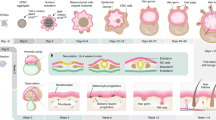
Abstract
In this protocol, we describe how to isolate keratinocytes from adult mouse epidermis, fractionate them into different sub-populations on the basis of cell surface markers and examine their function in an in vivo skin reconstitution assay with disaggregated neonatal dermal cells. We also describe how the isolated keratinocytes can be subjected to clonal analysis in vitro and in vivo and how to enrich for hair follicle-inducing dermal papilla cells in the dermal preparation. Using these approaches, it is possible to compare the capacity of different populations of adult epidermal stem cells to proliferate and to generate progeny that differentiate along the different epidermal lineages. Isolating, fractionating and grafting cells for the skin reconstitution assay is normally spread over 2 d. Clonal growth in culture is assessed after 14 d, while evaluation of the grafts is carried out after 4–5 weeks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Chu, D.H. Overview of biology, development and structure of skin. In Fitzpatrick's Dermatology in General Medicine, 7th edn. Vol. 1 (eds. Wolff, K. et al.) 57–73 (McGraw-Hill, New York, 2008).
Schneider, M.R., Schmidt-Ullrich, R. & Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 19, R132–R142 (2009).
Chuong, C.M. Regenerative biology: new hair from healing wounds. Nature 447, 265–266 (2007).
Jones, P.H., Simons, B.D. & Watt, F.M. Sic transit gloria: farewell to the epidermal transit amplifying cell? Cell Stem Cell 1, 371–381 (2007).
Watt, F.M. & Jensen, K.B. Epidermal stem cell diversity and quiescence. EMBO Mol. Med. 1, 260–267 (2009).
Jones, P.H. & Watt, F.M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 73, 713–724 (1993).
Kamimura, J., Lee, D., Baden, H.P., Brissette, J. & Dotto, G.P. Primary mouse keratinocyte cultures contain hair follicle progenitor cells with multiple differentiation potential. J. Invest. Dermatol. 109, 534–540 (1997).
Lo Celso, C. et al. Characterization of bipotential epidermal progenitors derived from human sebaceous gland: contrasting roles of c-Myc and beta-catenin. Stem Cells 26, 1241–1252 (2008).
Jaks, V. et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat. Genet. 40, 1291–1299 (2008).
Silva-Vargas, V. et al. Beta-catenin and Hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Dev. Cell 9, 121–131 (2005).
Jensen, K.B. et al. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell 4, 427–439 (2009).
Jensen, K.B. & Watt, F.M. Single-cell expression profiling of human epidermal stem and transit-amplifying cells: Lrig1 is a regulator of stem cell quiescence. Proc. Natl. Acad. Sci. USA 103, 11958–11963 (2006).
Morris, R.J. Procedure for harvesting epidermal cells from the dorsal epidermis of adult mice for primary cell culture in 'high calcium' defined medium. In The Keratinocyte Handbook, 1st edn. (eds. Leigh, I.M., Lane, E.B. & Watt, F.M.) 25–31 (Cambridge University Press, Cambridge, UK, 1994).
Romero, M.R., Carroll, J.M. & Watt, F.M. Analysis of cultured keratinocytes from a transgenic mouse model of psoriasis: effects of suprabasal integrin expression on keratinocyte adhesion, proliferation and terminal differentiation. Exp. Dermatol. 8, 53–67 (1999).
Rheinwald, J.G. Human epidermal keratinocyte cell culture and xenograft systems: applications in the detection of potential chemical carcinogens and the study of epidermal transformation. Prog. Clin. Biol. Res. 298, 113–125 (1989).
Rheinwald, J.G. & Green, H. Formation of a keratinizing epithelium in culture by a cloned cell line derived from a teratoma. Cell 6, 317–330 (1975).
Watt, F.M., Broad, S. & Prowse, D.M. Cultivation and retroviral infection of human epidermal keratinocytes. In Cell Biology: A Laboratory Handbook 3rd edn., Vol. 1 (ed. Celis, J.E.) 133–138 (Elsevier, Amsterdam, the Netherlands, 2006).
Owens, D.M., Romero, M.R., Gardner, C. & Watt, F.M. Suprabasal alpha6beta4 integrin expression in epidermis results in enhanced tumourigenesis and disruption of TGFbeta signalling. J. Cell. Sci. 116, 3783–3791 (2003).
Popova, N.V. & Morris, R.J. Genetic regulation of mouse stem cells: identification of two keratinocyte stem cell regulatory loci. Curr. Top Microbiol. Immunol. 280, 111–137 (2004).
Barrandon, Y. & Green, H. Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl. Acad. Sci. USA 84, 2302–2306 (1987).
Lowell, S., Jones, P., Le Roux, I., Dunne, J. & Watt, F.M. Stimulation of human epidermal differentiation by delta-notch signalling at the boundaries of stem-cell clusters. Curr. Biol. 10, 491–500 (2000).
Claudinot, S., Nicolas, M., Oshima, H., Rochat, A. & Barrandon, Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc. Natl. Acad. Sci. USA 102, 14677–14682 (2005).
Morris, R.J. et al. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 22, 411–417 (2004).
Weinberg, W.C. et al. Reconstitution of hair follicle development in vivo: determination of follicle formation, hair growth, and hair quality by dermal cells. J. Invest. Dermatol. 100, 229–236 (1993).
Yuspa, S.H., Morgan, D.L., Walker, R.J. & Bates, R.R. The growth of fetal mouse skin in cell culture and transplantation to F1 mice. J. Invest. Dermatol. 55, 379–389 (1970).
Fusenig, N.E. et al. Growth and differentiation characteristics of transformed keratinocytes from mouse and human skin in vitro and in vivo. J. Invest. Dermatol. 81, 168s–175s (1983).
Schmidt, G.H., Blount, M.A. & Ponder, B.A. Immunochemical demonstration of the clonal organization of chimaeric mouse epidermis. Development 100, 535–541 (1987).
Driskell, R.R., Giangreco, A., Jensen, K.B., Mulder, K.W. & Watt, F.M. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development 136, 2815–2823 (2009).
Jones, P.H., Harper, S. & Watt, F.M. Stem cell patterning and fate in human epidermis. Cell 80, 83–93 (1995).
Tani, H., Morris, R.J. & Kaur, P. Enrichment for murine keratinocyte stem cells based on cell surface phenotype. Proc. Natl. Acad. Sci. USA 97, 10960–10965 (2000).
Trempus, C.S. et al. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J. Invest. Dermatol. 120, 501–511 (2003).
Blanpain, C., Lowry, W.E., Geoghegan, A., Polak, L. & Fuchs, E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118, 635–648 (2004).
Triel, C., Vestergaard, M.E., Bolund, L., Jensen, T.G. & Jensen, U.B. Side population cells in human and mouse epidermis lack stem cell characteristics. Exp. Cell Res. 295, 79–90 (2004).
Albert, M.R., Foster, R.A. & Vogel, J.C. Murine epidermal label-retaining cells isolated by flow cytometry do not express the stem cell markers CD34, Sca-1, or Flk-1. J. Invest. Dermatol. 117, 943–948 (2001).
Jensen, U.B. et al. A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. J. Cell. Sci. 121, 609–617 (2008).
Lyle, S. et al. The C8/144B monoclonal antibody recognizes cytokeratin 15 and defines the location of human hair follicle stem cells. J. Cell. Sci. 111, 3179–3188 (1998).
Nijhof, J.G. et al. The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development 133, 3027–3037 (2006).
Jahoda, C.A., Horne, K.A. & Oliver, R.F. Induction of hair growth by implantation of cultured dermal papilla cells. Nature 311, 560–562 (1984).
Ito, Y. et al. Isolation of murine hair-inducing cells using the cell surface marker prominin-1/CD133. J. Invest. Dermatol. 127, 1052–1060 (2007).
Berta, M.A., Baker, C.M., Cottle, D.L. & Watt, F.M. Dose and context dependent effects of Myc on epidermal stem cell proliferation and differentiation. EMBO Mol. Med. 2, 16–25 (2009).
Acknowledgements
We thank D. Owens and D. Roop for sharing their grafting protocols; S. Goldie for providing one of the figure panels; A. Giangreco and C. Collins for introducing the technique into our lab; and the excellent core facilities of the CRUK Cambridge Research Institute and the Wellcome Trust Centre for Stem Cell Research. This work was supported by Cancer Research UK, the Medical Research Council, the Wellcome Trust and the European Union. F. Watt gratefully acknowledges support from the University of Cambridge and Hutchison Whampoa.
Author information
Authors and Affiliations
Contributions
K.B.J. and R.R.D. tested the protocols, generated the figures and co-wrote the paper. F.M.W. co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Jensen, K., Driskell, R. & Watt, F. Assaying proliferation and differentiation capacity of stem cells using disaggregated adult mouse epidermis. Nat Protoc 5, 898–911 (2010). https://doi.org/10.1038/nprot.2010.39
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2010.39
This article is cited by
-
Myc-dependent dedifferentiation of Gata6+ epidermal cells resembles reversal of terminal differentiation
Nature Cell Biology (2023)
-
The stem cell quiescence and niche signaling is disturbed in the hair follicle of the hairpoor mouse, an MUHH model mouse
Stem Cell Research & Therapy (2022)
-
Transplantation of intestinal organoids into a mouse model of colitis
Nature Protocols (2022)
-
Novel pneumatically assisted atomization device for living cell delivery: application of sprayed mesenchymal stem cells for skin regeneration
Bio-Design and Manufacturing (2022)
-
Interfollicular epidermal stem-like cells for the recreation of the hair follicle epithelial compartment
Stem Cell Research & Therapy (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.