Key Points
-
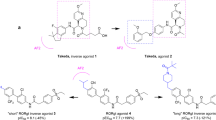
Small-molecule ligands that modulate the action of retinoic acid receptors (RARs) and retinoid X receptors (RXRs) — members of the nuclear receptor superfamily — have been used as anticancer drugs. However, their use can be limited by side effects.
-
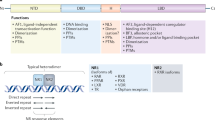
RARs and RXRs are each expressed from three isotypic genes (α,β and γ). The receptors are composed of a modular structure with several domains and associated functions. The main domains are the DNA-binding domain (DBD) and the ligand-binding domain (LBD).
-
Drug discovery efforts are focusing on isotype-selective modulators to reduce toxicity associated with current retinoid therapy; elicit more specific biological responses, because the tissue distribution of retinoid receptor isotypes is not uniform; better define the physiological role of each retinoid receptor isotype; and clarify the potential of RXR-targeted pharmacology.
-
Various crystal structures of RARs and RXRs have been elucidated, allowing for the correlation of ligand structure and (allosterically altered) receptor structure with the functional properties of the ligand-bound receptor.
-
This information will provide guidelines for the design of novel analogues, and advances in synthetic organic chemistry will also be useful in the development of isotype-selective ligands with a range of activities from agonists to inverse agonists through to powerful antagonists.
Abstract
Retinoic acid receptors (RARs) and retinoid X receptors (RXRs) are members of the nuclear receptor superfamily whose effects on cell growth and survival can be modulated therapeutically by small-molecule ligands. Although compounds that target these receptors are powerful anticancer drugs, their use is limited by toxicity. An improved understanding of the structural biology of RXRs and RARs and recent advances in the chemical synthesis of modified retinoid and rexinoid ligands should enable the rational design of more selective agents that might overcome such problems. Here, we review structural data for RXRs and RARs, discuss strategies in the design of selective RXR and RAR modulators, and consider lessons that can be learned for the design of selective nuclear-receptor modulators in general.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Laudet, V. & Gronemeyer, H. The Nuclear Receptor Facts Book (Academic Press, San Diego, 2002).
Gronemeyer, H., Gustafsson, J. A. & Laudet, V. Principles for modulation of the nuclear receptor superfamily. Nature Rev. Drug Disc. 3, 950–964 (2004).
Mark, M., Ghyselinck, N. B. & Chambon, P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signalling pathway during mouse embryogenesis. Annu. Rev. Pharmacol. Toxicol. 46, 451–480 (2005).
IJpenberg, A. et al. In vivo activation of PPAR target genes by RXR homodimers. EMBO J. 23, 2083–2091 (2004).
de Urquiza, A. M. et al. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science 290, 2140–2144 (2000).
Lengqvist, J. et al. Polyunsaturated fatty acids including docosahexaenoic and arachidonic acid bind to the retinoid X receptor α ligand-binding domain. Mol. Cell. Proteomics 3, 692–703 (2004).
Zusi, F. C., Lorenzi, M. V. & Vivat-Hannah, V. Selective retinoids and rexinoids in cancer therapy and chemo-prevention. Drug Discov. Today 7, 1165–1174 (2002).
Dawson, M. I. Synthetic retinoids and their nuclear receptors. Curr. Med. Chem. Anticancer Agents 4, 199–230 (2004).
Bourguet, W., Germain, P. & Gronemeyer, H. Nuclear receptor ligand-binding domains: three-dimensional structures, molecular interactions and pharmacological implications. Trends Pharmacol. Sci. 21, 381–388 (2000).
Bourguet, W., Ruff, M., Chambon, P., Gronemeyer, H. & Moras, D. Crystal structure of the ligand-binding domain of the human nuclear receptor RXR-α. Nature 375, 377–382 (1995). The first structural description of a nuclear receptor ligand binding domain.
Egea, P. F. et al. Crystal structure of the human RXRα ligand-binding domain bound to its natural ligand: 9-cis retinoic acid. EMBO J. 19, 2592–2601 (2000).
Wurtz, J. M. et al. A canonical structure for the ligand-binding domain of nuclear receptors. Nature Struct. Biol. 3, 87–94 (1996).
Hermanson, O., Glass, C. K. & Rosenfeld, M. G. Nuclear receptor coregulators: multiple modes of modification. Trends Endocrinol. Metab. 13, 55–60 (2002).
Smith, C. L. & O'Malley, B. W. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr. Rev. 25, 45–71 (2004).
Wu, R. C., Smith, C. L. & O'Malley, B. W. Transcriptional regulation by steroid receptor coactivator phosphorylation. Endocr. Rev. 26, 393–399 (2005).
Bourguet, W. et al. Crystal structure of a heterodimeric complex of RAR and RXR ligand- binding domains. Mol. Cell 5, 289–298 (2000). Describes the first structure of a heterodimer of nuclear receptor ligand binding domains.
Vivat, V. et al. A mutation mimicking ligand-induced conformational change yields a constitutive RXR that senses allosteric effects in heterodimers. EMBO J. 16, 5697–5709 (1997).
Vivat-Hannah, V., Bourguet, W., Gottardis, M. & Gronemeyer, H. Separation of retinoid X receptor homo- and heterodimerization functions. Mol. Cell. Biol. 23, 7678–7688 (2003).
Germain, P., Iyer, J., Zechel, C. & Gronemeyer, H. Coregulator recruitment and the mechanism of retinoic acid receptor synergy. Nature 415, 187–192 (2002). A demonstration showing that ligand design can differentially induce receptor activities, as revealed by co-regulator interaction. Description of the mechanism of RXR subordination.
Gampe, R. T. Jr et al. Asymmetry in the PPARγ/RXRα crystal structure reveals the molecular basis of heterodimerization among nuclear receptors. Mol. Cell 5, 545–555 (2000).
Brelivet, Y., Kammerer, S., Rochel, N., Poch, O. & Moras, D. Signature of the oligomeric behaviour of nuclear receptors at the sequence and structural level. EMBO Rep. 5, 423–429 (2004).
Shulman, A. I., Larson, C., Mangelsdorf, D. J. & Ranganathan, R. Structural determinants of allosteric ligand activation in RXR heterodimers. Cell 116, 417–429 (2004).
Nettles, K. W. et al. Allosteric control of ligand selectivity between estrogen receptors α and β: implications for other nuclear receptors. Mol. Cell 13, 317–327 (2004).
Aranda, A. & Pascual, A. Nuclear hormone receptors and gene expression. Physiol. Rev. 81, 1269–1304 (2001).
McKenna, N. J. & O'Malley, B. W. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108, 465–474 (2002).
Klein, E. S., Wang, J. W., Khalifa, B., Gavigan, S. A. & Chandraratna, R. A. Recruitment of nuclear receptor corepressor and coactivator to the retinoic acid receptor by retinoid ligands. Influence of DNA- heterodimer interactions. J. Biol. Chem. 275, 19401–19408 (2000).
Loeliger, P., Bollag, W. & Mayer, H. Arotinoids: a new class of highly active retinoids. Eur. J. Med. Chem. 15, 9–15 (1980). The term arotinoid was coined in this article, and the potency of TTNPB was described.
Domínguez, B., Alvarez, R. & de Lera, A. R. Recent Advances in the synthesis of retinoids. Org. Prep. Proc. Int. 35, 239–306 (2003).
Germain, P. et al. Rational design of RAR-selective ligands revealed by RARβ crystal stucture. EMBO Rep. 5, 877–882 (2004).
Klein, E. S. et al. Identification and functional separation of retinoic acid receptor neutral antagonists and inverse agonists. J. Biol. Chem. 271, 22692–22696 (1996).
Klaholz, B. P. et al. Conformational adaptation of agonists to the human nuclear receptor RARγ. Nature Struct. Biol. 5, 199–202 (1998).
Harmon, M. A., Boehm, M. F., Heyman, R. A. & Mangelsdorf, D. J. Activation of mammalian retinoid X receptors by the insect growth regulator methoprene. Proc. Natl Acad. Sci. USA 92, 6157–6160 (1995).
Kitareewan, S. et al. Phytol metabolites are circulating dietary factors that activate the nuclear receptor RXR. Mol. Cell. Biol. 7, 1153–1166 (1996).
LeMotte, P. K., Keidek, S. & Apfel, C. M. Phytanic acid is a retinoid X receptor ligand. Eur. J. Biochem. 236, 328–333 (1996).
Egea, P. F. et al. Crystal structure of the human RXRα ligand-binding domain bound to its natural ligand: 9-cis-retinoic acid. EMBO J. 19, 2592–2601 (2000).
Egea, P. F., Mitschler, A. & Moras, D. Molecular recognition of agonist ligands by RXRs. Mol. Endocrinol. 16, 987–997 (2002).
Nolte, R. T. et al. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-γ. Nature 395, 137–143 (1998). The first crystallographic study of the interaction of co-activators with nuclear receptors.
Uppenberg, J. et al. Crystal structure of the ligand binding domain of the human nuclear receptor PPARγ. J. Biol. Chem. 273, 31108–31112 (1998).
Xu, H. E. et al. Molecular recognition of fatty acids by peroxisome proliferator- activated receptors. Mol. Cell 3, 397–403 (1999).
Greschik, H. et al. Structural and functional evidence for ligand-independent transcriptional activation by the estrogen-related receptor 3. Mol. Cell 9, 303–313 (2002).
Kallen, J. et al. Evidence for ligand-independent transcriptional activation of the human estrogen-related receptor α (ERRα): crystal structure of ERRα ligand binding domain in complex with peroxisome proliferator-activated receptor coactivator-1α. J. Biol. Chem. 279, 49330–49337 (2004).
Wang, L. et al. X-ray crystal structures of the estrogen-related receptor-γ ligand binding domain in three functional states reveal the molecular basis of small molecule regulation. J. Biol. Chem. 281, 37773–37781 (2006).
Pissios, P., Tzameli, I., Kushner, P. & Moore, D. D. Dynamic stabilization of nuclear receptor ligand binding domains by hormone or corepressor binding. Mol. Cell 6, 245–253 (2000).
Tamrazi, A., Carlson, K. E. & Katzenellenbogen, J. A. Molecular sensors of estrogen receptor conformations and dynamics. Mol. Endocrinol. 17, 2593–2602 (2003).
Kallenberger, B. C., Love, J. D., Chatterjee, V. K. & Schwabe, J. W. A dynamic mechanism of nuclear receptor activation and its perturbation in a human disease. Nature Struct. Biol. 10, 136–140 (2003). The first experimental demonstration that the dynamic properties of the activation helix H12 are key to the regulation of the transcriptional activity of nuclear receptors.
Johnson, B. A. et al. Ligand-induced stabilization of PPARγ monitored by NMR spectroscopy: implications for nuclear receptor activation. J. Mol. Biol. 298, 187–194 (2000).
Boehm, M. F. et al. Synthesis and structure–activity relationships of novel retinoid X receptor-selective retinoids. J. Med. Chem. 37, 2930–2941 (1994).
Faul, M. M., Ratz, A. M., Sullivan, K. A., Trankle, W. G. & Winneroski, L. L. Synthesis of novel retinoid X receptor-selective retinoids. J. Org. Chem. 66, 5772–5782 (2001).
Gèhin, M. et al. Structural basis for engineering of retinoic acid receptor isotype-selective agonists and antagonists. Chem. Biol. 6, 519–529 (1999).
Beard, R. L. et al. Synthesis and biological activity of retinoic acid receptor-α specific amides. Bioorg. Med. Chem. Lett. 12, 3145–3148 (2002).
Teng, M. et al. Identification of highly potent retinoic acid receptor α-selective antagonists. J. Med. Chem. 40, 2445–2451 (1997).
Ostrowski, J. et al. Serine 232 and methionine 272 define the ligand binding pocket in retinoic acid receptor subtypes. J. Biol. Chem. 273, 3490–3495 (1998).
Yu, K.-L. et al. Structural modifications of 6-naphthalene-2-carboxylate retinoids Bioorg. Med. Chem. Lett. 6, 2865–2870 (1996).
Klaholz, B. P., Mitschler A. & Moras, D. Structural basis for isotype selectivity of the human retinoic acid nuclear receptor. J. Mol. Biol. 302, 155–170 (2000).
Klaholz, B. P., Mitschler, A., Belema, M., Zusi, C. & Moras, D. Enantiomer discrimination illustrated by high-resolution crystal structures of the human nuclear receptor hRARγ. Proc. Natl. Acad. Sci. USA 97, 6322–6327 (2000).
Charpentier, B. et al. Synthesis, structure-affinity relationships, and biological activities of ligands binding to retinoic acid receptor subtypes. J. Med. Chem. 38, 4993–5006 (1995).
Szondy, Z. et al. Induction of apoptosis by retinoids and retinoic acid receptor γ-selective compounds in mouse thymocytes through a novel apoptosis pathway. Mol. Pharmacol. 51, 972–982 (1997).
Zhang, L. et al. Discovery of novel retinoic acid receptor agonists having potent antiproliferative activity in cervical cancer cells. J. Med. Chem. 39, 2659–2663 (1996).
Dawson, M. I. et al. 4-[3-(5,6,7,8-Tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)phenyl]benzoic acid and heterocyclic-bridged analogues are novel retinoic acid receptor subtype and retinoid X receptor α agonists. Bioorg. Med. Chem. Lett. 10, 1311–1313 (2000).
Acknowledgements
We thank Adán González for his help in preparing the movies and all the members of our laboratories for their contributions to, and discussions of the work described. Work in our laboratories is supported by grants from the European Union QLK3-CT2002-02029 and EPITRON LSHC-CT2005-518417 (L.A., A.R. de L., H.G.); the VINCI program and the Association pour la Recherche sur le Cancer (L.A., H.G.); the Ministero dell'Istruzione, Università e Ricerca (PRIN 2006052835_003) and la Regione Campania L.S. annualità 2005; SAF2004-07131-FEDER (to A.R. de L.); the Ligue contre le Cancer (équipe labelisé), the Institut National de Cancer, the Association for International Cancer Research (AICR) and the European Union grants CRESCENDO LSHM-CT2005-018652 and X-TRA-NET LSHG-CT2005-018882 (H.G.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
Crystal structures of apo- and holo-retinoid receptors (PDF 438 kb)
Supplementary information S2 (movie)
Activation of RXR by a rexinoid and the mouse-trap mechanism (GIF 6232 kb)
Supplementary information S3 (movie)
Mechanism of antagonistic action in RAR (GIF 3746 kb)
Supplementary information S4 (box)
Structure of selective retinoids, rexinoids and RXR modulators. (PDF 141 kb)
Supplementary information S5 (box)
Hydrophobic, linker and polar motifs for retinoid build-up. (PDF 168 kb)
Supplementary information S6 (box)
Hydrophobic, linker and polar motifs for rexinoid build-up (PDF 159 kb)
Supplementary information S7 (table)
Atypical retinoids and retinoid-related molecules (RRMs) (PDF 306 kb)
Supplementary information S8 (box)
Enantioselective synthesis of BMS270,394 (PDF 132 kb)
Supplementary information S9 (box)
Enantioselective synthesis of AGN194,204 (PDF 131 kb)
Supplementary information S10 (movie)
Isotype specificity of RAR ligand-binding domains (GIF 6482 kb)
Supplementary information S11 (table)
Selective RXR modulators (PDF 194 kb)
Related links
Glossary
- Agonist
-
An endogenous substance or a drug that can interact with a receptor and initiate a physiological or a pharmacological response characteristic of that receptor.
- Antagonist
-
A drug or a compound that opposes the physiological effect of another. At the receptor level, it is a chemical entity that opposes the receptor-associated response normally induced by another bioactive agent.
- Co-activators
-
(CoAs). Proteins that cooperate with nuclear hormone receptors to activate transcription. Two classes are known: the p160 family, which recruits histone acetyltransferases, and the TRAP–DRIP–SMCC complex, which is thought to interact with the basal transcription machinery.
- Co-repressors
-
(CoRs). Proteins that cooperate with nuclear hormone receptors to repress transcription. CoRs tether histone deacetylase-containing complexes to target gene promoters. The two main nuclear receptor CoRs are nuclear receptor CoRs and silencing mediator of retinoid and thyroid hormone receptors.
- Selective nuclear receptor modulators
-
(SNuRMs). Ligands that selectively modulate different receptor isotypes and/or act in a cell-selective manner. Note that the term is used in a broader context than for the oestrogen receptors (SERMs) to include receptor isotype selectivity, RAR/RXR selectivity and cell-selective functionality (for example, agonist or antagonist action) of NR ligands.
- Teratogenicity
-
Teratogenicity is when a substance (teratogen) produces a malformation in an embryo.
- Partial agonist
-
An agonist that is unable to induce maximal activation of a receptor population, regardless of the amount of drug applied.
- Inverse agonist
-
A ligand that stabilizes an inactive conformation of a receptor, for example, by increasing co-repressor interaction, thereby decreasing signalling below basal levels.
- Analogue
-
An analogue is a drug that has a structure that is related to that of another drug but whose chemical and biological properties may be quite different.
- Pan-agonist
-
A ligand that activates all isotypes of a receptor (for example, retinoic acid receptor-α (RARα), RARβ and RARγ) similarly.
- Trans-activation
-
Activation of transcription by the binding of a transcription factor to a DNA-regulatory sequence.
- Transcriptomics
-
Technology by which the expression of a large number of genes, or of the entire genome, are monitored.
- ChIP-on-chip
-
Immunoprecipitated DNA from a chromatin immunoprecipitation (ChIP) experiment is amplified, labelled and used as a probe to interrogate the entire genome, spotted as 'tiling arrays' on chips, for binding sites or chromatin modifications. These tiling arrays cover the entire genome as precisely defined consecutive arrays of oligonucleotides.
Rights and permissions
About this article
Cite this article
de Lera, A., Bourguet, W., Altucci, L. et al. Design of selective nuclear receptor modulators: RAR and RXR as a case study. Nat Rev Drug Discov 6, 811–820 (2007). https://doi.org/10.1038/nrd2398
Issue Date:
DOI: https://doi.org/10.1038/nrd2398
This article is cited by
-
Prospecting the therapeutic edge of a novel compound (B12) over berberine in the selective targeting of Retinoid X Receptor in colon cancer
Journal of Molecular Modeling (2021)
-
Meglumine acridone acetate, the ionic salt of CMA and N-methylglucamine, induces apoptosis in human PBMCs via the mitochondrial pathway
Scientific Reports (2019)
-
Oncogenic potential of truncated RXRα during colitis-associated colorectal tumorigenesis by promoting IL-6-STAT3 signaling
Nature Communications (2019)
-
A new regulatory mechanism for Raf kinase activation, retinoic acid-bound Crabp1
Scientific Reports (2019)
-
Tuning artificial intelligence on the de novo design of natural-product-inspired retinoid X receptor modulators
Communications Chemistry (2018)