561–571 (2012).
Lu, A. et al. Plasticity in PYD assembly revealed by cryo-EM structure of the PYD filament of AIM2. Cell Discov. 1, 15013 (2015).
Franklin, B. S. et al. The adaptor ASC has extracellular and 'prionoid' activities that propagate inflammation. Nat. Immunol. 15, 727–737 (2014).
Baroja-Mazo, A. et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat. Immunol. 15, 738–748 (2014).
Lu, A. et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156, 1193–1206 (2014). This study used a combination of cryo-electron microscopy and biochemical methods to characterize the structure of the ASC filament and proposed a unified mechanism for how inflammasomes are assembled.
Sborgi, L. et al. Structure and assembly of the mouse ASC inflammasome by combined NMR spectroscopy and cryo-electron microscopy. Proc. Natl Acad. Sci. USA 112, 13237–13242 (2015).
Broz, P., von Moltke, J., Jones, J. W., Vance, R. E. & Monack, D. M. Differential requirement for caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 8, 471–483 (2010).
Guey, B., Bodnar, M., Manie, S. N., Tardivel, A. & Petrilli, V. Caspase-1 autoproteolysis is differentially required for NLRP1b and NLRP3 inflammasome function. Proc. Natl Acad. Sci. USA 111, 17254–17259 (2014).
Fink, S. L. & Cookson, B. T. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 73, 1907–1916 (2005).
He, W. T. et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 25, 1285–1298 (2015).
Pierini, R. et al. AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ. 19, 1709–1721 (2012).
Yang, D., He, Y., Munoz-Planillo, R., Liu, Q. & Nunez, G. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity 43, 923–932 (2015).
Vanden Berghe, T. et al. Passenger mutations confound interpretation of all genetically modified congenic mice. Immunity 43, 200–209 (2015).
Dinarello, C. A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 (2009).
Monteleone, M., Stow, J. L. & Schroder, K. Mechanisms of unconventional secretion of IL-1 family cytokines. Cytokine 74, 213–218 (2015).
Chen, K. W. et al. The neutrophil NLRC4 inflammasome selectively promotes IL-1β maturation without pyroptosis during acute Salmonella challenge. Cell Rep. 8, 570–582 (2014).
Humke, E. W., Shriver, S. K., Starovasnik, M. A., Fairbrother, W. J. & Dixit, V. M. ICEBERG: a novel inhibitor of interleukin-1β generation. Cell 103, 99–111 (2000).
Lamkanfi, M. et al. INCA, a novel human caspase recruitment domain protein that inhibits interleukin-1β generation. J. Biol. Chem. 279, 51729–51738 (2004).
Druilhe, A., Srinivasula, S. M., Razmara, M., Ahmad, M. & Alnemri, E. S. Regulation of IL-1β generation by Pseudo-ICE and ICEBERG, two dominant negative caspase recruitment domain proteins. Cell Death Differ. 8, 649–657 (2001).
Karasawa, T. et al. Oligomerized CARD16 promotes caspase-1 assembly and IL-1β processing. FEBS Open Bio 5, 348–356 (2015).
Saleh, M. et al. Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature 429, 75–79 (2004).
Saleh, M. et al. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature 440, 1064–1068 (2006).
Skeldon, A. M. et al. Caspase-12, but not caspase-11, inhibits obesity and insulin resistance. J. Immunol. 196, 437–447 (2016).
de Almeida, L. et al. The PYRIN domain-only protein POP1 inhibits inflammasome assembly and ameliorates inflammatory disease. Immunity 43, 264–276 (2015).
Bedoya, F., Sandler, L. L. & Harton, J. A. Pyrin-only protein 2 modulates NF-κB and disrupts ASC:CLR interactions. J. Immunol. 178, 3837–3845 (2007).
Dorfleutner, A. et al. Cellular pyrin domain-only protein 2 is a candidate regulator of inflammasome activation. Infect. Immun. 75, 1484–1492 (2007).
Khare, S. et al. The PYRIN domain-only protein POP3 inhibits ALR inflammasomes and regulates responses to infection with DNA viruses. Nat. Immunol. 15, 343–353 (2014). This study shows that POP3 is an IFN-inducible endogenous regulator of the AIM2 inflammasome that inhibits receptor signalling by directly binding to its PYD.
Yin, Q. et al. Molecular mechanism for p202-mediated specific inhibition of AIM2 inflammasome activation. Cell Rep. 4, 327–339 (2013).
Qu, Y. et al. Phosphorylation of NLRC4 is critical for inflammasome activation. Nature 490, 539–542 (2012).
Qu, Y. et al. NLRP3 recruitment by NLRC4 during Salmonella infection. J. Exp. Med. 213, 877–885 (2016).
Suzuki, S. et al. Shigella type III secretion protein MxiI is recognized by Naip2 to induce Nlrc4 inflammasome activation independently of Pkcδ. PLoS Pathog. 10, e1003926 (2014).
Py, B. F., Kim, M. S., Vakifahmetoglu-Norberg, H. & Yuan, J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol. Cell 49, 331–338 (2013).
Juliana, C. et al. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J. Biol. Chem. 287, 36617–36622 (2012).
Lu, B. et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature 488, 670–674 (2012).
He, Y., Franchi, L. & Nunez, G. The protein kinase PKR is critical for LPS-induced iNOS production but dispensable for inflammasome activation in macrophages. Eur. J. Immunol. 43, 1147–1152 (2013).
Hara, H. et al. Phosphorylation of the adaptor ASC acts as a molecular switch that controls the formation of speck-like aggregates and inflammasome activity. Nat. Immunol. 14, 1247–1255 (2013).
Gross, O. et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459, 433–436 (2009).
Okada, M., Matsuzawa, A., Yoshimura, A. & Ichijo, H. The lysosome rupture-activated TAK1–JNK pathway regulates NLRP3 inflammasome activation. J. Biol. Chem. 289, 32926–32936 (2014).
Martin, B. N. et al. IKKα negatively regulates ASC-dependent inflammasome activation. Nat. Commun. 5, 4977 (2014).
Guarda, G. et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34, 213–223 (2011).
Mayer-Barber, K. D. et al. Innate and adaptive interferons suppress IL-1α and IL-1β production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity 35, 1023–1034 (2011).
Mishra, B. B. et al. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1β. Nat. Immunol. 14, 52–60 (2013).
Man, S. M. et al. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat. Immunol. 16, 467–475 (2015).
Rathinam, V. A. et al. TRIF licenses caspase-11- dependent NLRP3 inflammasome activation by Gram-negative bacteria. Cell 150, 606–619 (2012).
Meunier, E. et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature 509, 366–370 (2014).
Meunier, E. et al. Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat. Immunol. 16, 476–484 (2015).
MacMicking, J. D. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat. Rev. Immunol. 12, 367–382 (2012).
Kravets, E. et al. Guanylate binding proteins (GBPs) directly attack via supramolecular complexes. eLife 5, e11479 (2016).
Pilla, D. M. et al. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc. Natl Acad. Sci. USA 111, 6046–6051 (2014).
Shenoy, A. R. et al. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science 336, 481–485 (2012).
Coll, R. C. et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21, 248–255 (2015). This study describes MCC950, a small molecule that specifically blocks the NLRP3 inflammasome in vitro and in mouse models of cryopyrin-associated periodic syndrome and experimental autoimmune encephalomyelitis.
Youm, Y. H. et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 21, 263–269 (2015).
Tanaka, S., Mizushina, Y., Kato, Y., Tamura, M. & Shiroishi, T. Functional conservation of Gsdma cluster genes specifically duplicated in the mouse genome. G3 (Bethesda) 3, 1843–1850 (2013).
Op de Beeck, K. et al. The DFNA5 gene, responsible for hearing loss and involved in cancer, encodes a novel apoptosis-inducing protein. Eur. J. Hum. Genet. 19, 965–973 (2011).
Saeki, N. et al. GASDERMIN, suppressed frequently in gastric cancer, is a target of LMO1 in TGF-β-dependent apoptotic signalling. Oncogene 26, 6488–6498 (2007).
Yang, C. S. et al. Small heterodimer partner interacts with NLRP3 and negatively regulates activation of the NLRP3 inflammasome. Nat. Commun. 6, 6115 (2015).
Matsushita, K. et al. A splice variant of ASC regulates IL-1β release and aggregates differently from intact ASC. Mediators Inflamm. 2009, 287387 (2009).
Bryan, N. B. et al. Differential splicing of the apoptosis-associated speck like protein containing a caspase recruitment domain (ASC) regulates inflammasomes. J. Inflamm. (Lond.) 7, 23 (2010).
Acknowledgements
P.B. is supported by the Swiss National Science Foundation (PP00P3_139120/1). The authors apologize to investigators whose contributions were not cited more extensively because of space limitations.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
V.M.D. is an employee of Genentech Inc. P.B. declares no competing interests.
Glossary
- Pyroptosis
-
A specialized form of programmed cell death that requires caspases 1 or 11 in mice and caspases 1, 4 or 5 in humans. It is characterized by cytoplasmic swelling, early plasma membrane rupture, nuclear condensation and internucleosomal DNA fragmentation. The cytoplasmic content is released into the extracellular space, and this is thought to augment inflammatory and repair responses.
- Muckle–Wells syndrome
-
(MWS). A rare autosomal dominant disease caused by mutations in NLRP3 that lead to the autoactivation of the receptor and increased production of interleukin-1β. The chronic inflammation associated with MWS can lead to deafness and amyloidosis.
- Type 3 secretion systems
-
(T3SSs). A virulence-associated specialized molecular machine present in some bacteria that facilitates the translocation of bacterial proteins into host cells.
- B30.2 domain
-
(Also known as SPRY/PRY domain). Defined by a sequence repeat discovered in SplA kinase and ryanodine receptors. B30.2 domains are found in more than 100 human and 70 mouse proteins and are implicated in mediating protein–protein interactions in innate and adaptive immunity.
- Familial Mediterranean fever
-
(FMF). The most common familial inflammatory disease that is characterized by self-limited attacks of fever and serositis. FMF is transmitted in an autosomal recessive pattern and is caused by mutations in the B30.2 domain of MEFV, which encodes pyrin.
- Type I IFNs
-
A multi-gene cytokine family that encodes 13 IFNα subtypes in humans (14 in mice), a single IFNβ and several poorly defined single gene products. Type I IFNs mediate the inhibition of viral replication, activate natural killer cells and macrophages, and increase antigen presentation to T cells.
- Type III IFNs
-
A group of interferons consisting of IFNλ1, IFNλ2 and IFNλ3 (also known as IL-29, IL-28A and IL-28B, respectively), and the recently identified IFNλ4. They have similar functions to cytokines of the type I IFN family.
- Apoptosome
-
A large multimeric protein complex that is formed by apoptotic protease-activating factor 1 (APAF1) following the recognition of cytochrome c release from damaged mitochondria and that activates caspase 9.
- Secondary necrosis
-
A process that occurs in apoptotic cells that are not cleared by phagocytes. The integrity of the plasma membrane is lost and the constituents of the cell are released.
- Alarmins
-
Endogenous mediators that are passively released as a result of lytic cell death (for example, necrosis, pyroptosis and necroptosis) in response to infection or injury and that interact with pattern-recognition receptors to activate innate immune cells.
- Cryopyrin-associated autoinflammatory syndrome
-
(CAPS). A family of autoinflammatory syndromes, including familial cold autoinflammatory syndrome, Muckle–Wells syndrome and neonatal-onset multisystem inflammatory disease. They are characterized by NLRP3 inflammasome hyperactivity and the excessive release of interleukin-1β, which leads to an autoinflammatory disease phenotype with periodic fever episodes, urticaria and often severe arthritis.
- Type II IFNs
-
Consists of a single gene product, IFNγ, that is predominantly produced by T cells and natural killer cells, and can act on a broad range of cell types that express the IFNγ receptor.
- Guanylate-binding proteins
-
(GBPs). A group of interferon-inducible GTPases produced by the host cell that often target pathogen-containing vacuoles, contributing to the release of pathogens from the vacuole and mediating pathogen killing.
- Cell-autonomous immunity
-
A defence mechanism used by cells to control infection that is not traditionally considered to be part of the immune system. Examples include compartmentalization to prevent inappropriate entry of bacteria into the cytoplasm within a eukaryotic cell and production of nitric oxide synthases to mediate killing of an invading microorganism.
Rights and permissions
About this article
Cite this article
Broz, P., Dixit, V. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 16, 407–420 (2016). https://doi.org/10.1038/nri.2016.58
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri.2016.58
This article is cited by
-
ChemR23 signaling ameliorates brain injury via inhibiting NLRP3 inflammasome-mediated neuronal pyroptosis in ischemic stroke
Journal of Translational Medicine (2024)
-
Inter-relationships of galectin-3 and NLR family pyrin domain containing 3 inflammasomes with oral lichen planus: a preliminary cross-sectional in vitro study
BMC Oral Health (2024)
-
Increased expression of NLRP3 associated with elevated levels of HMGB1 in children with febrile seizures: a case–control study
BMC Pediatrics (2024)
-
Copper homeostasis and cuproptosis in atherosclerosis: metabolism, mechanisms and potential therapeutic strategies
Cell Death Discovery (2024)
-
A cytomegalovirus inflammasome inhibitor reduces proinflammatory cytokine release and pyroptosis
Nature Communications (2024)