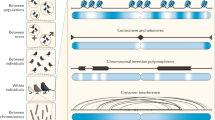
Key Points
-
Recombination events in many organisms are concentrated in highly localized areas termed 'hot spots'.
-
Hot spots can be detected by statistical analysis of genetic variation, analysis of pedigrees and analysis of sperm genomes.
-
Hot spot activities vary in orders of magnitude and can show sex specificity and imprinting effects.
-
The location and relative activity of hot spots is determined by both their own DNA sequence, acting in a cis manner on each chromatid, and by trans-acting factors, such as the zinc finger histone trimethylase PR domain-containing 9 (PRDM9).
-
The two sexes differ in their overall recombination activity and the distribution of crossing over along individual chromosomes.
-
Total recombination activity is genetically regulated and is affected by the number of chromosomes and chromosomal arms in each species.
-
Recombination hot spots evolve rapidly.
Abstract
Recombination, together with mutation, generates the raw material of evolution, is essential for reproduction and lies at the heart of all genetic analysis. Recent advances in our ability to construct genome-scale, high-resolution recombination maps and new molecular techniques for analysing recombination products have substantially furthered our understanding of this important biological phenomenon in humans and mice: from describing the properties of recombination hot spots in male and female meiosis to the recombination landscape along chromosomes. This progress has been accompanied by the identification of trans-acting systems that regulate the location and relative activity of individual hot spots.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Arnheim, N., Calabrese, P. & Tiemann-Boege, I. Mammalian meiotic recombination hot spots. Annu. Rev. Genet. 41, 369–399 (2007).
Buard, J. & de Massy, B. Playing hide and seek with mammalian meiotic crossover hotspots. Trends Genet. 23, 301–309 (2007).
de Massy, B. Distribution of meiotic recombination sites. Trends Genet. 19, 514–522 (2003).
Coop, G. & Przeworski, M. An evolutionary view of human recombination. Nature Rev. Genet. 8, 23–34 (2007).
Kauppi, L., Jeffreys, A. J. & Keeney, S. Where the crossovers are: recombination distributions in mammals. Nature Rev. Genet. 5, 413–424 (2004).
Steinmetz, M. et al. A molecular map of the immune response region from the major histocompatibility complex of the mouse. Nature 300, 35–42 (1982). The paper in which a recombination hot spot was first described and in which the term 'hot spot' was first used.
Chakravarti, A. et al. Nonuniform recombination within the human β-globin gene cluster. Am. J. Hum. Genet. 36, 1239–1258 (1984).
Chakravarti, A., Elbein, S. C. & Permutt, M. A. Evidence for increased recombination near the human insulin gene: implication for disease association studies. Proc. Natl Acad. Sci. USA 83, 1045–1049 (1986).
Kelmenson, P. M. et al. A torrid zone on mouse chromosome 1 containing a cluster of recombinational hotspots. Genetics 169, 833–841 (2005).
Buchner, D. A., Trudeau, M., George, A. L. Jr, Sprunger, L. K. & Meisler, M. H. High-resolution mapping of the sodium channel modifier Scnm1 on mouse chromosome 3 and identification of a 1.3-kb recombination hot spot. Genomics 82, 452–459 (2003).
Bois, P. R. A highly polymorphic meiotic recombination mouse hot spot exhibits incomplete repair. Mol. Cell. Biol. 27, 7053–7062 (2007).
Shiroishi, T., Sagai, T., Hanzawa, N., Gotoh, H. & Moriwaki, K. Genetic control of sex-dependent meiotic recombination in the major histocompatibility complex of the mouse. EMBO J. 10, 681–686 (1991). The initial demonstration that sequences outside the hot spot itself can determine hot spot activity and sex specificity.
Jeffreys, A. J., Kauppi, L. & Neumann, R. Intensely punctate meiotic recombination in the class II region of the major histocompatibility complex. Nature Genet. 29, 217–222 (2001).
Jeffreys, A. J. & May, C. A. Intense and highly localized gene conversion activity in human meiotic crossover hot spots. Nature Genet. 36, 151–156 (2004).
Jeffreys, A. J. & Neumann, R. Factors influencing recombination frequency and distribution in a human meiotic crossover hotspot. Hum. Mol. Genet. 14, 2277–2287 (2005).
Jeffreys, A. J. & Neumann, R. Reciprocal crossover asymmetry and meiotic drive in a human recombination hot spot. Nature Genet. 31, 267–271 (2002). This paper and reference 13 are excellent examples of the use of sperm assays to define the molecular details of recombination products at human hot spots.
Jeffreys, A. J. & Neumann, R. The rise and fall of a human recombination hot spot. Nature Genet. 41, 625–629 (2009). An intriguing paper that analysed the evolutionary appearance of a new hot spot and documented its eventual decline.
Jeffreys, A. J., Neumann, R., Panayi, M., Myers, S. & Donnelly, P. Human recombination hot spots hidden in regions of strong marker association. Nature Genet. 37, 601–606 (2005).
Jeffreys, A. J., Ritchie, A. & Neumann, R. High resolution analysis of haplotype diversity and meiotic crossover in the human TAP2 recombination hotspot. Hum. Mol. Genet. 9, 725–733 (2000).
Neumann, R. & Jeffreys, A. J. Polymorphism in the activity of human crossover hotspots independent of local DNA sequence variation. Hum. Mol. Genet. 15, 1401–1411 (2006).
Gabriel, S. B. et al. The structure of haplotype blocks in the human genome. Science 296, 2225–2229 (2002).
Crawford, D. C. et al. Evidence for substantial fine-scale variation in recombination rates across the human genome. Nature Genet. 36, 700–706 (2004).
McVean, G. A. et al. The fine-scale structure of recombination rate variation in the human genome. Science 304, 581–584 (2004).
Myers, S., Bottolo, L., Freeman, C., McVean, G. & Donnelly, P. A fine-scale map of recombination rates and hotspots across the human genome. Science 310, 321–324 (2005).
Myers, S., Freeman, C., Auton, A., Donnelly, P. & McVean, G. A common sequence motif associated with recombination hot spots and genome instability in humans. Nature Genet. 40, 1124–1129 (2008).
Tiemann-Boege, I., Calabrese, P., Cochran, D. M., Sokol, R. & Arnheim, N. High-resolution recombination patterns in a region of human chromosome 21 measured by sperm typing. PLoS Genet. 2, e70 (2006).
Tapper, W. et al. A map of the human genome in linkage disequilibrium units. Proc. Natl Acad. Sci. USA 102, 11835–11839 (2005).
Coop, G., Wen, X., Ober, C., Pritchard, J. K. & Przeworski, M. High-resolution mapping of crossovers reveals extensive variation in fine-scale recombination patterns among humans. Science 319, 1395–1398 (2008).
Kauppi, L., Stumpf, M. P. & Jeffreys, A. J. Localized breakdown in linkage disequilibrium does not always predict sperm crossover hot spots in the human MHC class II region. Genomics 86, 13–24 (2005).
Kong, A. et al. A high-resolution recombination map of the human genome. Nature Genet. 31, 241–247 (2002).
Broman, K. W., Murray, J. C., Sheffield, V. C., White, R. L. & Weber, J. L. Comprehensive human genetic maps: individual and sex-specific variation in recombination. Am. J. Hum. Genet. 63, 861–869 (1998).
Paigen, K. et al. The recombinational anatomy of a mouse chromosome. PLoS Genet. 4, e1000119 (2008).
Li, H. H. et al. Amplification and analysis of DNA sequences in single human sperm and diploid cells. Nature 335, 414–417 (1988).
Cullen, M., Perfetto, S. P., Klitz, W., Nelson, G. & Carrington, M. High-resolution patterns of meiotic recombination across the human major histocompatibility complex. Am. J. Hum. Genet. 71, 759–776 (2002).
Arnheim, N., Calabrese, P. & Nordborg, M. Hot and cold spots of recombination in the human genome: the reason we should find them and how this can be achieved. Am. J. Hum. Genet. 73, 5–16 (2003).
Guillon, H., Baudat, F., Grey, C., Liskay, R. M. & de Massy, B. Crossover and noncrossover pathways in mouse meiosis. Molecular Cell 20, 563–573 (2005).
Guillon, H. & de Massy, B. An initiation site for meiotic crossing-over and gene conversion in the mouse. Nature Genet. 32, 296–299 (2002).
May, C. A., Shone, A. C., Kalaydjieva, L., Sajantila, A. & Jeffreys, A. J. Crossover clustering and rapid decay of linkage disequilibrium in the Xp/Yp pseudoautosomal gene SHOX. Nature Genet. 31, 272–275 (2002).
Baudat, F. & de Massy, B. Cis- and trans-acting elements regulate the mouse Psmb9 meiotic recombination hotspot. PLoS Genet. 3, e100 (2007).
Ng, S. H., Parvanov, E., Petkov, P. M. & Paigen, K. A quantitative assay for crossover and noncrossover molecular events at individual recombination hotspots in both male and female gametes. Genomics 92, 204–209 (2008).
Huang, X. et al. High-throughput genotyping by whole-genome resequencing. Genome Res. 19, 1068–1076 (2009).
Baudat, F. & de Massy, B. Regulating double-stranded DNA break repair towards crossover or non-crossover during mammalian meiosis. Chromosome Res. 15, 565–577 (2007).
Holloway, K., Lawson, V. E. & Jeffreys, A. J. Allelic recombination and de novo deletions in sperm in the human β-globin gene region. Hum. Mol. Genet. 15, 1099–1111 (2006).
Jeffreys, A. J. et al. Meiotic recombination hot spots and human DNA diversity. Phil. Trans. R. Soc. Lond. B 359, 141–152 (2004).
Lercher, M. J. & Hurst, L. D. Imprinted chromosomal regions of the human genome have unusually high recombination rates. Genetics 165, 1629–1632 (2003).
Paldi, A., Gyapay, G. & Jami, J. Imprinted chromosomal regions of the human genome display sex-specific meiotic recombination frequencies. Curr. Biol. 5, 1030–1035 (1995).
Robinson, W. P. & Lalande, M. Sex-specific meiotic recombination in the Prader–Willi/Angelman syndrome imprinted region. Hum. Mol. Genet. 4, 801–806 (1995).
Ng, S. H. et al. Parental origin of chromosomes influences crossover activity within the Kcnq1 transcriptionally imprinted domain of Mus musculus. BMC Mol. Biol. 10, 43 (2009). This study provides direct evidence that the parental origin of chromatids affects their recombinatorial behaviour.
Sandovici, I. et al. Human imprinted chromosomal regions are historical hot-spots of recombination. PLoS Genet. 2, e101 (2006).
Lucifero, D., Mann, M. R., Bartolomei, M. S. & Trasler, J. M. Gene-specific timing and epigenetic memory in oocyte imprinting. Hum. Mol. Genet. 13, 839–849 (2004).
Kono, T., Obata, Y., Yoshimzu, T., Nakahara, T. & Carroll, J. Epigenetic modifications during oocyte growth correlates with extended parthenogenetic development in the mouse. Nature Genet. 13, 91–94 (1996).
Sato, S., Yoshimizu, T., Sato, E. & Matsui, Y. Erasure of methylation imprinting of Igf2r during mouse primordial germ-cell development. Mol. Reprod. Dev. 65, 41–50 (2003).
Hajkova, P. et al. Epigenetic reprogramming in mouse primordial germ cells. Mech. Dev. 117, 15–23 (2002).
Han, Z., Mtango, N. R., Patel, B. G., Sapienza, C. & Latham, K. E. Hybrid vigor and transgenerational epigenetic effects on early mouse embryo phenotype. Biol. Reprod. 79, 638–648 (2008).
Rakyan, V. K. et al. Transgenerational inheritance of epigenetic states at the murine AxinFu allele occurs after maternal and paternal transmission. Proc. Natl Acad. Sci. USA 100, 2538–2543 (2003).
Morgan, H. D., Sutherland, H. G., Martin, D. I. & Whitelaw, E. Epigenetic inheritance at the agouti locus in the mouse. Nature Genet. 23, 314–318 (1999).
Sutherland, H. G. et al. Reactivation of heritably silenced gene expression in mice. Mamm. Genome 11, 347–355 (2000).
Herman, H. et al. Trans allele methylation and paramutation-like effects in mice. Nature Genet. 34, 199–202 (2003).
Cuzin, F., Grandjean, V. & Rassoulzadegan, M. Inherited variation at the epigenetic level: paramutation from the plant to the mouse. Curr. Opin. Genet. Dev. 18, 193–196 (2008).
Rassoulzadegan, M. et al. RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature 441, 469–474 (2006).
Cedar, H. & Bergman, Y. Linking DNA methylation and histone modification: patterns and paradigms. Nature Rev. Genet. 10, 295–304 (2009).
Probst, A. V., Dunleavy, E. & Almouzni, G. Epigenetic inheritance during the cell cycle. Nature Rev. Mol. Cell Biol. 10, 192–206 (2009).
Steiner, W. W. & Smith, G. R. Optimizing the nucleotide sequence of a meiotic recombination hotspot in Schizosaccharomyces pombe. Genetics 169, 1973–1983 (2005).
Parvanov, E. D., Ng, S. H., Petkov, P. M. & Paigen, K. Trans-regulation of mouse meiotic recombination hotspots by Rcr1. PLoS Biol. 7, e1000036 (2009).
Grey, C., Baudat, F. & de Massy, B. Genome-wide control of the distribution of meiotic recombination. PLoS Biol. 7, e35 (2009).
Mihola, O., Trachtulec, Z., Vlcek, C., Schimenti, J. C. & Forejt, J. A mouse speciation gene encodes a meiotic histone H3 methyltransferase. Science 323, 373–375 (2009).
Birtle, Z. & Ponting, C. P. Meisetz and the birth of the KRAB motif. Bioinformatics 22, 2841–2845 (2006).
Hayashi, K., Yoshida, K. & Matsui, Y. A histone H3 methyltransferase controls epigenetic events required for meiotic prophase. Nature 438, 374–378 (2005).
Buard, J., Barthes, P., Grey, C. & de Massy, B. Distinct histone modifications define initiation and repair of meiotic recombination in the mouse. EMBO J. 28, 2616–2624 (2009).
Borde, V. et al. Histone H3 lysine 4 trimethylation marks meiotic recombination initiation sites. EMBO J. 28, 99–111 (2009).
Parvanov, E. D., Petkov, P. M. & Paigen, K. Prdm9 controls activation of mammalian recombination hotspots. Science 31 Dec 2009 (doi:10.1126/science.1181495).
Baudat, F. et al. PRDM9 is a major determinant of meiotic recombination hotspots in humans and mice. Science 31 Dec 2009 (doi:10.1126/science.1183439).
Myers, S. et al. Drive against hotspot motifs in primates implicates the PRDM9 gene in meiotic recombination. Science 31 Dec 2009 (doi:10.1126/science.1182363). References 71–73 provide evidence that PRDM9 is a major trans -acting regulator of hot spot activity in humans and mice.
Boulton, A., Myers, R. S. & Redfield, R. J. The hotspot conversion paradox and the evolution of meiotic recombination. Proc. Natl Acad. Sci. USA 94, 8058–8063 (1997). The first paper to point out the paradox that hot spots are prevalent despite strong selection against their survival from mutations that diminish their activity.
Jeffreys, A. J. & Neumann, R. Factors influencing recombination frequency and distribution in a human meiotic crossover hotspot. Hum. Mol. Genet. 14, 2277–2287 (2005).
Oliver, P. L. et al. Accelerated evolution of the Prdm9 speciation gene across diverse metazoan taxa. PLoS Genet. 5, e1000753 (2009).
Thomas, J. H., Emerson, R. O. & Shendure, J. Extraordinary molecular evolution in the PRDM9 fertility gene. PLoS ONE 4, e8505 (2009).
Neff, M. W. et al. A second-generation genetic linkage map of the domestic dog, Canis familiaris. Genetics 151, 803–820 (1999).
Mikawa, S. et al. A linkage map of 243 DNA markers in an intercross of Göttingen miniature and Meishan pigs. Anim. Genet. 30, 407–417 (1999).
Lynn, A., Ashley, T. & Hassold, T. Variation in human meiotic recombination. Annu. Rev. Genomics Hum. Genet. 5, 317–349 (2004).
Cox, A. et al. A new standard genetic map for the laboratory mouse. Genetics 182, 1335–1344 (2009).
Drouaud, J. et al. Sex-specific crossover distributions and variations in interference level along Arabidopsis thaliana chromosome 4. PLoS Genet. 3, e106 (2007).
Kappes, S. M. et al. A second-generation linkage map of the bovine genome. Genome Res. 7, 235–249 (1997).
Maddox, J. F. et al. An enhanced linkage map of the sheep genome comprising more than 1,000 loci. Genome Res. 11, 1275–1289 (2001).
Borner, G. V., Kleckner, N. & Hunter, N. Crossover/noncrossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell 117, 29–45 (2004).
Kleckner, N. et al. A mechanical basis for chromosome function. Proc. Natl Acad. Sci. USA 101, 12592–12597 (2004).
Petkov, P. M., Broman, K. W., Szatkiewicz, J. P. & Paigen, K. Crossover interference underlies sex differences in recombination rates. Trends Genet. 23, 539–542 (2007). This paper shows that CO interference underlying sex differences in overall recombination rates is a function of the reduced compaction of the SC in females relative to males.
de Boer, E., Stam, P., Dietrich, A. J., Pastink, A. & Heyting, C. Two levels of interference in mouse meiotic recombination. Proc. Natl Acad. Sci. USA 103, 9607–9612 (2006).
Tease, C. & Hulten, M. A. Inter-sex variation in synaptonemal complex lengths largely determine the different recombination rates in male and female germ cells. Cytogenet. Genome Res. 107, 208–215 (2004).
Mancera, E., Bourgon, R., Brozzi, A., Huber, W. & Steinmetz, L. M. High-resolution mapping of meiotic crossovers and non-crossovers in yeast. Nature 454, 479–485 (2008).
Barchi, M. et al. ATM promotes the obligate XY crossover and both crossover control and chromosome axis integrity on autosomes. PLoS Genet. 4, e1000076 (2008).
Mets, D. G. & Meyer, B. J. Condensins regulate meiotic DNA break distribution, thus crossover frequency, by controlling chromosome structure. Cell 139, 73–86 (2009).
Tsai, C. J. et al. Meiotic crossover number and distribution are regulated by a dosage compensation protein that resembles a condensin subunit. Genes Dev. 22, 194–211 (2008).
Roig, I. et al. Female-specific features of recombinational double-stranded DNA repair in relation to synapsis and telomere dynamics in human oocytes. Chromosoma 113, 22–33 (2004).
Pfeifer, C., Scherthan, H. & Thomsen, P. D. Sex-specific telomere redistribution and synapsis initiation in cattle oogenesis. Dev. Biol. 255, 206–215 (2003).
Tankimanova, M., Hulten, M. A. & Tease, C. The initiation of homologous chromosome synapsis in mouse fetal oocytes is not directly driven by centromere and telomere clustering in the bouquet. Cytogenet. Genome Res. 105, 172–181 (2004).
Lynn, A. et al. Covariation of synaptonemal complex length and mammalian meiotic exchange rates. Science 296, 2222–2225 (2002).
Hassold, T. et al. Cytological studies of meiotic recombination in human males. Cytogenet. Genome Res. 107, 249–255 (2004).
Sun, F. et al. Variation in MLH1 distribution in recombination maps for individual chromosomes from human males. Hum. Mol. Genet. 15, 2376–2391 (2006).
Laurie, D. A. & Hulten, M. A. Further studies on bivalent chiasma frequency in human males with normal karyotypes. Ann. Hum. Genet. 49, 189–201 (1985).
Koehler, K. E., Cherry, J. P., Lynn, A., Hunt, P. A. & Hassold, T. J. Genetic control of mammalian meiotic recombination. I. Variation in exchange frequencies among males from inbred mouse strains. Genetics 162, 297–306 (2002).
Dumont, B. L., Broman, K. W. & Payseur, B. A. Variation in genomic recombination rates among heterogeneous stock mice. Genetics 182, 1345–1349 (2009).
Charlesworth, B. & Charlesworth, D. Genetic variation in recombination in Drosophila. I. Responses to selection and preliminary genetic analysis. Heredity 54, 71–83 (1985).
Charlesworth, B. & Charlesworth, D. Genetic variation in recombination in Drosophila. II. Genetic analysis of a high recombination stock. Heredity 54, 85–98 (1985).
Detlefsen, J. A. & Roberts, E. Studies on crossing over. I. The effect of selection on crossover values. J. Exp. Zoology 32, 333–354 (1921).
Stefansson, H. et al. A common inversion under selection in Europeans. Nature Genet. 37, 129–137 (2005).
Kong, A. et al. Sequence variants in the RNF212 gene associate with genome-wide recombination rate. Science 319, 1398–1401 (2008). Describes the use of a genome-wide association study to map genes that determine overall recombination rates in humans.
Chowdhury, R., Bois, P. R., Feingold, E., Sherman, S. L. & Cheung, V. G. Genetic analysis of variation in human meiotic recombination. PLoS Genet. 5, e1000648 (2009).
Martini, E., Diaz, R. L., Hunter, N. & Keeney, S. Crossover homeostasis in yeast meiosis. Cell 126, 285–295 (2006).
Moens, P. B. et al. The time course and chromosomal localization of recombination-related proteins at meiosis in the mouse are compatible with models that can resolve the early DNA–DNA interactions without reciprocal recombination. J. Cell Sci. 115, 1611–1622 (2002). A detailed description of the physical locations of a number of key molecules relative to the SC during recombination.
Moens, P. B., Marcon, E., Shore, J. S., Kochakpour, N. & Spyropoulos, B. Initiation and resolution of interhomolog connections: crossover and non-crossover sites along mouse synaptonemal complexes. J. Cell Sci. 120, 1017–1027 (2007).
Dutrillaux, B. [Role of chromosomes in evolution: a new interpretation]. Ann. Genet. 29, 69–75 (1986) (in French).
Pardo-Manuel de Villena, F. & Sapienza, C. Recombination is proportional to the number of chromosome arms in mammals. Mamm. Genome 12, 318–322 (2001).
Fledel-Alon, A. et al. Broad-scale recombination patterns underlying proper disjunction in humans. PLoS Genet. 5, e1000658 (2009).
Dumas, D. & Britton-Davidian, J. Chromosomal rearrangements and evolution of recombination: comparison of chiasma distribution patterns in standard and Robertsonian populations of the house mouse. Genetics 162, 1355–1366 (2002).
Ptak, S. E. et al. Fine-scale recombination patterns differ between chimpanzees and humans. Nature Genet. 37, 429–434 (2005).
Winckler, W. et al. Comparison of fine-scale recombination rates in humans and chimpanzees. Science 308, 107–111 (2005). References 116 and 117 demonstrate the rapid evolution of hot spots among primate species.
Dumont, B. L. & Payseur, B. A. Evolution of the genomic rate of recombination in mammals. Evolution 62, 276–294 (2008).
Allers, T. & Lichten, M. Differential timing and control of noncrossover and crossover recombination during meiosis. Cell 106, 47–57 (2001).
Schwacha, A. & Kleckner, N. Identification of joint molecules that form frequently between homologs but rarely between sister chromatids during yeast meiosis. Cell 76, 51–63 (1994).
Bishop, D. K. RecA homologs Dmc1 and Rad51 interact to form multiple nuclear complexes prior to meiotic chromosome synapsis. Cell 79, 1081–1092 (1994).
Schwacha, A. & Kleckner, N. Interhomolog bias during meiotic recombination: meiotic functions promote a highly differentiated interhomolog-only pathway. Cell 90, 1123–1135 (1997).
Thompson, D. A. & Stahl, F. W. Genetic control of recombination partner preference in yeast meiosis. Isolation and characterization of mutants elevated for meiotic unequal sister-chromatid recombination. Genetics 153, 621–641 (1999).
Niu, H. et al. Mek1 kinase is regulated to suppress double-strand break repair between sister chromatids during budding yeast meiosis. Mol. Cell. Biol. 27, 5456–5467 (2007).
Niu, H. et al. Regulation of meiotic recombination via Mek1-mediated Rad54 phosphorylation. Mol. Cell 36, 393–404 (2009).
Kon, N., Krawchuk, M. D., Warren, B. G., Smith, G. R. & Wahls, W. P. Transcription factor Mts1/Mts2 (Atf1/Pcr1, Gad7/Pcr1) activates the M26 meiotic recombination hotspot in Schizosaccharomyces pombe. Proc. Natl Acad. Sci. USA 94, 13765–13770 (1997).
Hirota, K., Mizuno, K., Shibata, T. & Ohta, K. Distinct chromatin modulators regulate the formation of accessible and repressive chromatin at the fission yeast recombination hotspot ade6-M26. Mol. Biol. Cell 19, 1162–1173 (2008).
Pryce, D. W. & McFarlane, R. J. The meiotic recombination hotspots of Schizosaccharomyces pombe. Genome Dyn. 5, 1–13 (2009).
White, M. A., Dominska, M. & Petes, T. D. Transcription factors are required for the meiotic recombination hotspot at the HIS4 locus in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 90, 6621–6625 (1993).
Merker, J. D. et al. The histone methylase Set2p and the histone deacetylase Rpd3p repress meiotic recombination at the HIS4 meiotic recombination hotspot in Saccharomyces cerevisiae. DNA Repair (Amst.) 7, 1298–1308 (2008).
Gottlieb, S. & Esposito, R. E. A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell 56, 771–776 (1989).
Heng, H. H. et al. Regulation of meiotic chromatin loop size by chromosomal position. Proc. Natl Acad. Sci. USA 93, 2795–2800 (1996).
Moens, P. B. et al. Rad51 immunocytology in rat and mouse spermatocytes and oocytes. Chromosoma 106, 207–215 (1997).
Anderson, L. K., Hooker, K. D. & Stack, S. M. The distribution of early recombination nodules on zygotene bivalents from plants. Genetics 159, 1259–1269 (2001).
Anderson, L. K., Reeves, A., Webb, L. M. & Ashley, T. Distribution of crossing over on mouse synaptonemal complexes using immunofluorescent localization of MLH1 protein. Genetics 151, 1569–1579 (1999).
Foss, E., Lande, R., Stahl, F. W. & Steinberg, C. M. Chiasma interference as a function of genetic distance. Genetics 133, 681–691 (1993).
Foss, E. J. & Stahl, F. W. A test of a counting model for chiasma interference. Genetics 139, 1201–1209 (1995).
McPeek, M. S. & Speed, T. P. Modeling interference in genetic recombination. Genetics 139, 1031–1044 (1995).
Broman, K. W. & Weber, J. L. Characterization of human crossover interference. Am. J. Hum. Genet. 66, 1911–1926 (2000).
Acknowledgements
The authors are thankful to M. A. Handel for her thoughtful comments. This work was supported by US National Institutes of Health grants GM078643 and GM083408 to K.P., grant GM078452 to P.P., project grant GM076468 (part of which is managed by P.P.) to G. Churchill and grant CA34196 to The Jackson Laboratory.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Cosmid
-
A bacterial recombination vector that contains long inserted DNA sequences.
- Single-nucleotide polymorphism
-
Single-nucleotide polymorphisms (SNPs) distinguish the chromosomes of two individuals or mouse strains. There are millions of SNPs in mammalian genomes, and they have become the preferred markers for genetic studies.
- Haplotype
-
A set of genetic markers that are present on a single chromosome and that show complete or nearly complete linkage disequilibrium — that is, they are inherited through generations without being changed by crossing over or other recombination mechanisms.
- Linkage disequilibrium
-
Preferential association of allelic combinations among groups of nearby genes.
- Genetic drift
-
The random fluctuations in allele frequencies over time that are due to chance alone.
- Coalescent-based statistical methods
-
Methods of reconstructing population history by simulating the genealogy of genes back to the most recent common ancestor of all alleles currently in the population.
- Genome-wide association study
-
An examination of common genetic variation across the genome that is designed to identify associations with traits, such as common diseases.
- Imprinting
-
The epigenetic marking of a gene on the basis of parental origin, which in somatic tissues results in monoallelic expression.
- Crossover
-
A reciprocal exchange of DNA along chromatids such that the proximal end of one homologue becomes attached to the distal end of the other.
- Double-strand break
-
A cut made across a DNA molecule in which both strands are broken. In recombination the cut is made by the enzyme sporulation-specific 11 (SPO11).
- Pseudoautosomal
-
A region on a sex chromosome that is homologous between the X chromosome and the Y chromosome. Successful meiosis in males requires a crossover in this pseudoautosomal region.
- Gene conversion
-
The process during recombination in which a short segment of DNA on the initiating chromatid is replaced by the DNA sequence of its partner without the exchange of flanking markers.
- Holliday junction
-
The point at which the strands of two dsDNA molecules exchange partners as an intermediate step in crossing over. Typically, two Holliday junctions are formed in the recombination pathway that gives rise to crossovers.
- Topoisomerase
-
An ATP-dependent enzyme that normally creates transient breaks in both strands of the DNA sugar-phosphate backbone, then passes one strand through the other and reseals the break. In the case of the topoisomerase sporulation-specific 11 (SPO11), which initiates recombination, the breaks are not immediately resealed because the 5′ strand on each end is rapidly resected, leaving a free 3′ overhang.
- Chromatid
-
The product of chromosome replication in meiosis I. Chromatids are distinguished from chromosomes by the fact that the two daughter chromatids of one chromosome remain attached at their centromeres through meiosis I cell division.
- Recombination nodules
-
The early, visible manifestations of sites of chiasmata and crossing over. They are recognized by immunochemical staining, typically for the protein MutL homologue 1, which is a component of late recombination nodules.
- Zinc finger
-
A protein loop in which cysteine or cysteine-histidine residues coordinate a zinc ion to form the base of the loop. Three of the amino acids in the loop cooperate to recognize three base pairs of DNA, and a tandem array of zinc fingers can show considerable DNA-binding specificity.
- Positive selection
-
A process by which natural selection favours a single beneficial genotype over other genotypes and may drive this genotype to a high frequency in a population.
- Minisatellite
-
A region of DNA in which repeat units of 10–50 bp are tandemly arranged in arrays 0.5–30 kb in length.
- Genetic interference
-
The presence of a recombinational event in one region that affects the occurrence of recombinational events in adjacent regions. Positive interference, which is seen in eukaryotes, reduces the probability of using nearby hot spots in the same meiosis and causes a more even spacing of crossovers than would occur by chance.
- Synaptonemal complex
-
A linear protein complex that forms the backbone of each chromatid during prophase I of meiosis and promotes genetic recombination. The DNA of the chromatid is attached to the complex in long loops. The name is derived from the word synapsis, which has been used to describe chromatid pairing.
- Bouquet formation
-
The clustering of telomeres together on the nuclear membrane early in meiosis.
- Chiasmata
-
A chiasma (plural chiasmata) is the cytologically visible physical connection between homologous chromatids during meiosis that corresponds to the sites of genetic crossing over.
- MutL homologue 1 foci
-
Sites of staining for MutL homologue 1 that identify sites of genetic crossing over.
- RAD51
-
The human homologue of bacterial RecA. RAD51 is required for homologous recombination, during which it promotes strand invasion, forming nucleoprotein filaments around ssDNA. Immunohistochemical staining of RAD51 foci identifies sites of DNA double-strand breaks.>>
Rights and permissions
About this article
Cite this article
Paigen, K., Petkov, P. Mammalian recombination hot spots: properties, control and evolution. Nat Rev Genet 11, 221–233 (2010). https://doi.org/10.1038/nrg2712
Issue Date:
DOI: https://doi.org/10.1038/nrg2712
This article is cited by
-
Molecular mechanisms and regulation of recombination frequency and distribution in plants
Theoretical and Applied Genetics (2024)
-
Genome-wide recombination map construction from single sperm sequencing in cattle
BMC Genomics (2022)
-
Histone deacetylase inhibition leads to regulatory histone mark alterations and impairs meiosis in oocytes
Epigenetics & Chromatin (2021)
-
Proteome landscape and spatial map of mouse primordial germ cells
Science China Life Sciences (2021)
-
Male recombination map of the autosomal genome in German Holstein
Genetics Selection Evolution (2020)