Abstract
We describe here a highly efficient procedure for conditional mutagenesis in Plasmodium. The procedure uses the site-specific recombination FLP-FRT system of yeast and targets the pre-erythrocytic stages of the rodent Plasmodium parasite P. berghei, including the sporozoite stage and the subsequent liver stage. The technique consists of replacing the gene under study by an FRTed copy (i.e., flanked by FRT sites) in the erythrocytic stages of a parasite clone that expresses the flip (FLP) recombinase stage-specifically—called the 'deleter' clone. We present the available deleter clones, which express FLP at different times of the parasite life cycle, as well as the schemes and tools for constructing new deleter parasites. We also outline and discuss the various strategies for exchanging a wild-type gene with an FRTed copy and for generating conditional gene knockout or knockdown parasite clones. Finally, we detail the protocol for obtaining sporozoites that lack a protein of interest and for monitoring sporozoite-specific DNA excision and depletion of the target protein. The protocol should allow the functional analysis of any essential protein in the sporozoite, liver stage or hepatic merozoite stages of rodent Plasmodium parasites.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
References
Wu, Y., Kirkman, L.A. & Wellems, T.E. Transformation of Plasmodium falciparum malaria parasites by homologous integration of plasmids that confer resistance to pyrimethamine. Proc. Natl. Acad. Sci. USA 93, 1130–1134 (1996).
van Dijk, M.R., Janse, C.J. & Waters, A.P. Expression of a Plasmodium gene introduced into subtelomeric regions of Plasmodium berghei chromosomes. Science 271, 662–665 (1996).
Carvalho, T.G. & Ménard, R. Manipulating the Plasmodium genome. Curr. Issues Mol. Biol. 7, 39–55 (2005).
Cowman, A.F. et al. Functional analysis of proteins involved in Plasmodium falciparum merozoite invasion of red blood cells. FEBS Lett. 476, 84–88 (2000).
Ménard, R., Heussler, V., Yuda, M. & Nussenzweig, V. Plasmodium pre-erythrocytic stages: what's new? Trends Parasitol. 24, 564–569 (2008).
Vaughan, A.M., Aly, A.S. & Kappe, S.H. Malaria parasite pre-erythrocytic stage infection: gliding and hiding. Cell Host & Microbe 4, 209–218 (2008).
Casares, S., Brumeanu, T.D. & Richie, T.L. The RTS,S malaria vaccine. Vaccine 12, 4880–4894 (2010).
Ménard, R. et al. Circumsporozoite protein is required for development of malaria sporozoites in mosquitoes. Nature 385, 336–340 (1997).
Gossen, M. & Bujard, H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 89, 5547–5551 (1992).
Gossen, M. & Bujard, H. Studying gene function in eukaryotes by conditional gene inactivation. Annu. Rev. Genet. 36, 153–173 (2002).
Meissner, M. et al. Tetracycline analogue-regulated transgene expression in Plasmodium falciparum blood stages using Toxoplasma gondii transactivators. Proc. Natl. Acad. Sci. USA 102, 2980–2985 (2005).
Meissner, M., Schlüter, D. & Soldati, D. Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science 298, 837–840 (2002).
Mital, J., Meissner, M., Soldati, D. & Ward, G.E. Conditional expression of Toxoplasma gondii apical membrane antigen-1 (TgAMA1) demonstrates that TgAMA1 plays a critical role in host cell invasion. Mol. Biol. Cell 16, 4341–4349 (2005).
Huynh, M.H. & Carruthers, V.B. Toxoplasma MIC2 is a major determinant of invasion and virulence. PLoS Pathog. 2, e84 (2006).
Banaszynski, L.A., Chen, L.-C., Maynard-Smith, L.A., Ooi, A.G. & Wandless, T.J. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell 126, 995–1004 (2006).
Armstrong, C.M. & Goldberg, D.E. An FKBP destabilization domain modulates protein levels in Plasmodium falciparum. Nat. Methods 4, 1007–1009 (2007).
Herm-Götz, A. et al. Rapid control of protein level in the apicomplexan Toxoplasma gondii. Nat. Methods 4, 1003–1005 (2007).
Agop-Nersesian, C. et al. Biogenesis of the inner membrane complex is dependent on vesicular transport by the alveolate specific GTPase Rab11B. PLoS Pathog. 6, e1001029 (2010).
Branda, C.S. & Dymecki, S.M. Talking about a revolution: the impact of site-specific recombinases on genetic analyses in mice. Dev. Cell 6, 7–28 (2004).
Gil Carvalho, T., Thiberge, S., Sakamoto, H. & Ménard, R. Conditional mutagenesis using site-specific recombination in Plasmodium berghei. Proc. Natl. Acad. Sci. USA 101, 14931–14936 (2004).
Combe, A. et al. Clonal conditional mutagenesis in malaria parasites. Cell Host & Microbe 5, 386–396 (2009).
Falae, A. et al. Role of Plasmodium berghei cGMP-dependent protein kinase in late liver stage development. J. Biol. Chem. 285, 3282–3288 (2010).
Ringrose, L. et al. Comparative kinetic analysis of FLP and Cre recombinases: mathematical models for DNA binding and recombination. J. Mol. Biol. 284, 363–384 (1998).
Abremski, K. & Hoess, R. Bacteriophage P1 site-specific recombination. Purification and properties of the Cre recombinase protein. J. Biol. Chem. 259, 1509–1514 (1984).
Buchholz, F., Angrand, P.-O. & Stewart, A.F. Improved properties of FLP recombinase evolved by cycling mutagenesis. Nat. Biotech. 16, 657–662 (1998).
Rodriguez, C.I. et al. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat. Gen. 25, 139–140 (2000).
Buchholz, F., Ringrose, L., Angrand, P.-O., Rossi, F. & Stewart, A.F. Different thermostabilities of FLP and Cre recombinases: implications for applied site-specific recombination. Nucleic Acids Res. 24, 4256–4262 (1996).
Senecoff, J.F., Rossmeissl, P.J. & Cox, M.M. DNA recognition by the FLP recombinase of the yeast 2m plasmid: a mutational analysis of the FLP binding site. J. Mol. Biol. 201, 405–421 (1988).
Matuschewski, K. et al. Infectivity-associated changes in the transcriptional repertoire of the malaria parasite sporozoite stage. J. Biol. Chem. 277, 41948–41953 (2002).
Rosinski-Chupin, I. et al. Serial analysis of gene expression in Plasmodium berghei salivary gland sporozoites. BMC Genomics 8, 466 (2007).
Ishino, T., Orito, Y., Chinzei, Y. & Yuda, M. A calcium-dependent protein kinase regulates Plasmodium ookinetes access to the midgut epithelial cell. Mol. Microbiol. 59, 1175–1184 (2006).
Amino, R. et al. Host cell traversal is important for progression of the malaria parasite through the dermis to the liver. Cell Host & Microbe 3, 88–96 (2008).
O'Donnell, R.A., Saul, A., Cowman, A.F. & Crabb, B.S. Functional conservation of the malaria vaccine antigen MSP1-19 across distantly related Plasmodium species. Nat. Med. 6, 91–95 (2000).
Triglia, T. et al. Apical membrane antigen 1 plays a central role in erythrocyte invasion by Plasmodium species. Mol. Microbiol. 38, 706–718 (2000).
Nunes, A. et al. Subtle mutagenesis by ends-in recombination in malaria parasites. Mol. Cell Biol. 19, 2895–2902 (1999).
Tycowski, K.T., Kolev, N.G., Conrad, N.K., Fok, V. & Steitz, J.A. The ever-growing world of small nuclear ribonucleoproteins in the RNA world. (eds. Gesteland, R.F., Cech, T. & Atkins, J.F.) 331 (Cold Spring Harbor Laboratory Press, 2006).
Janse, C.J., Ramesar, J. & Waters, A.P. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malarial parasite Plasmodium berghei. Nat. Protoc. 1, 346–356 (2006).
Thiberge, S. et al. In vivo imaging of malaria parasites in the murine liver. Nat. Protoc. 2, 1811–1818 (2007).
Mair, G.R. et al. Regulation of sexual development of Plasmodium by translational repression. Science 313, 667–669 (2006).
Applied Biosystems. Real-time PCR Systems: Chemistry Guide, Part no. 4348358, Rev. E http://www3.appliedbiosystems.com/cms/groups/mcb_marketing/documents/generaldocuments/cms_041440.pdf (2005).
Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970).
Towbin, H., Staehelin, T. & Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76, 4350–4354 (1979).
Acknowledgements
We thank C. Bourgouin and I. Thiéry and the other members of the Center for Production and Infection of Anopheles of Institut Pasteur for rearing the mosquitoes. This work was performed with the financial help of the American Heart Association (to P.B.); of the 'Institut Carnot-Pasteur Maladies Infectieuses' (to J.-C.B.); and of the Institut Pasteur, the 'Agence Nationale pour la Recherche' and the Howard Hughes Medical Institute International Program (to R.M.).
Author information
Authors and Affiliations
Contributions
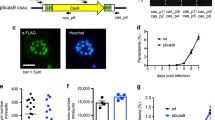
C.L. performed all transfections, contributed to the characterization of conditional clones and is the protocol expert; D.G. and A.C. constructed the deleters, and inactivated and characterized MSP1, AMA1 and RON4; S.S. characterized the AMA1 and RON4 mutant; D.P. and P.B. inactivated PKG and provided Figure 6; D.B., L.T. and J.-C.B. helped to refine the 3′ excision strategy; S.T. helped with transfections; T.G.C. demonstrated the feasibility of the clonal conditional approach; and R.M. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lacroix, C., Giovannini, D., Combe, A. et al. FLP/FRT-mediated conditional mutagenesis in pre-erythrocytic stages of Plasmodium berghei. Nat Protoc 6, 1412–1428 (2011). https://doi.org/10.1038/nprot.2011.363
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2011.363
This article is cited by
-
An essential vesicular-trafficking phospholipase mediates neutral lipid synthesis and contributes to hemozoin formation in Plasmodium falciparum
BMC Biology (2021)
-
Lineage tracing: technology tool for exploring the development, regeneration, and disease of the digestive system
Stem Cell Research & Therapy (2020)
-
Plasmodium translocon component EXP2 facilitates hepatocyte invasion
Nature Communications (2020)
-
Generation of transgenic rodent malaria parasites by transfection of cell culture-derived merozoites
Malaria Journal (2017)
-
Recent advances in genetic modification systems for Actinobacteria
Applied Microbiology and Biotechnology (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.