Abstract
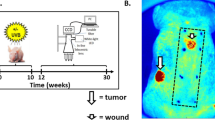
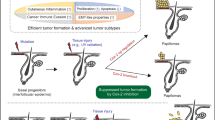
For more than 60 years, the chemical induction of tumors in mouse skin has been used to study mechanisms of epithelial carcinogenesis and evaluate modifying factors. In the traditional two-stage skin carcinogenesis model, the initiation phase is accomplished by the application of a sub-carcinogenic dose of a carcinogen. Subsequently, tumor development is elicited by repeated treatment with a tumor-promoting agent. The initiation protocol can be completed within 1–3 h depending on the number of mice used; whereas the promotion phase requires twice weekly treatments (1–2 h) and once weekly tumor palpation (1–2 h) for the duration of the study. Using the protocol described here, a highly reproducible papilloma burden is expected within 10–20 weeks with progression of a portion of the tumors to squamous cell carcinomas within 20–50 weeks. In contrast to complete skin carcinogenesis, the two-stage model allows for greater yield of premalignant lesions, as well as separation of the initiation and promotion phases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
DiGiovanni, J. Multistage carcinogenesis in mouse skin. Pharmacol. Ther. 54, 63–128 (1992).
Kemp, C.J. Multistep skin cancer in mice as a model to study the evolution of cancer cells. Semin. Cancer Biol. 15, 460–473 (2005).
Verma, A.K., Wheeler, D.L., Aziz, M.H. & Manoharan, H. Protein kinase Cepsilon and development of squamous cell carcinoma, the nonmelanoma human skin cancer. Mol. Carcinog. 45, 381–388 (2006).
Bornstein, S., Hoot, K., Han, G.W., Lu, S.L. & Wang, X.J. Distinct roles of individual Smads in skin carcinogenesis. Mol. Carcinog. 46, 660–664 (2007).
Zhang, J. & Bowden, G.T. Targeting Bcl-X(L) for prevention and therapy of skin cancer. Mol. Carcinog. 46, 665–670 (2007).
Kim, D.J., Chan, K.S., Sano, S. & DiGiovanni, J. Signal transducer and activator of transcription 3 (Stat3) in epithelial carcinogenesis. Mol. Carcinog. 46, 725–731 (2007).
Yuspa, S.H. The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis. J. Dermatol. Sci. 17, 1–7 (1998).
Yuspa, S.H. The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis–Thirty-third G. H. A. Clowes Memorial Award Lecture. Cancer Res. 54, 1178–1189 (1994).
Slaga, T.J. Cellular and molecular mechanisms involved in multistage skin carcinogenesis. in Carcinogenesis: A comprehensive Survey Skin Tumors: Experimental and Clinical Aspects Vol. 11 (eds. Conti, C.J., Slaga, T.J. & Klein-Szanto, A.J.P.) 1–18 (Raven Press, New York, 1989).
Rundhaug, J.E. & Fischer, S.M. Tumor promoters and models of promotion. in Comprehensive Toxicology Vol. 12 (eds. Sipes, I.G., McQueen, C.A. & Gandolfi, A.J.) 325–348 (Elsevier Sciences Ltd., New York, 1997).
Kundu, J.K., Shin, Y.K. & Surh, Y.J. Resveratrol modulates phorbol ester-induced pro-inflammatory signal transduction pathways in mouse skin in vivo: NF-kappaB and AP-1 as prime targets. Biochem. Pharmacol. 72, 1506–1515 (2006).
Fujiki, H., Atsumasa, K. & Suganuma, M. Chemoprevention of cancer. in Comprehensive Toxicology Vol. 12 (eds. Bowden, G.T. & Fischer, S.M.) 453–471 (Pergamon, Oxford, UK, 1997).
DiGiovanni, J. Modification of multistage skin carcinogenesis in mice. in Modification of Tumor Development in Rodents Vol. 33 (eds. Ito, N. & Sugano, H.) 192–229 (Karger, Basel, Switzerland, 1991).
Wilker, E. et al. Role of PI3K/Akt signaling in insulin-like growth factor-1 (IGF-1) skin tumor promotion. Mol. Carcinog. 44, 137–145 (2005).
Amornphimoltham, P., Leelahavanichkul, K., Molinolo, A., Patel, V. & Gutkind, J.S. Inhibition of Mammalian target of rapamycin by rapamycin causes the regression of carcinogen-induced skin tumor lesions. Clin. Cancer Res. 14, 8094–8101 (2008).
Brown, K., Strathdee, D., Bryson, S., Lambie, W. & Balmain, A. The malignant capacity of skin tumours induced by expression of a mutant H-ras transgene depends on the cell type targeted. Curr. Biol. 8, 516–524 (1998).
Kemp, C.J., Donehower, L.A., Bradley, A. & Balmain, A. Reduction of p53 gene dosage does not increase initiation or promotion but enhances malignant progression of chemically induced skin tumors. Cell 74, 813–822 (1993).
Glick, A.B. et al. Targeted deletion of the TGF-beta 1 gene causes rapid progression to squamous cell carcinoma. Genes Dev. 8, 2429–2440 (1994).
Han, G. et al. Distinct mechanisms of TGF-beta1-mediated epithelial-to-mesenchymal transition and metastasis during skin carcinogenesis. J. Clin. Invest. 115, 1714–1723 (2005).
Matsumoto, T. et al. Targeted expression of c-Src in epidermal basal cells leads to enhanced skin tumor promotion, malignant progression, and metastasis. Cancer Res. 63, 4819–4828 (2003).
Chan, K.S. et al. Disruption of Stat3 reveals a critical role in both the initiation and the promotion stages of epithelial carcinogenesis. J. Clin. Invest. 114, 720–728 (2004).
Rundhaug, J.E., Pavone, A., Kim, E. & Fischer, S.M. The effect of cyclooxygenase-2 overexpression on skin carcinogenesis is context dependent. Mol. Carcinog. 46, 981–992 (2007).
Segrelles, C. et al. Deregulated activity of Akt in epithelial basal cells induces spontaneous tumors and heightened sensitivity to skin carcinogenesis. Cancer Res. 67, 10879–10888 (2007).
Rundhaug, J.E. et al. Changes in protein expression during multistage mouse skin carcinogenesis. Mol. Carcinog. 20, 125–136 (1997).
Bassi, D.E. & Klein-Szanto, A.J.P. Carcinogen-induced animal models of head and neck squamous cell carcinoma. in Current Protocols in Pharmacology Supplement 37 14.12.11–14.12.19 (John Wiley & Sons, Hoboken, NJ, USA, 2007).
Ward, J.M., Rehm, S., Devor, D., Hennings, H. & Wenk, M.L. Differential carcinogenic effects of intraperitoneal initiation with 7,12-dimethylbenz(a)anthracene or urethane and topical promotion with 12-O-tetradecanoylphorbol-13-acetate in skin and internal tissues of female SENCAR and BALB/c mice. Environ. Health Perspect. 68, 61–68 (1986).
Ise, K. et al. Targeted deletion of the H-ras gene decreases tumor formation in mouse skin carcinogenesis. Oncogene. 19, 2951–2956 (2000).
Pierceall, W.E., Kripke, M.L. & Ananthaswamy, H.N. N-ras mutation in ultraviolet radiation-induced murine skin cancers. Cancer Res. 52, 3946–3951 (1992).
Rehman, I. et al. Frequent codon 12 Ki-ras mutations in mouse skin tumors initiated by N-methyl-N'-nitro-N-nitrosoguanidine and promoted by mezerein. Mol. Carcinog. 27, 298–307 (2000).
Nelson, M.A., Futscher, B.W., Kinsella, T., Wymer, J. & Bowden, G.T. Detection of mutant Ha-ras genes in chemically initiated mouse skin epidermis before the development of benign tumors. Proc. Natl. Acad. Sci. USA 89, 6398–6402 (1992).
Balmain, A., Ramsden, M., Bowden, G.T. & Smith, J. Activation of the mouse cellular Harvey-ras gene in chemically induced benign skin papillomas. Nature 307, 658–660 (1984).
Brown, K., Buchmann, A. & Balmain, A. Carcinogen-induced mutations in the mouse c-Ha-ras gene provide evidence of multiple pathways for tumor progression. Proc. Natl. Acad. Sci. USA 87, 538–542 (1990).
Spalding, J.W., Momma, J., Elwell, M.R. & Tennant, R.W. Chemically induced skin carcinogenesis in a transgenic mouse line (TG.AC) carrying a v-Ha-ras gene. Carcinogenesis 14, 1335–1341 (1993).
Morris, R.J. A perspective on keratinocyte stem cells as targets for skin carcinogenesis. Differentiation 72, 381–386 (2004).
Klein-Szanto, A.J.P. Pathology of human and experimental skin tumors. in Skin Tumors: Experimental and Clinical Aspects (eds. Conti, C.J., Slaga, T.J. & Klein-Szanto, A.J.P.) 19–53 (Raven Press, New York, 1989).
Yuspa, S.H., Ben, T., Hennings, H. & Lichti, U. Divergent responses in epidermal basal cells exposed to the tumor promoter 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 42, 2344–2349 (1982).
Karen, J. et al. 12-O-tetradecanoylphorbol-13-acetate induces clonal expansion of potentially malignant keratinocytes in a tissue model of early neoplastic progression. Cancer Res. 59, 474–481 (1999).
Hennings, H., Michael, D., Lichti, U. & Yuspa, S.H. Response of carcinogen-altered mouse epidermal cells to phorbol ester tumor promoters and calcium. J. Invest. Dermatol. 88, 60–65 (1987).
Parkinson, E.K. Defective responses of transformed keratinocytes to terminal differentiation stimuli. Their role in epidermal tumour promotion by phorbol esters and by deep skin wounding. Br. J. Cancer 52, 479–493 (1985).
Woodworth, C.D. et al. Strain-dependent differences in malignant conversion of mouse skin tumors is an inherent property of the epidermal keratinocyte. Carcinogenesis 25, 1771–1778 (2004).
Gimenez-Conti, I.B., et al. Dissociation of sensitivities to tumor promotion and progression in outbred and inbred SENCAR mice. Cancer Res. 52, 3432–3435 (1992).
Hennings, H. et al. FVB/N mice: an inbred strain sensitive to the chemical induction of squamous cell carcinomas in the skin. Carcinogenesis 14, 2353–2358 (1993).
Ewing, M.W., Conti, C.J., Kruszewski, F.H., Slaga, T.J. & DiGiovanni, J. Tumor progression in Sencar mouse skin as a function of initiator dose and promoter dose, duration, and type. Cancer Res. 48, 7048–7054 (1988).
DuBowski, A. et al. Papillomas at high risk for malignant progression arising both early and late during two-stage carcinogenesis in SENCAR mice. Carcinogenesis 19, 1141–1147 (1998).
Aldaz, C.M. & Conti, C.J. The premalignant nature of mouse skin papillomas: histopathologic, cytogenetic, and biochemical evidence. Carcinog. Compr. Surv. 11, 227–242 (1989).
Aldaz, C.M., Conti, C.J., Klein-Szanto, A.J. & Slaga, T.J. Progressive dysplasia and aneuploidy are hallmarks of mouse skin papillomas: relevance to malignancy. Proc. Natl. Acad. Sci. USA 84, 2029–2032 (1987).
Conti, C.J., Aldaz, C.M., O'Connell, J., Klein-Szanto, A.J. & Slaga, T.J. Aneuploidy, an early event in mouse skin tumor development. Carcinogenesis 7, 1845–1848 (1986).
Ruggeri, B. et al. Alterations of the p53 tumor suppressor gene during mouse skin tumor progression. Cancer Res. 51, 6615–6621 (1991).
Aldaz, C.M., Trono, D., Larcher, F., Slaga, T.J. & Conti, C.J. Sequential trisomization of chromosomes 6 and 7 in mouse skin premalignant lesions. Mol. Carcinog. 2, 22–26 (1989).
Chan, K.S. et al. Forced expression of a constitutively active form of Stat3 in mouse epidermis enhances malignant progression of skin tumors induced by two-stage carcinogenesis. Oncogene 27, 1087–1094 (2008).
Navarro, P. et al. A role for the E-cadherin cell-cell adhesion molecule during tumor progression of mouse epidermal carcinogenesis. J. Cell. Biol. 115, 517–533 (1991).
Caulin, C., Bauluz, C., Gandarillas, A., Cano, A. & Quintanilla, M. Changes in keratin expression during malignant progression of transformed mouse epidermal keratinocytes. Exp. Cell. Res. 204, 11–21 (1993).
Hennings, H. et al. Malignant conversion and metastasis of mouse skin tumors: a comparison of SENCAR and CD-1 mice. Environ. Health Perspect. 68, 69–74 (1986).
Boutwell, R.K. Some biological aspects of skin carcinogenesis. Prog. Exp. Tumor Res. 4, 207–250 (1964).
Digiovanni, J. Genetic Determinants of Susceptibility to Mouse Skin Tumor Promotions in Inbred Mice (Marcel Dekker, Inc., New York, 1989).
Mahler, K.L. et al. Sequence divergence of Mus spretus and Mus musculus across a skin cancer susceptibility locus. BMC Genomics 9, 626 (2008).
Mock, B.A. et al. Multigenic control of skin tumor susceptibility in SENCARA/Pt mice. Carcinogenesis 19, 1109–1115 (1998).
Nagase, H. et al. Distinct genetic loci control development of benign and malignant skin tumours in mice. Nat. Genet. 10, 424–429 (1995).
Fujiwara, K., Igarashi, J., Irahara, N., Kimura, M. & Nagase, H. New chemically induced skin tumour susceptibility loci identified in a mouse backcross between FVB and dominant resistant PWK. BMC Genet. 8, 39 (2007).
Peissel, B. et al. Use of intercross outbred mice and single nucleotide polymorphisms to map skin cancer modifier loci. Mamm. Genome. 12, 291–294 (2001).
Angel, J.M., Caballero, M. & DiGiovanni, J. Identification of novel genetic loci contributing to 12-O-tetradecanoylphorbol-13-acetate skin tumor promotion susceptibility in DBA/2 and C57BL/6 mice. Cancer Res. 63, 2747–2751 (2003).
Angel, J.M. & DiGiovanni, J. Genetics of skin tumor promotion. Prog. Exp. Tumor Res. 35, 143–157 (1999).
de Koning, J.P., Wakabayashi, Y., Nagase, H., Mao, J.H. & Balmain, A. Convergence of congenic mapping and allele-specific alterations in tumors for the resolution of the Skts1 skin tumor susceptibility locus. Oncogene 26, 4171–4178 (2007).
DiGiovanni, J. Role of transforming growth factor-a and the epidermal growth factor receptor in multistage mouse skin carcinogenesis. in Skin Cancer: Mechanisms and Human Relevance (ed. Mukhtar, H.) 181–197 (CRC Press, Inc., Boca Raton, FL, 1995).
DiGiovanni, J., Bhatt, T.S. & Walker, S.E. C57BL/6 mice are resistant to tumor promotion by full thickness skin wounding. Carcinogenesis 14, 319–321 (1993).
DiGiovanni, J., Walker, S.C., Beltran, L., Naito, M. & Eastin Jr., W.C. Evidence for a common genetic pathway controlling susceptibility to mouse skin tumor promotion by diverse classes of promoting agents. Cancer Res. 51, 1398–1405 (1991).
Imamoto, A. et al. Comparison of 12-O-tetradecanoylphorbol-13-acetate and teleocidin for induction of epidermal hyperplasia, activation of epidermal PKC isozymes and skin tumor promotion in SENCAR and C57BL/6 mice. Carcinogenesis 14, 719–724 (1993).
Hanahan, D. & Weinberg, R.A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Brabletz, T., Jung, A., Spaderna, S., Hlubek, F. & Kirchner, T. Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat. Rev. Cancer 5, 744–749 (2005).
Leedham, S.J. & Wright, N.A. Expansion of a mutated clone: from stem cell to tumour. J. Clin. Pathol. 61, 164–171 (2008).
Wistuba, I.I. et al. Sequential molecular abnormalities are involved in the multistage development of squamous cell lung carcinoma. Oncogene 18, 643–650 (1999).
Segditsas, S. et al. APC and the three-hit hypothesis. Oncogene 28, 146–155 (2009).
Kinzler, K.W. & Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 87, 159–170 (1996).
Kangsamaksin, T., Park, H.J., Trempus, C.S. & Morris, R.J. A perspective on murine keratinocyte stem cells as targets of chemically induced skin cancer. Mol. Carcinog. 46, 579–584 (2007).
Trempus, C.S. et al. CD34 expression by hair follicle stem cells is required for skin tumor development in mice. Cancer Res. 67, 4173–4181 (2007).
Rundhaug, J.E., Fuscher, S.M. & Bowden, G.T. Tumor Promoters and Models of Promotion. in Comprehensive Toxicology Vol. 12 (eds. Bowden, G.T. & Fischer, S.M.) 325–347 (Pergamon, Oxford, UK, 1997).
Pitot, H.C. & Dragan, Y.P. Facts and theories concerning the mechanisms of carcinogenesis. FASEB J. 5, 2280–2286 (1991).
Klein, E.A. Can prostate cancer be prevented? Nat. Clin. Pract. Urol. 2, 24–31 (2005).
Chen, J. & Roop, D.R. Genetically engineered mouse models for skin research: taking the next step. J. Dermatol. Sci. 52, 1–12 (2008).
Kleiner, H.E., Vulimiri, S.V., Starost, M.F., Reed, M.J. & DiGiovanni, J. Oral administration of the citrus coumarin, isopimpinellin, blocks DNA adduct formation and skin tumor initiation by 7,12-dimethylbenz[a]anthracene in SENCAR mice. Carcinogenesis 23, 1667–1675 (2002).
Gills, J.J. et al. Sulforaphane prevents mouse skin tumorigenesis during the stage of promotion. Cancer Lett. 236, 72–79 (2006).
Singh, R.P., Tyagi, A.K., Zhao, J. & Agarwal, R. Silymarin inhibits growth and causes regression of established skin tumors in SENCAR mice via modulation of mitogen-activated protein kinases and induction of apoptosis. Carcinogenesis 23, 499–510 (2002).
Birt, D.F., Pinch, H.J., Barnett, T., Phan, A. & Dimitroff, K. Inhibition of skin tumor promotion by restriction of fat and carbohydrate calories in SENCAR mice. Cancer Res. 53, 27–31 (1993).
Moore, T. et al. Dietary energy balance modulates signaling through the Akt/mammalian target of rapamycin pathways in multiple epithelial tissues. Cancer Prev. Res. (Phila Pa) 1, 65–76 (2008).
Stewart, J.W. et al. Prevention of mouse skin tumor promotion by dietary energy restriction requires an intact adrenal gland and glucocorticoid supplementation restores inhibition. Carcinogenesis 26, 1077–1084 (2005).
Benjamin, C.L. & Ananthaswamy, H.N. p53 and the pathogenesis of skin cancer. Toxicol. Appl. Pharmacol. 224, 241–248 (2007).
Bos, J.L. ras oncogenes in human cancer: a review. Cancer Res. 49, 4682–4689 (1989).
Kwei, K.A., Finch, J.S., Ranger-Moore, J. & Bowden, G.T. The role of Rac1 in maintaining malignant phenotype of mouse skin tumor cells. Cancer Lett. 231, 326–338 (2006).
Hirakawa, S. et al. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 109, 1010–1017 (2007).
Casanova, M.L. et al. A critical role for ras-mediated, epidermal growth factor receptor-dependent angiogenesis in mouse skin carcinogenesis. Cancer Res. 62, 3402–3407 (2002).
Hirakawa, S. et al. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J. Exp. Med. 201, 1089–1099 (2005).
Hoot, K.E. et al. Keratinocyte-specific Smad2 ablation results in increased epithelial-mesenchymal transition during skin cancer formation and progression. J. Clin. Invest. 118, 2722–2732 (2008).
Naito, M., Naito, Y. & DiGiovanni, J. Comparison of the histological changes in the skin of DBA/2 and C57BL/6 mice following exposure to various promoting agents. Carcinogenesis 8, 1807–1815 (1987).
DiGiovanni, J. et al. Further genetic analyses of skin tumor promoter susceptibility using inbred and recombinant inbred mice. Carcinogenesis 13, 525–531 (1992).
Stern, M.C. et al. Analysis of two inbred strains of mice derived from the SENCAR stock with different susceptibility to skin tumor progression. Carcinogenesis 19, 125–132 (1998).
DiGiovanni, J., Slaga, T.J. & Boutwell, R.K. Comparison of the tumor-initiating activity of 7,12-dimethylbenz[a]anthracene and benzo[a]pyrene in female SENCAR and CD-1 mice. Carcinogenesis 1, 381–389 (1980).
Boutwell, R.K. The biochemistry of preneoplasia in mouse skin. Cancer Res. 36, 2631–2635 (1976).
Slaga, T.J. Overview of tumor promotion in animals. Environ. Health Perspect. 50, 3–14 (1983).
Slaga, T. & Nesnow, S. SENCAR mouse skin tumorigenesis. in Handbook of Carcinogen Testing (eds. Milman, H.A. & Weisburger, E.K.) 230–250 (Noyes Publication, Park Ridge, IL, 1985).
Markel, P. et al. Theoretical and empirical issues for marker-assisted breeding of congenic mouse strains. Nat. Genet. 17, 280–284 (1997).
Haseman, J.K. Statistical issues in the design, analysis and interpretation of animal carcinogenicity studies. Environ. Health Perspect. 58, 385–392 (1984).
Festing, M.F. Design and statistical methods in studies using animal models of development. ILAR J. 47, 5–14 (2006).
Subcommittee on Laboratory Animal Nutrition, C.o.A.N., Board on Agriculture, National Research Council. Nutrient Requirements of Laboratory Animals, (National Academy Press, Washington, DC, 1995).
Virador, V.M. et al. The human promyelocytic leukemia protein is a tumor suppressor for murine skin carcinogenesis. Mol. Carcinog. 48, 599–609 (2008).
Santos, M. et al. Susceptibility of pRb-deficient epidermis to chemical skin carcinogenesis is dependent on the p107 allele dosage. Mol. Carcinog. 47, 815–821 (2008).
Roop, D.R., Krieg, T.M., Mehrel, T., Cheng, C.K. & Yuspa, S.H. Transcriptional control of high molecular weight keratin gene expression in multistage mouse skin carcinogenesis. Cancer Res. 48, 3245–3252 (1988).
Nischt, R. et al. Aberrant expression during two-stage mouse skin carcinogenesis of a type I 47-kDa keratin, K13, normally associated with terminal differentiation of internal stratified epithelia. Mol. Carcinog. 1, 96–108 (1988).
Gimenez-Conti, I. et al. Early expression of type I K13 keratin in the progression of mouse skin papillomas. Carcinogenesis 11, 1995–1999 (1990).
Aldaz, C.M. et al. Sequential development of aneuploidy, keratin modifications, and gamma-glutamyltransferase expression in mouse skin papillomas. Cancer Res. 48, 3253–3257 (1988).
Diaz-Guerra, M. et al. Expression of simple epithelial cytokeratins in mouse epidermal keratinocytes harboring Harvey ras gene alterations. Cancer Res. 52, 680–687 (1992).
Larcher, F. et al. Aberrant expression of the simple epithelial type II keratin 8 by mouse skin carcinomas but not papillomas. Mol. Carcinog. 6, 112–121 (1992).
Mehrel, T. et al. Identification of a major keratinocyte cell envelope protein, loricrin. Cell 61, 1103–1112 (1990).
Steven, A.C., Bisher, M.E., Roop, D.R. & Steinert, P.M. Biosynthetic pathways of filaggrin and loricrin–two major proteins expressed by terminally differentiated epidermal keratinocytes. J. Struct. Biol. 104, 150–162 (1990).
Bickenbach, J.R., Greer, J.M., Bundman, D.S., Rothnagel, J.A. & Roop, D.R. Loricrin expression is coordinated with other epidermal proteins and the appearance of lipid lamellar granules in development. J. Invest. Dermatol. 104, 405–410 (1995).
Chiba, M., Maley, M.A. & Klein-Szanto, A.J. Sequential study of gamma-glutamyltransferase during complete and two-stage mouse skin carcinogenesis. Cancer Res. 46, 259–263 (1986).
Takeichi, M. Morphogenetic roles of classic cadherins. Curr. Opin. Cell Biol. 7, 619–627 (1995).
Margulis, A. et al. Loss of intercellular adhesion activates a transition from low- to high-grade human squamous cell carcinoma. Int. J. Cancer 118, 821–831 (2006).
Brouxhon, S. et al. Sequential down-regulation of E-cadherin with squamous cell carcinoma progression: loss of E-cadherin via a prostaglandin E2-EP2 dependent posttranslational mechanism. Cancer Res. 67, 7654–7664 (2007).
Holden, P.R., McGuire, B., Stoler, A., Balmain, A. & Pitts, J.D. Changes in gap junctional intercellular communication in mouse skin carcinogenesis. Carcinogenesis 18, 15–21 (1997).
Kim, D.J. et al. Targeted disruption of Bcl-x(L) in mouse keratinocytes inhibits both UVB- and chemically induced skin carcinogenesis. Mol. Carcinog. 23 March 2009 (Epub ahead of print).
Darwiche, N. et al. Expression profile of skin papillomas with high cancer risk displays a unique genetic signature that clusters with squamous cell carcinomas and predicts risk for malignant conversion. Oncogene 26, 6885–6895 (2007).
Hennings, H., Shores, R., Mitchell, P., Spangler, E.F. & Yuspa, S.H. Induction of papillomas with a high probability of conversion to malignancy. Carcinogenesis 6, 1607–1610 (1985).
Hennings, H. et al. Malignant conversion of mouse skin tumours is increased by tumour initiators and unaffected by tumour promoters. Nature 304, 67–69 (1983).
Hennings, H. et al. Enhanced malignant conversion of benign mouse skin tumors by cisplatin. J. Natl. Cancer Inst. 82, 836–840 (1990).
Abel, E. & DiGiovanni, J. Environmental carcinogenesis. in The Molecular Basis of Cancer (eds. Mendelsohn, J., Howley, P.M., Israel, M., Gray, J.W. & Thompson, C.B.) 91–113 (Elsevier, Philadelphia, 2008).
DiGiovanni, J., Kruszewski, F.H. & Chenicek, K.J. Modulation of chrysarobin skin tumor promotion. Carcinogenesis 9, 1445–1450 (1988).
Slaga, T.J. SENCAR mouse skin tumorigenesis model versus other strains and stocks of mice. Environ. Health Perspect. 68, 27–32 (1986).
Reiners Jr., J.J. & Singh, K.P. Susceptibility of 129/SvEv mice in two-stage carcinogenesis protocols to 12-O-tetradecanoylphorbol-13-acetate promotion. Carcinogenesis 18, 593–597 (1997).
Naito, M. & DiGiovanni, J. Genetic background and development of skin tumors. Carcinog. Compr. Surv. 11, 187–212 (1989).
Naito, M., Chenicek, K.J., Naito, Y. & DiGiovanni, J. Susceptibility to phorbol ester skin tumor promotion in (C57BL/6 × DBA/2) F1 mice is inherited as an incomplete dominant trait: evidence for multi-locus involvement. Carcinogenesis 9, 639–645 (1988).
Sundberg, J.P., Sundberg, B.A. & Beamer, W.G. Comparison of chemical carcinogen skin tumor induction efficacy in inbred, mutant, and hybrid strains of mice: morphologic variations of induced tumors and absence of a papillomavirus cocarcinogen. Mol. Carcinog. 20, 19–32 (1997).
Bol, D.K. et al. Cyclooxygenase-2 overexpression in the skin of transgenic mice results in suppression of tumor development. Cancer Res. 62, 2516–2521 (2002).
Moore, T. et al. Reduced susceptibility to two-stage skin carcinogenesis in mice with low circulating insulin-like growth factor I levels. Cancer Res. 68, 3680–3688 (2008).
Acknowledgements
This work was supported by NIH grants ES015718, ES016623, CA076520, CA37111, CA016672 and the National Institute of Environmental Health Sciences Center Grant ES007784. We would like to thank the Histology and Tissue Processing Core Facility for their technical assistance in immunohistochemical analyses and Dr. J. Rundhaug for providing tumor samples. We also thank S. Johnson for her assistance in the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
E.L.A. and J.D. were responsible for overall manuscript writing including compilation of the supporting data and procedure descriptions. J.M.A. was responsible for compiling and describing the data in Table 1. K.K. was responsible for creating and describing the results of Figure 2. Numerous laboratory groups and individuals have contributed to the design and refinement of the two-stage skin carcinogenesis protocol in mice.
Corresponding author
Rights and permissions
About this article
Cite this article
Abel, E., Angel, J., Kiguchi, K. et al. Multi-stage chemical carcinogenesis in mouse skin: Fundamentals and applications. Nat Protoc 4, 1350–1362 (2009). https://doi.org/10.1038/nprot.2009.120
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.120
This article is cited by
-
Tumor-derived interleukin-1 receptor antagonist exhibits immunosuppressive functions and promotes pancreatic cancer
Cell & Bioscience (2023)
-
Tissue memory relies on stem cell priming in distal undamaged areas
Nature Cell Biology (2023)
-
Sulforaphane Functionalized Chitosan Nanoparticles Serve as an Effective Nanocomplex System for the Treatment of Human Cervical Cancer
Journal of Cluster Science (2023)
-
Three-dimensional in vitro culture models in oncology research
Cell & Bioscience (2022)
-
Synthetic lethal kinases in Ras/p53 mutant squamous cell carcinoma
Oncogene (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.