Abstract
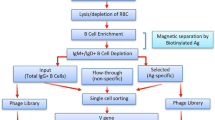
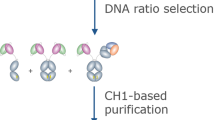
Single-domain intracellular antibodies are antibody variable segments that bind to specific target proteins inside cells. These antigen-binding variable regions can interfere with protein function or perturb protein–protein interactions and can be used as tools for research, especially functional genomics and proteomics and interfering with the protein interactome. This protocol (Intracellular Antibody Capture, IAC3) describes the isolation of functional variable heavy (VH) or variable light (VL) segments from diverse libraries. The protocol comprises four principle steps: validation of a bait antigen; initial screening in yeast of a single domain library; generation of sub-libraries after the initial screen; and finally confirmation of the positive clones interaction with antigen in yeast and mammalian cells. Each library (initial, second and third) screening takes upto 2 weeks and the overall procedure <3 months. This third generation IAC method has many advantages, including isolation of single domain intracellular antibodies with high affinity and specificity by direct in vivo genetic selection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Köhler, G. & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497 (1975).
Reichert, J.M., Rosensweig, C.J., Faden, L.B. & Dewitz, M.C. Monoclonal antibody successes in the clinic. Nat. Biotechnol. 23, 1073–1078 (2005).
Lobato, M.N. & Rabbitts, T.H. Intracellular antibodies and challenges facing their use as therapeutic agents. Trends in Mol. Med. 9, 390–396 (2003).
Tanaka, T., Williams, R.L. & Rabbitts, T.H. Tumour prevention by a single antibody domain inhibiting binding of signal transduction molecules to activated RAS. EMBO J. 26, 3250–3259 (2007).
Ward, E.S. et al. Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature 341, 544–546 (1989).
Hamers-Casterman, C. et al. Naturally occurring antibodies devoid of light chains. Nature 363, 446–448 (1993).
Roux, K.H. et al. Structural analysis of the nurse shark (new) antigen receptor (NAR): molecular convergence of NAR and unusual mammalian immunoglobulins. Proc. Natl. Acad. Sci. USA 95, 11804–11809 (1998).
Nuttall, S.D. et al. Isolation of the new antigen receptor from wobbegong sharks, and use as a scaffold for the display of protein loop libraries. Mol. Immunol. 38, 313–326 (2001).
Tanaka, T., Lobato, M.N. & Rabbitts, T.H. Single domain intracellular antibodies: a minimal fragment for direct in vivo selection of antigen-specific intrabodies. J. Mol. Biol. 331, 1109–1120 (2003).
Colby, D.W. et al. Potent inhibition of huntingtin aggregation and cytotoxicity by a disulfide bond-free single domain intracellular antibody. Proc. Natl. Acad. Sci. USA 101, 17616–17621 (2004).
Aires da Silva, F. et al. Camelized rabbit-derived VH single domain intrabodies against Vif strongly neutralize HIV-1 infectivity. J. Mol. Biol. 340, 525–542 (2004).
Paz, K. et al. Human single domain neutralizing intrabodies directed against Etk kinase: a novel approach to impair cellular transformation. Mol. Cancer Ther. 4, 1801–1809 (2005).
Verheesen, P. et al. Prevention of oculopharyngeal muscular dystrophy-associated aggregation of nuclear polyA-binding protein with a single domain intracellular antibody. Hum. Mol. Genet. 15, 105–111 (2006).
Gueorguieva, D. et al. Identification of single domain, Bax-specific intrabodies that confer resistance to mammalian cells against oxidative-stress-induced apoptosis. FASEB. J. 20, 2636–2638 (2006).
Serruys, B. et al. Llama-derived single domain intrabodies inhibit secretion of hepatitis B virions in mice. Hepatology 49, 39–49 (2009).
Tanaka, T. & Rabbitts, T.H. Functional intracellular antibody fragments do not require invariant intra-domain disulfide bonds. J. Mol. Biol. 376, 749–757 (2008).
Huston, J.S. et al. Protein engineering of antibody binding sites: Recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc. Natl. Acad. Sci. USA 85, 5879–5883 (1988).
Fields, S. & Song, O. A novel genetic system to detect protein-protein interactions. Nature 340, 245–246 (1989).
Visintin, M. et al. The intracellular antibody capture technology (IACT): towards a consensus sequence for intracellular antibodies. J. Mol. Biol. 317, 73–83 (2002).
Tse, E. et al. Intracellular antibody capture technology: application to selection of single chain Fv recognising the BCR-ABL oncogenic protein. J. Mol. Biol. 317, 85–94 (2002).
Tanaka, T. & Rabbitts, T.H. Intrabodies based on intracellular capture frameworks that bind the RAS protein with high affinity and impair oncogenic transformation. EMBO J. 22, 1025–1035 (2003).
Nam, C.H. et al. An antibody inhibitor of the LMO2-protein complex blocks its normal and tumorigenic functions. Oncogene 27, 4962–4968 (2008).
Winter, G., Griffiths, A.D., Hawkins, R.E. & Hoogenboom, H.R. Making antibodies by phage display technology. Ann. Rev. Imm. 12, 433–455 (1994).
Sheets, M.D. et al. Efficient construction of a large nonimmune phage antibody library: the production of high-affinity human single-chain antibodies to protein antigens. Proc. Natl. Acad. Sci. USA 95, 6157–6162 (1998).
de Wildt, R.M., Mundy, C.R., Gorick, B.D. & Tomlinson, I.M. Antibody arrays for high-throughput screening of antibody-antigen interactions. Nat. Biotechnol. 18, 989–994 (2000).
Buckler, D.R. et al. Screening isolates from antibody phage-display libraries. Drug Discov. Today 13, 318–324 (2008).
Gietz, R.D. & Woods, R.A. Transformation of yeast by the Liac/ss carrier DNA/PEG method. Methods Enzymol. 350, 87–96 (2002).
Tanaka, T. et al. De novo production of diverse intracellular antibody libraries. Nucleic Acids Res. 31, e23 (2003).
Vojtek, A.B., Hollenberg, S.M. & Cooper, J.A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell 74, 205–214 (1993).
Vidal, M. et al. Reverse two-hybrid and one-hybrid systems to detect dissociation of protein-protein and DNA-protein interactions. Proc. Natl. Acad. Sci. USA 93, 10315–10320 (1996).
Visintin, M. et al. Selection of antibodies for intracellular function using a two-hybrid in vivo system. Proc. Natl. Acad. Sci. USA 96, 11723–11728 (1999).
Sadowski, I., Bell, B., Broad, P. & Hollis, M. GAL4 fusion vectors for expression in yeast or mammalian cells. Gene 118, 137–141 (1992).
Sambrook, J. & Russell, P.W. (eds.) in Molecular Cloning A Laboratory Manual 3rd ed. 1.1–1.162, (Cold Spring Harbor Laboratory Press, New York, 2001).
Lundblad, V. et al. Saccharomyces cerevisiae. in Short Protocols in Molecular Biology 3rd ed. (eds. Ausubel, F.M., et al.) 13 (Wiley Publishing, New York, 1992).
Gallagher, S. et al. Immunoblotting and immunodetection. in Short Protocols in Molecular Biology 3rd ed. (eds. Ausubel, F.M., et al.) 10.7 (Wiley Publishing, New York, 1992).
Engebrecht, J. et al. Escherichia coli, plasmids, and bacteriophages. in Short Protocols in Molecular Biology 3rd ed. (eds. Ausubel, F.M., et al.) 1.6–1.8 (Wiley Publishing, New York, 1992).
Acknowledgements
This protocol was developed from work funded by the National Foundation for Cancer Research, Medical Research Council (UK), the Leukaemia Research Fund (UK) and the University of Leeds. We would like to thank Drs. Hanif Ali and Andrew Dixon for their comments and editing of the detailed protocol.
Author information
Authors and Affiliations
Contributions
T.T. and T.H.R. wrote the manuscript.
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Tanaka, T., Rabbitts, T. Protocol for the selection of single-domain antibody fragments by third generation intracellular antibody capture. Nat Protoc 5, 67–92 (2010). https://doi.org/10.1038/nprot.2009.199
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.199
This article is cited by
-
Pan RAS-binding compounds selected from a chemical library by inhibiting interaction between RAS and a reduced affinity intracellular antibody
Scientific Reports (2021)
-
Cancer cell killing by target antigen engagement with engineered complementary intracellular antibody single domains fused to pro-caspase3
Scientific Reports (2019)
-
A genetically encoded probe for imaging nascent and mature HA-tagged proteins in vivo
Nature Communications (2019)
-
Small molecule inhibitors of RAS-effector protein interactions derived using an intracellular antibody fragment
Nature Communications (2018)
-
Intracellular immunization against HIV infection with an intracellular antibody that mimics HIV integrase binding to the cellular LEDGF protein
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.