Abstract
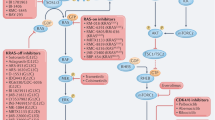
Farnesyltransferase (FTase) inhibitors (FTIs) were developed originally as anti-RAS compounds and novel target-based drugs for cancer treatment. The analyses of FTIs continue in the clinic, but the antitumour activity cannot be ascribed simply to inhibition of RAS. Although FTI action is due to inhibition of FTase, and RAS proteins are indeed substrates for this enzyme, the RAS proteins that are most frequently mutated in human cancers escape FTI inhibition. RHOB has been suggested as a target, but is this issue resolved or do the crucial targets of FTIs remain to be identified?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Shields, J. M., Pruitt, K., McFall, A., Shaub, A. & Der, C. J. Understanding Ras: 'it ain't over 'til it's over'. Trends Cell Biol. 10, 147–154 (2000).
Sahai, E. & Marshall, C. J. RHO-GTPases and cancer. Nature Rev. Cancer 2, 133–142 (2002).
Ellis, C. A. et al. Rig is a novel Ras-related protein and potential neural tumor suppressor. Proc. Natl Acad. Sci. USA 99, 9876–9881 (2002).
Finlin, B. S. et al. RERG is a novel ras-related, estrogen-regulated and growth-inhibitory gene in breast cancer. J. Biol. Chem. 276, 42259–42267 (2001).
Hamaguchi, M. et al. DBC2, a candidate for a tumor suppressor gene involved in breast cancer. Proc. Natl Acad. Sci. USA 99, 13647–13652 (2002).
Yu, Y. et al. NOEY2 (ARHI), an imprinted putative tumor suppressor gene in ovarian and breast carcinomas. Proc. Natl Acad. Sci. USA 96, 214–219 (1999).
Cox, A. D. & Der, C. J. Ras family signaling: therapeutic targeting. Cancer Biol. Ther. 1, 599–606 (2002).
Downward, J. Targeting RAS signalling pathways in cancer therapy. Nature Rev. Cancer 3, 11–22 (2003).
Barbacid, M. Ras genes. Annu. Rev. Biochem. 56, 779–827 (1987).
Sebti, S. M. & Hamilton, A. D. Anticancer activity of farnesyltransferase and geranylgeranyltransferase I inhibitors: prospects for drug development. Exp. Opin. Invest. Drugs 6, 1711–1714 (1997).
Sebti, S. M. & Hamilton, A. D. (eds.) Farnesyltransferase Inhibitors in Cancer Therapy 197–219 (Humana Press, Totowa, New Jersey, 2000).
Cox, A. D. & Der, C. J. Farnesyltransferase inhibitors and cancer treatment: targeting simply Ras? Biochim. Biophys. Acta 1333, F51–F71 (1997).
Gibbs, J. B. & Oliff, A. The potential of farnesyltransferase inhibitors as cancer chemotherapeutics. Annu. Rev. Pharmacol. Toxicol. 37, 143–166 (1997).
Sebti, S. M. & Hamilton, A. D. Inhibition of Ras prenylation: a novel approach to cancer chemotherapy. Pharmacol. Ther. 74, 103–114 (1997).
Reiss, Y., Goldstein, J. L., Seabra, M. C., Casey, P. J. & Brown, M. S. Inhibition of purified p21ras farnesyl:protein transferase by Cys-AAX tetrapeptides. Cell 62, 81–88 (1990).
Sun, J. et al. Antitumor efficacy of a novel class of non-thiol-containing peptidomimetic inhibitors of farnesyltransferase and geranylgeranyltransferase I: combination therapy with the cytotoxic agents cisplatin, Taxol, and gemcitabine. Cancer Res. 59, 4919–4926 (1999).
James, G. L., Goldstein, J. L. & Brown, M. S. Polylysine and CVIM sequences of K-RasB dictate specificity of prenylation and confer resistance to benzodiazepine peptidomimetic in vitro. J. Biol. Chem. 270, 6221–6226 (1995).
Whyte, D. B. et al. K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J. Biol. Chem. 272, 14459–14464 (1997).
Rowell, C. A., Kowalczyk, J. J., Lewis, M. D. & Garcia, A. M. Direct demonstration of geranylgeranylation and farnesylation of Ki-Ras in vivo. J. Biol. Chem. 272, 14093–14097 (1997).
Lerner, E. C. et al. Inhibition of the prenylation of K-Ras, but not H- or N-Ras, is highly resistant to CAAX peptidomimetics and requires both a farnesyltransferase and a geranylgeranyltransferase I inhibitor in human tumor cell lines. Oncogene 15, 1283–1288 (1997).
Sun, J., Qian, Y., Hamilton, A. D. & Sebti, S. M. Both farnesyltransferase and geranylgeranyltransferase I inhibitors are required for inhibition of oncogenic K-Ras prenylation but each alone is sufficient to suppress human tumor growth in nude mouse xenografts. Oncogene 16, 1467–1473 (1998).
Lerner, E. C. et al. Ras CAAX peptidomimetic FTI-277 selectively blocks oncogenic Ras signaling by inducing cytoplasmic accumulation of inactive Ras-Raf complexes. J. Biol. Chem. 270, 26802–26806 (1995).
Bredel, M., Pollack, I. F., Freund, J. M., Hamilton, A. D. & Sebti, S. M. Inhibition of Ras and related G-proteins as a therapeutic strategy for blocking malignant glioma growth. Neurosurgery 43, 124–131 (1998).
Pollack, I. F., Bredel, M., Erff, M., Hamilton, A. D. & Sebti, S. M. Inhibition of Ras and related guanosine triphosphate-dependent proteins as a therapeutic strategy for blocking malignant glioma growth: II—preclinical studies in a nude mouse model. Neurosurgery 45, 1208–1214 (1999).
Lantry, L. E. et al. Effect of farnesyltransferase inhibitor FTI-276 on established lung adenomas from A/J mice induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis 21, 113–116 (2000).
Zhang, Z. et al. Farnesyltransferase inhibitors are potent lung cancer chemopreventive agents in A/J mice with a dominant-negative p53 and/or heterozygous deletion of Ink4a/Arf. Oncogene 22, 6257–6265 (2003).
Gibbs, J. B. et al. Farnesyltransferase Inhibitors in Cancer Therapy (ed. Hamilton, S. M.) 65–70 (Humana Press, Totowa, New Jersey, 2000).
Sun, J. et al. Geranylgeranyltransferase I inhibitor, GGTI-2154, induces breast carcinoma apoptosis and tumor regression in H-Ras transgenic mice. Cancer Res. (in the press).
Omer, C. A. et al. Mouse mammary tumor virus-Ki-rasB transgenic mice develop mammary carcinomas that can be growth-inhibited by a farnesyl:protein transferase inhibitor. Cancer Res. 2680–2688 (2000).
Vogt, A., Sun, J., Qian, Y., Hamilton, A. D. & Sebti, S. M. The geranylgeranyltransferase-I inhibitor GGTI-298 arrests human tumor cells in G0/G1 and induces p21WAF1/CIP1/SDI1 in a p53-independent manner. J. Biol. Chem. 272, 27224–27229 (1997).
Ashar, H. R. et al. Farnesyl transferase inhibitors block the farnesylation of CENP-E and CENP-F and alter the association of CENP-E with the microtubules. J. Biol. Chem. 275, 30451–30457 (2000).
Crespo, N. C., Ohkanda, J., Yen, T. J., Hamilton, A. D. & Sebti, S. M. The farnesyltransferase inhibitor, FTI-2153, blocks bipolar spindle formation and chromosome alignment and causes prometaphase accumulation during mitosis of human lung cancer cells. J. Biol. Chem. 276, 16161–16167 (2001).
Crespo, N. C. et al. The farnesyltransferase inhibitor, FTI-2153, inhibits bipolar spindle formation during mitosis independently of transformation and Ras and p53 mutation status. Cell Death Differ. 9, 702–709 (2002).
Hussein, D. & Taylor, S. S. Farnesylation of Cenp-F is required for G2/M progression and degradation after mitosis. J. Cell Sci. 115, 3403–3414 (2002).
Suzuki, N., Urano, J. & Tamanoi, F. Farnesyltransferase inhibitors induce cytochrome c release and caspase 3 activation preferentially in transformed cells. Proc. Natl Acad. Sci. USA 95, 15356–15361 (1998).
Lebowitz, P. F., Sakamuro, D. & Prendergast, G. C. Farnesyl transferase inhibitors induce apoptosis of Ras-transformed cells denied substratum attachment. Cancer Res. 57, 708–713 (1997).
Jiang, K. et al. The phosphoinositide 3-OH kinase/AKT2 pathway as a critical target for farnesyltransferase inhibitor-induced apoptosis. Mol. Cell. Biol. 20, 139–148 (2000).
Liu, A. & Prendergast, G. C. Geranylgeranylated RhoB is sufficient to mediate tissue-specific suppression of Akt kinase activity by farnesyltransferase inhibitors. FEBS Lett. 481, 205–208 (2000).
Du, W. & Prendergast, G. C. Geranylgeranylated RhoB mediates suppression of human tumor cell growth by farnesyltransferase inhibitors. Cancer Res. 59, 5492–5496 (1999).
Kohl, N. E. et al. Selective inhibition of ras-dependent transformation by a farnesyltransferase inhibitor. Science 260, 1934–1937 (1993).
James, G. L. et al. Benzodiazepine peptidomimetics: potent inhibitors of Ras farnesylation in animal cells. Science 260, 1937–1942 (1993).
Sepp-Lorenzino, L. et al. A peptidomimetic inhibitor of farnesyl:protein transferase blocks the anchorage-dependent and-independent growth of human tumor cell lines. Cancer Res. 55, 5302–5309 (1995).
Fiordalisi, J. J. et al. High affinity for farnesyl transferase and alternative prenylation contribute individually to K-ras4B resistance to farnesyl transferase inhibitors. J. Biol. Chem. 278, 41718–41727 (2003).
Voice, J. K., Klemke, R. L., Le, A. & Jackson, J. H. Four human ras homologs differ in their abilities to activate Raf-1, induce transformation, and stimulate cell motility. J. Biol. Chem. 274, 17164–17170 (1999).
Yan, J., Roy, S., Apolloni, A., Lane, A. & Hancock, J. F. Ras isoforms vary in their ability to activate Raf-1 and phosphoinositide 3-kinase. J. Biol. Chem. 273, 24052–24056 (1998).
Armstrong, S. A., Hannah, V. C., Goldstein, J. L. & Brown, M. S. CAAX geranylgeranyl transferase transfers farnesyl as efficiently as geranylgeranyl to RhoB. J. Biol. Chem. 270, 7864–7868 (1995).
Lebowitz, P. F., Casey, P. J., Prendergast, G. C. & Thissen, J. A. Farnesyltransferase inhibitors alter the prenylation and growth-stimulating function of RhoB. J. Biol. Chem. 272, 15591–15594 (1997).
Du, W., Liu, A. & Prendergast, G. C. Activation of the PI3K-AKT pathway masks the proapoptotic effects of farnesyltransferase inhibitors. Cancer Res. 59, 4208–4212 (1999).
Liu, A., Du, W., Liu, J. P., Jessell, T. M. & Prendergast, G. C. RhoB alteration is necessary for apoptotic and antineoplastic responses to farnesyltransferase inhibitors. Mol. Cell. Biol. 20, 6105–6113 (2000).
Liu, A. X., Rane, N., Liu, J. P. & Prendergast, G. C. RhoB is dispensable for mouse development, but it modifies susceptibility to tumor formation as well as cell adhesion and growth factor signaling in transformed cells. Mol. Cell. Biol. 21, 6906–6912 (2001).
Chen, Z. et al. Both farnesylated and geranylgeranylated RhoB inhibit malignant transformation and suppress human tumor growth in nude mice. J. Biol. Chem. 275, 17974–17978 (2000).
Baron, R. et al. RhoB prenylation is driven by the three carboxyl-terminal amino acids of the protein: evidenced in vivo by an anti-farnesyl cysteine antibody. Proc. Natl Acad. Sci. USA 97, 11626–11631 (2000).
Adnane, J., Muro-Cacho, C., Mathews, L., Sebti, S. M. & Munoz-Antonia, T. Suppression of rho B expression in invasive carcinoma from head and neck cancer patients. Clin. Cancer Res. 8, 2225–2232 (2002).
Forget, M. A. et al. The expression of rho proteins decreases with human brain tumor progression: potential tumor markers. Clin. Exp. Metastasis 19, 9–15 (2002).
Fritz, G., Brachetti, C., Bahlmann, F., Schmidt, M. & Kaina, B. Rho GTPases in human breast tumours: expression and mutation analyses and correlation with clinical parameters. Br. J. Cancer 87, 635–644 ( 2002).
Hancock, J. F., Cadwallader, K., Paterson, H. & Marshall, C. J. A CAAX or a CAAL motif and a second signal are sufficient for plasma membrane targeting of ras proteins. EMBO J. 10, 4033–4039 (1991).
Cox, A. D., Hisaka, M. M., Buss, J. E. & Der, C. J. Specific isoprenoid modification is required for function of normal, but not oncogenic, Ras protein. Mol. Cell. Biol. 12, 2606–2615 (1992).
Allal, C. et al. RhoA prenylation is required for promotion of cell growth and transformation and cytoskeleton organization but not for induction of serum response element transcription. J. Biol. Chem. 275, 31001–31008 (2000).
Solski, P. A., Helms, W., Keely, P. J., Su, L. & Der, C. J. RhoA biological activity is dependent on prenylation but independent of specific isoprenoid modification. Cell Growth Differ. 13, 363–373 (2002).
Rose, W. C. et al. Preclinical antitumor activity of BMS-214662, a highly apoptotic and novel farnesyltransferase inhibitor. Cancer Res. 61, 7507–7517 (2001).
Alton, G., Cox, A. D., Toussaint, L. G. & Westwick, J. K. Functional proteomics analysis of GTPase signaling networks. Methods Enzymol. 332, 300–316 (2001).
Cates, C. A. et al. Prenylation of oncogenic human PTP(CAAX) protein tyrosine phosphatases. Cancer Lett. 110, 49–55 (1996).
Zeng, Q. et al. Prenylation-dependent association of protein-tyrosine phosphatases PRL-1,-2, and-3 with the plasma membrane and the early endosome. J. Biol. Chem. 275, 21444–21452 (2000).
Zeng, Q. et al. PRL-3 and PRL-1 promote cell migration, invasion, and metastasis. Cancer Res. 63, 2716–2722 (2003).
Wang, Q., Holmes, D. I., Powell, S. M., Lu, Q. L. & Waxman, J. Analysis of stromal-epithelial interactions in prostate cancer identifies PTPCAAX2 as a potential oncogene. Cancer Lett. 175, 63–69 (2002).
Saha, S. et al. A phosphatase associated with metastasis of colorectal cancer. Science 294, 1343–1346 (2001).
Guasch, R. M., Scambler, P., Jones, G. E. & Ridley, A. J. RhoE regulates actin cytoskeleton organization and cell migration. Mol. Cell. Biol. 18, 4761–4771 (1998).
Nobes, C. D. et al. A new member of the Rho family, Rnd1, promotes disassembly of actin filament structures and loss of cell adhesion. J. Cell Biol. 141, 187–197 (1998).
Wennerberg, K. et al. Rnd proteins function as RhoA antagonists by activating p190 RhoGAP. Curr. Biol. 13, 1106–1115 (2003).
Riento, K., Guasch, R. M., Garg, R., Jin, B. & Ridley, A. J. RhoE binds to ROCK I and inhibits downstream signaling. Mol. Cell. Biol. 23, 4219–4229 (2003).
Hansen, S. H. et al. Induced expression of Rnd3 is associated with transformation of polarized epithelial cells by the Raf-MEK-extracellular signal-regulated kinase pathway. Mol. Cell. Biol. 20, 9364–9375 (2000).
Clark, G. J. et al. The Ras-related protein Rheb is farnesylated and antagonizes Ras signaling and transformation. J. Biol. Chem. 272, 10608–10615 (1997).
Saucedo, L. J. et al. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nature Cell Biol. 5, 566–571 (2003).
Stocker, H. et al. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nature Cell Biol. 5, 559–565 (2003).
Inoki, K., Li, Y., Xu, T. & Guan, K. L. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 17, 1829–1834 (2003).
Tee, A. R., Manning, B. D., Roux, P. P., Cantley, L. C. & Blenis, J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr. Biol. 13, 1259–1268 (2003).
Garami, A. et al. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol. Cell 11, 1457–1466 (2003).
Castro, A. F., Rebhun, J. F., Clark, G. G. & Quilliam, L. A. Rheb binds TSC2 and promotes S6 kinase activation in a rapamycin- and farnesylation-dependent manner. J. Biol. Chem. 278, 32493–32496 (2003).
Zhang, Y. et al. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nature Cell Biol. 5, 578–581 (2003).
Garami, A. et al. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol. Cell 11, 1457–1466 (2003).
Yang, W., Tabancay, A. P. Jr, Urano, J. & Tamanoi, F. Failure to farnesylate Rheb protein contributes to the enrichment of G0/G1 phase cells in the Schizosaccharomyces pombe farnesyltransferase mutant. Mol. Microbiol. 41, 1339–1347 (2001).
Patel, P. H. et al. Drosophila Rheb GTPase is required for cell cycle progression and cell growth. J. Cell Sci. 116, 3601–3610 (2003).
Gromov, P. S., Madsen, P., Tomerup, N. & Celis, J. E. A novel approach for expression cloning of small GTPases: identification, tissue distribution and chromosome mapping of the human homolog of rheb. FEBS Lett. 377, 221–226 (1995).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sebti, S., Der, C. Searching for the elusive targets of farnesyltransferase inhibitors. Nat Rev Cancer 3, 945–951 (2003). https://doi.org/10.1038/nrc1234
Issue Date:
DOI: https://doi.org/10.1038/nrc1234
This article is cited by
-
Optimal functional levels of activation-induced deaminase specifically require the Hsp40 DnaJa1
The EMBO Journal (2012)
-
The nuclear envelope environment and its cancer connections
Nature Reviews Cancer (2012)
-
The Farnesyl Transferase Inhibitor Lonafarnib Inhibits mTOR Signaling and Enforces Sorafenib-Induced Apoptosis in Melanoma Cells
Journal of Investigative Dermatology (2011)
-
Measurement of protein farnesylation and geranylgeranylation in vitro, in cultured cells and in biopsies, and the effects of prenyl transferase inhibitors
Nature Protocols (2011)
-
Differential requirement of CAAX-mediated posttranslational processing for Rheb localization and signaling
Oncogene (2010)