Key Points
-
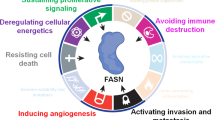
A wide variety of tumours and their precursor lesions undergo exacerbated de novo biogenesis of fatty acids (FAs) irrespective of the levels of circulating lipids. Neoplastic lipogenesis is reflected by significantly increased activity and coordinate expression of several lipogenic enzymes in tumour cells. Upregulation of fatty acid synthase (FASN), the key metabolic multi-enzyme that is responsible for the terminal catalytic step in FA synthesis, represents a nearly-universal phenotypic alteration in most human malignancies.
-
Although the same disturbances in signalling pathways responsible for oncogenic transformation can contribute to increased lipogenesis in tumours, FASN hyperactivity and overexpression is not only a secondary phenomenon that results from the induction of other pathways during carcinogenesis. Rather, it is directly selected for because it provides a growth and/or survival advantage achieved through multiple mechanisms.
-
Early upregulation of FASN in precursor lesions might represent an obligatory metabolic acquisition in response to the microenvironment of pre-invasive lesions (that is, poor oxygenation and high acidity, and/or lack of nutrients), which continue to occur in invasive and/or metastatic stages. The functional and temporal linkage of the 'glycolytic-switch' and the FASN-related lipogenic phenotype may represent co-evolved essential components of the malignant phenotype and, therefore, hallmarks of invasive cancers.
-
Both the persistent prevalence of the exacerbated de novo FA biosynthesis in primary and metastatic malignancy and the existence of bi-directional linkages of FASN with cancer-controlling networks (such as oestrogen receptor and ERBB2), strongly suggest that FASN can work as a previously unrecognized metabolic intermediate of oncogenesis linking energy, anabolism and malignant transformation.
-
As exacerbated lipogenesis emerges early in carcinogenesis, it might represent an exploitable target in cancer prevention by retarding the progression of pre-malignant lesions. At later stages, a more complete understanding of the molecular and physiological consequences of its specific inhibition might lead to targeted therapies for the treatment of advanced or metastatic carcinomas.
Abstract
There is a renewed interest in the ultimate role of fatty acid synthase (FASN) — a key lipogenic enzyme catalysing the terminal steps in the de novo biogenesis of fatty acids — in cancer pathogenesis. Tumour-associated FASN, by conferring growth and survival advantages rather than functioning as an anabolic energy-storage pathway, appears to necessarily accompany the natural history of most human cancers. A recent identification of cross-talk between FASN and well-established cancer-controlling networks begins to delineate the oncogenic nature of FASN-driven lipogenesis. FASN, a nearly-universal druggable target in many human carcinomas and their precursor lesions, offers new therapeutic opportunities for metabolically treating and preventing cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Warburg, O. On the origin of cancer cells. Science 123, 309–314 (1956).
Garber, K. Energy boost: the Warburg effect returns in a new theory of cancer. J. Natl Cancer Inst. 96, 1805–1806 (2004).
Shaw, R. J. Glucose metabolism and cancer. Curr. Opin. Cell Biol. 18, 598–608 (2006).
Bui, T. & Thompson, C. B. Cancer's sweet tooth. Cancer Cell 9, 419–420 (2006).
Garber, K. Energy deregulation: licensing tumors to grow. Science 312, 1158–1159 (2006).
Clemens, M. J. Targets and mechanisms for the regulation of translation in malignant transformation. Oncogene 23, 3180–3188 (2004).
Averous, J. & Proud, C. G. When translation meets transformation: the mTOR story. Oncogene 25, 6423–6435 (2006).
Voeller, D., Rahman, L. & Zajac-Kaye, M. Elevated levels of thymidylate synthase linked to neoplastic transformation of mammalian cells. Cell Cycle 3, 1005–1007 (2004).
Rahman, L. et al. Thymidylate synthase as an oncogene: a novel role for an essential DNA synthesis enzyme. Cancer Cell 5, 341–351 (2004).
Kuhajda, F. P. Fatty-acid Synthase and human cancer: new perspectives on its role in tumor biology. Nutrition 16, 202–208 (2000). First review documenting the relationship between FASN-catalysed endogenous FA biogenesis and tumour biology.
Wakil, S. J. Structure and function of animal fatty acid synthase. Lipids 39, 1045–1053 (2004).
Asturias, F. J. et al. Structure and molecular organization of mammalian fatty acid synthase. Nature Struct. Mol. Biol. 12, 225–232 (2005).
Maier, T., Jenni, S. & Ban, N. Architecture of mammalian fatty acid synthase at 4.5 Å resolution. Science 311, 1258–1262 (2006). Revealed the architecture of mammalian FASN at 4.5 Å resolution for the first time.
Weiss, L. et al. Fatty-acid biosynthesis in man, a pathway of minor importance. Purification, optimal assay conditions, and organ distribution of fatty-acid synthase. Biol. Chem. Hoppe Seyler 367, 905–912 (1986).
Wagle, S. et al. Hormonal regulation and cellular localization of fatty acid synthase in human fetal lung. Am. J. Physiol. 277, 381–390 (1999).
Kusakabe, T. et al. Fatty acid synthase is expressed mainly in adult hormone-sensitive cells or cells with high lipid metabolism and in proliferating fetal cells. J. Histochem. Cytochem. 48, 613–622 (2000).
Pizer, E. S. et al. Expression of fatty acid synthase is closely linked to proliferation and stromal decidualization in cycling endometrium. Int. J. Gynecol. Pathol. 16, 45–51 (1997).
Anderson, S. M. et al. Key stages in mammary gland development. Secretory activation in the mammary gland: it's not just about milk protein synthesis! Breast Cancer Res. 9, 204 (2007). An up-to-date review exhaustively describing all the hormonal regulation pathways that are involved in the mammary gland transition from pregnancy to lactating breast, a highly efficient lipid synthetic organ.
Medes, G., Thomas, A. & Weinhouse, S. Metabolism of neoplastic tissue IV: a study of lipid synthesis in neoplastic tissue slices in vitro. Cancer Res. 13, 27–29 (1953). One of the pioneer studies describing the existence of exacerbated endogenous FA metabolism in tumour tissues.
Szutowicz, A., Kwiatkowski, J. & Angielski, S. Lipogenetic and glycolytic enzyme activities in carcinoma and nonmalignant diseases of the human breast. Br. J. Cancer 39, 681–687 (1979).
Kuhajda, F. P., Piantadosi, S. & Pasternack, G. P. Haptoglobin-related protein (Hpr) epitopes in breast cancer as a predictor of recurrence of the disease. N. Engl. J. Med. 321, 636–641 (1989).
Kuhajda, F. P. et al. Fatty acid synthesis: a potential selective target for antineoplastic therapy. Proc. Natl Acad. Sci. USA 91, 6379–6383 (1994). Key finding characterizing the breast tumour-associated protein OA-519 as the enzyme FASN.
Menendez, J. A. & Lupu, R. Fatty acid synthase-catalyzed de novo fatty acid biosynthesis: from anabolic-energy-storage pathway in normal tissues to jack-of-all-trades in cancer cells. Arch. Immunol. Ther. Exp. (Warsz.) 52, 414–426 (2004).
Swinnen, J. V., Brusselmans, K. & Verhoeven, G. Increased lipogenesis in cancer cells: new players, novel targets. Curr. Opin. Clin. Nutr. Metab. Care 9, 358–365 (2006).
Menendez, J. A. & Lupu, R. Oncogenic properties of the endogenous fatty acid metabolism: molecular pathology of fatty acid synthase in cancer cells. Curr. Opin. Clin. Nutr. Metab. Care 9, 346–357 (2006).
Kuhajda, F. P. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. 66, 5977–5980 (2006).
Jackowski, S. Coordination of membrane phospholipid synthesis with cell cycle. J. Biol. Chem. 269, 3858–3867 (1994).
Costello, L. C. & Franklin, R. B. Tumor cell metabolism: the marriage of molecular genetics and proteomics with cellular intermediary metabolism; proceed with caution. Molecular Cancer 5, 59 (2006).
Costello, L. C. & Franklin, R. B. “Why do tumour cells glycolyse”?: from glycolysis through citrate to lipogenesis. Mol. Cell. Biochem. 280, 1–8 (2005).
Pizer, E. S. et al. Fatty acid synthase (FAS): A target for cytotoxic metabolites in HL60 promyelocitic leukemia cells. Cancer Res. 56, 745–751 (1996).
Swinnen, J. V. et al. Fatty acid synthase drives the synthesis of phospholipids partitioning into detergent-resistant membrane microdomains. Biochem. Biophys. Res. Commun. 302, 898–903 (2003).
Bauer, D. E. et al. ATP citrate lyase is an important component of cell growth and transformation. Oncogene 24, 6314–6322 (2005).
Hatzivassiliou, G. et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell 8, 311–321 (2005).
Tong, L. Acetyl-coenzyme A carboxylase: crucial metabolic enzyme and attractive target for drug discovery. Cell. Mol. Life Sci. 62, 1784–1803 (2005).
Milgraum, L. Z. et al. Enzymes of the fatty acid synthase pathway are highly expressed in in situ breast carcinoma. Clin. Cancer Res. 3, 2115–2120 (1997).
Brusselmans, K. et al. RNA interference-mediated silencing of the acetyl-CoA carboxylase-α gene induces growth inhibition and apoptosis of prostate cancer cells. Cancer Res. 65, 6719–6725 (2005).
Chajès, V. et al. Acetyl-CoA carboxylase α is essential to breast cancer cell survival. Cancer Res. 66, 5287–5294 (2006).
Sinilnikova, O. M. et al. Acetyl-CoA carboxylase α gene and breast cancer susceptibility. Carcinogenesis 25, 2417–2424 (2004).
Sinilnikova, O. M. et al. Haplotype-based analysis of common variation in the acetyl-CoA carboxylase a gene and breast cancer risk: A case-control study nested within the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol. Biomarkers Prev. 16, 409–415 (2007).
Chin, K. et al. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell 10, 529–541 (2006).
Jensen, V. et al. The prognostic value of oncogenic antigen-519 (OA-519) expression and proliferative activity detected by antibody MIB-1 in node-negative breast cancer. J. Pathol. 176, 343–352 (1995).
Alo, P. L. et al. Expression of fatty acid synthase (FAS) as a predictor of recurrence in stage I breast carcinoma patients. Cancer 77, 474–482 (1996).
Gansler, T. S. et al. Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Hum. Pathol. 28, 686–692 (1997).
Alo, P. L. et al. Fatty acid synthase (FAS) predictive strength in poorly differentiated early breast carcinomas. Tumori. 85, 35–40 (1999).
Visca, P. et al. Fatty acid synthase (FAS) is a marker of increased risk of recurrence in lung carcinoma. Anticancer Res. 24, 4169–4173 (2004).
Sebastian, V. et al. Fatty acid synthase is a marker of increased risk of recurrence in endometrial carcinoma. Gynecol. Oncol. 92, 101–105 (2004).
Bandyopadhyay, S. et al. FAS expression inversely correlates with PTEN level in prostate cancer and a PI 3-kinase inhibitor synergizes with FAS siRNA to induce apoptosis. Oncogene 24, 5389–5395 (2005).
Sinclair, C. S. et al. The 17q23 amplicon and breast cancer. Breast Cancer Res. Treat. 78, 313–322 (2003).
Shah, U. S. et al. Fatty acid synthase gene overexpression and copy number gain in prostate adenocarcinoma. Hum. Pathol. 37, 401–409 (2006).
Katsurada, A. et al. Effects of nutrients and hormones on transcriptional and post-transcriptional regulation of fatty acid synthase in liver. Eur. J. Biochem. 190, 427–433 (1990).
Sul, H. S. & Wang, D. Nutritional and hormonal regulation of enzymes in fat synthesis: studies of fatty acid synthase and mitochondrial glycerol-3-phosphate acyltransferase gene transcription. Annu. Rev. Nutr. 18, 331–351 (1998).
Fukuda, H. et al. Transcriptional regulation of fatty acid synthase by insulin/glucose, polyunsaturated fatty acids and leptin in hepatocytes and adipocytes in normal and genetically obese rats. Eur. J. Biochem. 260, 505–511 (1999).
Swinnen, J. V. et al. Stimulation of tumor-associated fatty acid synthase expression by growth factor activation of the sterol regulatory element-binding protein pathway. Oncogene 19, 5173–5181 (2000). First study to reveal how exacerbated GF–GFR signalling in cancer cells constitutively upregulates tumour-associated FASN.
Oskouian, B. Overexpression of fatty acid synthase in SKBR3 breast cancer cell line is mediated via a transcriptional mechanism. Cancer Lett. 149, 43–51 (2000).
Kumar-Sinha, C. et al. Transcriptome analysis of HER2 reveals a molecular connection to fatty acid synthesis. Cancer Res. 63, 132–139 (2003).
Menendez, J. A. et al. Overexpression and hyperactivity of breast cancer-associated fatty acid synthase (oncogenic antigen-519) is insensitive to normal arachidonic fatty acid-induced suppression in lipogenic tissues but it is selectively inhibited by tumoricidal alpha-linolenic and gamma-linolenic fatty acids: a novel mechanism by which dietary fat can alter mammary tumorigenesis. Int. J. Oncol. 24, 1369–1383 (2004).
Menendez, J. A. et al. Pharmacological inhibition of fatty acid synthase (FAS): a novel therapeutic approach for breast cancer chemoprevention through its ability to suppress Her-2/neu (erbB-2) oncogene-induced malignant transformation. Mol. Carcinog. 41, 164–178 (2004).
Zhang, D. et al. Proteomic study reveals that proteins involved in metabolic and detoxification pathways are highly expressed in HER-2/neu-positive breast cancer. Mol. Cell Proteomics 4, 1686–1696 (2005).
Van de Sande, T. et al. Role of the phosphatidylinositol 3′-kinase/PTEN/Akt kinase pathway in the overexpression of fatty acid synthase in LNCaP prostate cancer cells. Cancer Res. 62, 642–646 (2002).
Porstmannm, T. et al. PKB/AKT induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene 24, 6465–6481 (2005).
Yang, Y. A. et al. Activation of fatty acid synthesis during neoplastic transformation: role of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Exp. Cell Res. 279, 80–90 (2002).
Wang, H. Q. et al. Positive feedback regulation between AKT activation and fatty acid synthase expression in ovarian carcinoma cells. Oncogene 24, 3574–3582 (2005).
Chalbos, D. et al. Fatty acid synthetase and its mRNA are induced by progestins in breast cancer cells. J. Biol. Chem. 262, 9923–9926 (1987).
Swinnen, J. V. et al. Androgens stimulate fatty acid synthase in the human prostate cancer cell line LNCaP. Cancer Res. 57, 1086–1090 (1997).
Menendez, J. A. et al. The estrogenic activity of synthetic progestins used in oral contraceptives enhances fatty acid synthase-dependent breast cancer cell proliferation and survival. Int. J. Oncol. 26, 1507–1515 (2005).
Lupu, R. & Menendez, J. A. Targeting fatty acid synthase in breast and endometrial cancer: An alternative to selective estrogen receptor modulators? Endocrinology 147, 4056–4066 (2006). This review provides new insights on the cross-talk between tumour-associated FASN and oestrogen receptor (ER) in hormone-sensitive human tumours.
Rawson, R. B. The SREBP pathway—insights from Insigs and insects. Nature Rev. Mol. Cell Biol. 4, 631–640 (2003).
Eberle, D. et al. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie. 86, 839–848 (2004). References 67 and 68 brilliantly review the role of SREBP transcription factors as master regulators of lipid homeostasis.
Kim, J. B. et al. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J. Clin. Invest. 101, 1–9 (1998).
Shimano, H. et al. Sterol regulatory element-binding protein 1 as a key transcription factor for nutritional induction of lipogenic enzymes genes. J. Biol. Chem. 274, 35832–35839 (1999).
Fleischmann, M. & Iynedjian, P. B. Regulation of sterol regulatory-element binding protein 1 gene expression in liver: role of insulin and protein kinase B/cAkt. Biochem. J. 349, 13–17 (2000).
Kotzka, J. et al. Sterol regulatory element binding proteins (SREBP)-1a and SREBP-2 are linked to the MAP-kinase cascade. J. Lipid Res. 41, 99–108 (2000).
Kersten, S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2, 282–286 (2001).
Swinnen, J. V. et al. Coordinate regulation of lipogenic gene expression by androgens: evidence for a cascade mechanism involving sterol regulatory element binding proteins. Proc. Natl Acad. Sci. USA 94, 12975–12980 (1997).
Yang, Y. A. et al. Regulation of fatty acid synthase expression in breast cancer by sterol regulatory elements binding protein-1c. Exp. Cell Res. 282, 132–137 (2003).
Ettinger, S. L. et al. Dysregulation of sterol response element-binding proteins and downstream effectors in prostate cancer during progression to androgen independence. Cancer Res. 64, 2212–2221 (2004).
Menendez, J. A., Decker, J. P. & Lupu, R. In support of fatty acid synthase (FAS) as a metabolic oncogene: extracellular acidosis acts in an epigenetic fashion activating FAS gene expression in cancer cells. J. Cell. Biochem. 94, 1–4 (2005).
Heemers, H. et al. Androgens stimulate lipogenic gene expression in prostate cancer cells by activation of the sterol regulatory element-binding protein cleavage activating protein/sterol regulatory element-binding protein pathway. Mol. Endocrinol. 15, 1817–1828 (2001).
Menendez, J. A., Colomer, R. & Lupu, R. Why does tumor-associated fatty acid synthase (oncogenic antigen-519) ignore dietary fatty acids? Med. Hypotheses 64, 342–349 (2005).
Li, J. N. et al. Sterol regulatory element-binding protein-1 participates in the regulation of fatty acid synthase expression in colorectal neoplasia. Exp. Cell. Res. 261, 159–165 (2000).
Swinnen, J. V. Increased lipogenesis in steroid-responsive cancer cells: mechanisms of regulation, role in cancer cell biology and perspectives on clinical applications. Verh. K. Acad. Geneeskd. Belg. 63, 321–333 (2001).
Graner, E. et al. The isopeptidase USP2a regulates the stability of fatty acid synthase in prostate cancer. Cancer Cell 5, 253–261 (2004). The first study to describe how tumour-associated FASN overexpression can be achieved through protein stabilization.
Priolo, C. et al. The isopeptidase USP2a protects human prostate cancer from apoptosis. Cancer Res. 66, 8625–8632 (2006).
Swinnen, J. V. et al. Selective activation of the fatty acid synthesis pathway in human prostate cancer. Int. J. Cancer 88, 176–179 (2000).
Swinnen, J. V. et al. Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. Int. J. Cancer 98, 19–22 (2002).
Esslimani-Sahla, M. et al. Increased expression of fatty acid synthase and progesterone receptor in early steps of human mammary carcinogenesis. Int. J. Cancer 120, 224–229 (2007).
Pizer, E. S. et al. Inhibition of fatty acid synthesis delays disease progression in a xenograft model of ovarian cancer. Cancer Res. 56, 1189–1193 (1996).
Pizer, E. S. et al. Inhibition of fatty acid synthesis induces programmed cell death in human breast cancer cells. Cancer Res. 12, 2745–2747 (1996).
Pizer, E. S. et al. Pharmacological inhibitors of mammalian fatty acid synthase suppress DNA replication and induce apoptosis in tumor cell lines. Cancer Res. 58, 4611–4615 (1998).
Pizer, E. S. et al. Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res. 60, 213–218 (2000).
Li, J. N. et al. Pharmacological inhibition of fatty acid synthase activity produces both cytostatic and cytotoxic effects modulated by p53. Cancer Res. 61, 1493–1499 (2001).
Kuhajda, F. P. et al. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc. Natl Acad. Sci. USA 97, 3450–3454 (2000).
Thupari, J. N., Pinn, M. L. & Kuhajda, F. P. Fatty acid synthase inhibition in human breast cancer cells leads to malonyl-CoA-induced inhibition of fatty acid oxidation and cytotoxicity. Biochem. Biophys. Res. Commun. 285, 217–223 (2001).
Heiligtag, S. J., Bredehorst, R. & Davidm K. A. Key role of mitochondria in cerulenin-mediated apoptosis. Cell Death Differ. 9, 1017–1025 (2002).
Liu, B. et al. Triclosan inhibits enoyl-reductase of type I fatty acid synthase in vitro and is cytotoxic to MCF-7 and SKBr-3 breast cancer cells. Cancer Chemother. Pharmacol. 49, 187–193 (2002).
Zhou, W. et al. Fatty acid synthase inhibition triggers apoptosis during S phase in human cancer cells. Cancer Res. 63, 7330–7337 (2003).
Brusselmans, K. et al. Epigallocatechin-3-gallate is a potent natural inhibitor of fatty acid synthase in intact cells and selectively induces apoptosis in prostate cancer cells. Int. J. Cancer 106, 856–862 (2003).
Menendez, J. A. et al. Inhibition of tumor-associated fatty acid synthase activity antagonizes estradiol- and tamoxifen-induced agonist transactivation of estrogen receptor (ER) in human endometrial adenocarcinoma cells. Oncogene 23, 4945–4958 (2004).
Menendez, J. A. et al. Novel signaling molecules implicated in tumor-associated fatty acid synthase-dependent breast cancer cell proliferation and survival: role of exogenous dietary fatty acids, p53-p21WAF1/CIP1, ERK1/2 MAPK, p27KIP1, BRCA1, and NF-κB. Int. J. Oncol. 24, 591–608 (2004).
Kridel, S. J. et al. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res. 64, 2070–2075 (2004).
Knowles, L. M. et al. A fatty acid synthase blockade induces tumor cell-cycle arrest by down-regulating Skp2. J. Biol. Chem. 279, 30540–30545 (2004).
Menendez, J. A., Vellon, L. & Lupu, R. Antitumoral actions of the anti-obesity drug orlistat (Xenical™) in breast cancer cells: blockade of cell cycle progression, promotion of apoptotic cell death and PEA3-mediated transcriptional repression of Her2/neu (erbB-2) oncogene. Ann. Oncol. 16, 1253–1267 (2005).
Menendez, J. A. & Lupu, R. RNA interference-mediated silencing of the p53 tumor-suppressor protein drastically increases apoptosis after inhibition of endogenous fatty acid metabolism in breast cancer cells. Int. J. Mol. Med. 15, 33–40 (2005).
Bandyopadhyay, S. et al. Mechanism of apoptosis induced by the inhibition of fatty acid synthase in breast cancer cells. Cancer Res. 66, 5934–5940 (2006).
Zhao, W. et al. Fatty acid synthase: a novel target for antiglioma therapy. Br. J. Cancer 95, 869–878 (2006).
Lupu, R. & Menendez, J. A. Pharmacological inhibitors of fatty acid synthase (FASN)-catalyzed endogenous fatty acid biogenesis: a new family of anti-cancer agents? Curr. Pharm. Biotechnol. 7, 495–502 (2006).
Menendez, J. A. et al. Inhibition of fatty acid synthase (FAS) suppresses HER2/neu (erbB-2) oncogene overexpression in cancer cells. Proc. Natl Acad. Sci. USA 101, 10715–10720 (2004). First experimental evidence showing the ability of endogenous FA metabolism to specifically regulate well-characterized oncogenes such as ERBB2.
Menendez, J. A., Lupu, R. & Colomer, R. Targeting fatty acid synthase: potential for therapeutic intervention in Her-2/neu-overexpressing breast cancer. Drug News Perspect. 18, 375–385 (2005).
Gatenby, R. A. & Gawlinski, E. T. The glycolytic phenotype in carcinogenesis and tumor invasion: insights through mathematical models. Cancer Res. 63, 3847–3854 (2003).
Gatenby, R. A. & Gillies, R. J. Why do cancers have high aerobic glycolysis? Nature Rev. Cancer 4, 891–899 (2004). A compelling opinion piece, in which the authors convincingly propose that the glycolytic phenotype confers a significant proliferative advantage during somatic evolution of cancer and must, therefore, be a crucial component of the malignant phenotype.
Gillies, R. J. & Gatenby, R. A. Hypoxia and adaptive landscapes in the evolution of carcinogenesis. Cancer Metastasis Rev. 26, 311–317 (2007).
Gatenby, R. A. & Gillies, R. J. Glycolysis in cancer: a potential target for therapy. Int. J. Biochem. Cell Biol. 39, 1358–1366 (2007).
Hochachka, P. W. Living without oxygen. 1–181 (Harvard University Press, Cambridge, USA, 1980).
Hochachka, P. W. et al. Going malignant: the hypoxia-cancer connection in the prostate. Bioessays 24, 749–757 (2002).
Baron, A. et al. Fatty acid synthase: a metabolic oncogene in prostate cancer? J. Cell. Biochem. 91, 47–53 (2004).
Elstrom, R. L. et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 64, 3892–3899 (2004).
Buzzai, M. et al. The glucose dependence of Akt-transformed cells can be reversed by pharmacological activation of fatty acid-beta oxidation. Oncogene 24, 4165–4173 (2005).
Gatenby, R. A. et al. Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res. 66, 5216–5223 (2006).
Smallbone, K. et al. Metabolic changes during carcinogenesis: potential impact on invasiveness. J. Theor. Biol. 244, 703–713 (2007).
Visca, P. et al. Immunohistochemical expression and prognostic significance of FAS and GLUT1 in bladder carcinoma. Anticancer Res. 23, 335–339 (2003).
Alo, P. L. et al. Immunohistochemical expression of human erythrocyte glucose transporter and fatty acid synthase in infiltrating breast carcinomas and adjacent typical/atypical hyperplastic or normal breast tissue. Am. J. Clin. Pathol. 116, 129–134 (2001).
Robey, I. F. et al. Hypoxia-inducible factor-1α and the glycolytic phenotype in tumors. Neoplasia 7, 324–330 (2005).
Semenza, G. L. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol. Med. 8, 62–67 (2002).
Semenza, G. L. Targeting HIF-1 for cancer therapy. Nature Rev. Cancer 3, 721–732 (2003).
Laughner, E. et al. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1α) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol. Cell Biol. 21, 3995–4004 (2001).
Li, J. et al. Altered metabolic response to intermittent hypoxia in mice with partial deficiency of hypoxia-inducible factor-1α. Physiol. Genomics 25, 450–457 (2006).
Menendez, J. A . et al. Does endogenous fatty acid metabolism allow cancer cells to sense hypoxia and mediate hypoxic vasodilatation? Characterization of a novel molecular connection between fatty acid synthase (FAS) and hypoxia-inducible factor-1α (HIF-1α)-related expression of vascular endothelial growth factor (VEGF) in cancer cells overexpressing Her-2/neu oncogene. J. Cell. Biochem. 94, 857–863 (2005).
Pflug, B. R. et al. Increased fatty acid synthase expression and activity during progression of prostate cancer in the TRAMP model. Prostate 57, 245–254 (2003).
Rossi, S. et al. Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Mol. Cancer Res. 1, 707–715 (2003).
De Schrijver, E. et al. RNA interference-mediated silencing of the fatty acid synthase gene attenuates growth and induces morphological changes and apoptosis of LNCaP prostate cancer cells. Cancer Res. 63, 3799–3804 (2003).
Menendez, J. A. et al. Pharmacological and small interference RNA-mediated inhibition of breast cancer-associated fatty acid synthase (oncogenic antigen-519) synergistically enhances Taxol (paclitaxel)-induced cytotoxicity. Int. J. Cancer. 115, 19–35 (2005). First experimental evidence demonstrating a role for FASN in the response of cancer cells to chemotherapy.
Menendez, J. A., Colomer, R. & Lupu, R. Inhibition of tumor-associated fatty acid synthase activity enhances vinorelbine (Navelbine)-induced cytotoxicity and apoptotic cell death in human breast cancer cells. Oncol Rep. 12, 411–422 (2004).
Menendez, J. A., Lupu, R. & Colomer, R. Inhibition of tumor-associated fatty acid synthase hyperactivity induces synergistic chemosensitization of HER-2/neu-overexpressing human breast cancer cells to docetaxel (taxotere). Breast Cancer Res. Treat. 84, 183–195 (2004).
Lu, S. & Archer, M. C. Fatty acid synthase is a potential molecular target for the chemoprevention of breast cancer. Carcinogenesis 26, 153–157 (2005).
Alli, P. M. et al. Fatty acid synthase inhibitors are chemopreventive for mammary cancer in neu-N transgenic mice. Oncogene 24, 39–46 (2005). This in vivo study supports the notion of targeting FASN as a valuable approach in breast cancer chemoprevention.
Cunningham, B. A. et al. “Spot 14” protein: a metabolic integrator in normal and neoplastic cells. Thyroid 8, 815–825 (1998).
Moncur, J. T. et al. The “Spot 14” gene resides on the telomeric end of the 11q13 amplicon and is expressed in lipogenic breast cancers: implications for control of tumor metabolism. Proc. Natl Acad. Sci. USA 95, 6989–6994 (1998).
Wells, W. A. et al. Expression of “Spot 14” (THRSP) predicts disease free survival in invasive breast cancer: immunohistochemical analysis of a new molecular marker. Breast Cancer Res. Treat. 98, 231–240 (2006).
Martel, P. M. et al. S14 protein in breast cancer cells: direct evidence of regulation by SREBP-1c, superinduction with progestin, and effects on cell growth. Exp. Cell Res. 312, 278–288 (2006).
Kinlaw, W. B. et al. Spot 14: A marker of aggressive breast cancer and a potential therapeutic target. Endocrinology 147, 4048–4055 (2006).
Magnard, C. et al. BRCA1 interacts with acetyl-CoA carboxylase throuch its tandem of BRCT domains. Oncogene 21, 6729–6739 (2002).
Moreau, K. et al. BRCA1 affects lipid synthesis through its interaction with acetyl-CoA carboxylase. J. Biol. Chem. 281, 3172–3181 (2006). This study reveals that neoplastic lipogenesis may associate with an increased susceptibility to breast and ovarian cancer through BRCA1.
Brunet, J. et al. BRCA1 and Acetyl-CoA Carboxylase: The metabolic syndrome of breast cancer. Mol. Carcinog. 9 July 2007 [Epub ahead of print].
Hardie, D. G. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology 144, 5179–5183 (2003).
Long, Y. C. & Zierath, J. R. AMP-activated protein kinase signaling in metabolic regulation. J. Clin. Invest. 116, 1776–1783 (2006).
Luo, Z. et al. AMPK, the metabolic syndrome and cancer. Trends Pharmacol. Sci. 26, 69–76 (2005).
Swinnen, J. V. et al. Mimicry of a cellular low energy status blocks tumor cells anabolism and suppresses the malignant phenotype. Cancer Res. 65, 2441–2448 (2005). Provides clear evidence that the energy status of tumour cells is crucial in the maintenance of the transformed phenotype.
Guastamacchia, E. et al. Evidence for a putative relationship between type 2 diabetes and neoplasia with particular reference to breast cancer: Role of hormones, growth factors and specific receptors. Curr. Drug Targets Immune. Endocr. Metabol. Disord. 4, 59–66 (2004).
Resta, F. et al. The impact of body mass index and type 2 diabetes on breast cancer: current therapeutic measures of prevention. Curr. Drug Targets Immune Endocr. Metabol. Disord. 4, 327–333 (2004).
Zakikhani, M. et al. Metformin is an AMPK kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 66, 10269–10273 (2007).
Pemble, C. W. IIII et al. Crystal structure of the thioesterase domain of human fatty acid synthase inhibited by Orlistat. Struct. Mol. Biol. 14, 704–709 (2007). This report should provide an excellent base from which to develop new anti-FASN agents with therapeutic value.
Knowles, L. M. & Smith, J. W. Genome-wide changes accompanying knockdown of fatty acid synthase in breast cancer. BMC Genomics 8, 168 (2007).
Little, J. L. et al. Inhibition of fatty acid synthase induces endoplasmic reticulum stress in tumor cells. Cancer Res. 67, 1262–1269, 2007.
Buzzai, M. et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 67, 6745–6752 (2007).
Ogata, M. et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell Biol. 26, 9220–9231 (2006).
Yorimitsu, T. & Klionsky, D. J. Endoplasmic reticulum stress: a new pathway to induce autophagy. Autophagy 3, 160–162 (2007).
Degenhardt, K. et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 10, 51–64 (2006).
Jin, S. & White, E. Role of autophagy in cancer: management of metabolic stress. Autophagy 3, 28–31 (2007).
Jin, S. et al. Metabolic catastrophe as a means to cancer cell death. J.Cell. Sci. 120, 379–378 (2007).
Levine, B. Cell biology: autophagy and cancer. Nature 446, 745–747 (2007).
Rodriguez-Gonzalez, A. et al. Inhibition of choline kinase renders a highly selective cytotoxic effect in tumour cells through a mitochondrial independent mechanism. Int. J. Oncol. 26, 999–1008 (2005).
Simons, K. & Ikonen, E. Functional rafts in cell membranes. Nature 387, 569–572 (1997).
Helms, J. B. & Zurzolo, C. Lipids as targeting signals: lipid rafts and intracellular trafficking. Traffic 5, 247–254 (2004).
Nagy, P. et al. Lipid rafts and the local density of ErbB proteins influence the biological role of homo- and heteroassociations of ErbB2. J. Cell Sci. 115, 4251–4262 (2002).
Yarden, Y. & Sliwkowski, M. X. Untangling the ErbB signalling network. Nature Rev. Mol. Cell Biol. 2, 127–137 (2001).
Nahta, R. et al. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nature Clin. Pract. Oncol. 3, 269–280 (2006).
Xing, X. The ets protein PEA3 suppresses HER-2/neu overexpression and inhibits tumorigenesis. Nature Med. 6, 189–195 (2000).
Hurst, H. C. Update of HER-2 as a target for cancer therapy: the ERBB2 promoter and its exploitation for cancer treatment. Breast Cancer Res. 3, 395–398 (2001).
Menendez, J. A. et al. A genomic explanation connecting “Mediterranean diet”, olive oil and cancer: oleic acid, the main monounsaturated fatty acid of olive oil, induces formation of inhibitory “PEA3 transcription factor-PEA3 DNA binding site” complexes at the Her-2/neu (erbB-2) oncogene promoter in breast, ovarian and stomach cancer cells. Eur. J. Cancer 42, 2425–2432 (2006).
Citri, A. & Yarden, Y. EGF-ERBB signalling: towards the systems level. Nature Rev. Mol. Cell Biol. 7, 505–516 (2006).
Menendez, J. A., Vellon, L. & Lupu, R. Orlistat: from antiobesity drug to anticancer agent in Her-2/neu (erbB-2)-overexpressing gastrointestinal tumors? Exp. Biol. Med (Maywood) 230, 151–154 (2005).
Loftus, T. M. et al. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science 288, 2379–2381 (2000).
Wortman, M. D. et al. C75 inhibits food intake by increasing CNS glucose metabolism. Nature Med. 9, 483–485 (2003).
Wang, X. & Tian, W. Green tea epigallocatechin gallate: a natural inhibitor of fatty-acid synthase. Biochem. Biophys. Res. Commun. 288, 1200–1206 (2001).
Brusselmans, K. et al. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J. Biol. Chem. 280, 5636–5645 (2005).
Acknowledgements
We truly regret that we could not cite the work of many of our colleagues owing to space limitation. The authors are supported by funding from Instituto de Salud Carlos III (Ministerio de Sanidad y Consumo, Fondo de Investigación Sanitaria (FIS), Spain, grants CP05-00090, PI06-0778 and RD06-0020-0028) and grant BCTR0600,894 from the Susan G. Komen Breast Cancer Foundation to J.A.M. And the Extramural Funding Program of the US National Institutes of Health, RO1CA116,623, and the Breast Cancer Auxillary Program of the Evanston Northwestern Healthacare, to R.L.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
FURTHER INFORMATION
Girona Biomedical Research Institute
Glossary
- β-oxidation
-
The process by which fats, in the form of acyl coenzyme A (CoA) molecules, are broken down in the mitochondria to generate acetyl-CoA, the entry molecule for the Krebs cycle (also known as the citric acid cycle).
- Genome copy number abnormalities
-
Gains and losses of DNA sequences throughout the genome that associate with cancer development. Measurement of copy number variations at multiple loci simultaneously provides an important tool for studying cancer.
- Multi-enzyme
-
A protein possessing more than one catalytic function contributed by distinct parts of a polypeptide chain (domains), by distinct subunits, or both.
- Paget's disease of the vulva
-
A usually non-invasive adenocarcinoma of the skin on the vulva. It may be a primary lesion but in a small percentage of women an invasive cancer of the vulva is found below the area of Paget's.
- Prostatic intraepithelial neoplasia (PIN) lesions
-
The high-grade form of PIN has been postulated to be the precursor of invasive carcinoma of the prostate. Low-grade PIN corresponds to 'very mild' to 'mild' dysplasia.
- Sterol regulatory element binding protein-1c (SREBP1c)
-
A basic helix-loop-helix leucine zipper transcription factor that activates genes involved in the synthesis of cholesterol and fatty acids, including fatty acid synthase.
- Gene-set enrichment analysis
-
A microarray data analysis method that uses predefined gene sets and ranks of genes to identify significant biological changes when gene expression changes in a given microarray data set are minimal or moderate.
- Ductal carcinoma in situ (DCIS)
-
The earliest form of breast cancer, known as stage 0 (non-invasive) cancer, which stays inside the milk duct of the breast in which it started. It is not known how to predict which DCIS lesions will become invasive.
- Lobular carcinoma in situ (LCIS)
-
An overgrowth of cells in the lobules of the breast. These cells are not likely to turn into an invasive cancer, but having them means a higher risk of getting breast cancer.
- Lipid raft aggregates
-
Plasma membrane domains enriched with glycosphingolipids and cholesterol that are implicated in key biological processes including signal transduction, intracellular trafficking, cell polarization and cell migration. Rafts might function as platforms regulating receptor tyrosine kinase signalling pathways.
- Hypoxia-inducible factor-1α (HIF1α)
-
Part of a dimeric transcription factor formed of α and β subunits that is involved in the hypoxia-sensitive regulation of numerous genes, including glycolytic enzymes, glucose transporters and angiogenic factors.
- TRAMP
-
An autochthonous mouse model of prostate cancer. Various stages of progressive prostate disease can be observed in TRAMP mice, with focal adenocarcinomas developing between 10 and 20 weeks of age with 100% frequency.
- Neu-N-mice
-
This transgenic mouse model overexpresses the non-transforming rat homologue of ERBB2 (Neu) cDNA under control of the mouse mammary tumour virus (MMTV) promoter in a mammary-specific fashion and develops spontaneous focal NEU-expressing tumours.
- Endoplasmic reticulum (ER) stress
-
The inability of the ER to fold proteins properly. Accumulation of unfolded proteins initiates a stress response called the unfolded protein response (UPR), a set of pathways that decreases protein synthesis. Failure of the UPR to correct ER stress results in apoptosis.
- Autophagy
-
A cell survival mechanism that is activated in response to starvation and can lead to cell death. Subcellular membranes undergo dramatic morphological changes and portions of cytoplasm are degraded.
Rights and permissions
About this article
Cite this article
Menendez, J., Lupu, R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7, 763–777 (2007). https://doi.org/10.1038/nrc2222
Issue Date:
DOI: https://doi.org/10.1038/nrc2222
This article is cited by
-
TC2N inhibits distant metastasis and stemness of breast cancer via blocking fatty acid synthesis
Journal of Translational Medicine (2024)
-
FFAR2 expressing myeloid-derived suppressor cells drive cancer immunoevasion
Journal of Hematology & Oncology (2024)
-
LncRNA SOX9-AS1 triggers a transcriptional program involved in lipid metabolic reprogramming, cell migration and invasion in triple-negative breast cancer
Scientific Reports (2024)
-
A novel anoikis-related gene signature identifies LYPD1 as a novel therapy target for bladder cancer
Scientific Reports (2024)
-
Lipids as mediators of cancer progression and metastasis
Nature Cancer (2024)