Abstract
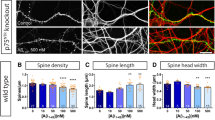
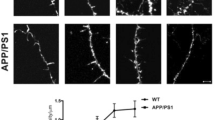
Excessive synaptic loss is thought to be one of the earliest events in Alzheimer's disease. Amyloid beta (Aβ), a peptide secreted in an activity-modulated manner by neurons, has been implicated in the pathogenesis of Alzheimer's disease by removing dendritic spines, sites of excitatory synaptic transmission. However, issues regarding the subcellular source of Aβ, as well as the mechanisms of its production and actions that lead to synaptic loss, remain poorly understood. In rat organotypic slices, we found that acute overproduction of either axonal or dendritic Aβ reduced spine density and plasticity at nearby (∼5–10 μm) dendrites. The production of Aβ and its effects on spines were sensitive to blockade of action potentials or nicotinic receptors; the effects of Aβ (but not its production) were sensitive to NMDA receptor blockade. Notably, only 30–60 min blockade of Aβ overproduction permitted induction of plasticity. Our results indicate that continuous overproduction of Aβ at dendrites or axons acts locally to reduce the number and plasticity of synapses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Terry, R.D. et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30, 572–580 (1991).
Walsh, D.M. & Selkoe, D.J. Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron 44, 181–193 (2004).
Lacor, P.N. et al. Synaptic targeting by Alzheimer's-related amyloid beta oligomers. J. Neurosci. 24, 10191–10200 (2004).
Hsieh, H. et al. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron 52, 831–843 (2006).
Shankar, G.M. et al. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci. 27, 2866–2875 (2007).
Shrestha, B.R. et al. Amyloid beta peptide adversely affects spine number and motility in hippocampal neurons. Mol. Cell. Neurosci. 33, 274–282 (2006).
Calabrese, B. et al. Rapid, concurrent alterations in pre- and postsynaptic structure induced by naturally-secreted amyloid-beta protein. Mol. Cell. Neurosci. 35, 183–193 (2007).
Evans, N.A. et al. Abeta(1–42) reduces synapse number and inhibits neurite outgrowth in primary cortical and hippocampal neurons: a quantitative analysis. J. Neurosci. Methods 175, 96–103 (2008).
Lacor, P.N. et al. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J. Neurosci. 27, 796–807 (2007).
Lanz, T.A., Carter, D.B. & Merchant, K.M. Dendritic spine loss in the hippocampus of young PDAPP and Tg2576 mice and its prevention by the ApoE2 genotype. Neurobiol. Dis. 13, 246–253 (2003).
Jacobsen, J.S. et al. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. USA 103, 5161–5166 (2006).
Spires, T.L. et al. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J. Neurosci. 25, 7278–7287 (2005).
Kamenetz, F. et al. APP processing and synaptic function. Neuron 37, 925–937 (2003).
Snyder, E.M. et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat. Neurosci. 8, 1051–1058 (2005).
Almeida, C.G. et al. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol. Dis. 20, 187–198 (2005).
Ting, J.T., Kelley, B.G., Lambert, T.J., Cook, D.G. & Sullivan, J.M. Amyloid precursor protein overexpression depresses excitatory transmission through both presynaptic and postsynaptic mechanisms. Proc. Natl. Acad. Sci. USA 104, 353–358 (2007).
Walsh, D.M. et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 (2002).
Chapman, P.F. et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat. Neurosci. 2, 271–276 (1999).
Stéphan, A., Laroche, S. & Davis, S. Generation of aggregated beta-amyloid in the rat hippocampus impairs synaptic transmission and plasticity and causes memory deficits. J. Neurosci. 21, 5703–5714 (2001).
Cleary, J.P. et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat. Neurosci. 8, 79–84 (2005).
Klyubin, I. et al. Amyloid beta protein immunotherapy neutralizes Abeta oligomers that disrupt synaptic plasticity in vivo. Nat. Med. 11, 556–561 (2005).
Hartman, R.E. et al. Treatment with an amyloid-beta antibody ameliorates plaque load, learning deficits and hippocampal long-term potentiation in a mouse model of Alzheimer's disease. J. Neurosci. 25, 6213–6220 (2005).
Walsh, D.M. et al. Certain inhibitors of synthetic amyloid beta-peptide (Abeta) fibrillogenesis block oligomerization of natural Abeta and thereby rescue long-term potentiation. J. Neurosci. 25, 2455–2462 (2005).
Morgan, D. et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature 408, 982–985 (2000).
Kopec, C.D., Li, B., Wei, W., Boehm, J. & Malinow, R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J. Neurosci. 26, 2000–2009 (2006).
Engert, F. & Bonhoeffer, T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399, 66–70 (1999).
Maletic-Savatic, M., Malinow, R. & Svoboda, K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science 283, 1923–1927 (1999).
Matsuzaki, M., Honkura, N., Ellis-Davies, G.C. & Kasai, H. Structural basis of long-term potentiation in single dendritic spines. Nature 429, 761–766 (2004).
Cirrito, J.R. et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron 48, 913–922 (2005).
Buckner, R.L. et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J. Neurosci. 25, 7709–7717 (2005).
Lazarov, O., Lee, M., Peterson, D.A. & Sisodia, S.S. Evidence that synaptically released beta-amyloid accumulates as extracellular deposits in the hippocampus of transgenic mice. J. Neurosci. 22, 9785–9793 (2002).
Buxbaum, J.D. et al. Alzheimer amyloid protein precursor in the rat hippocampus: transport and processing through the perforant path. J. Neurosci. 18, 9629–9637 (1998).
Koo, E.H. et al. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc. Natl. Acad. Sci. USA 87, 1561–1565 (1990).
Ferreira, A., Caceres, A. & Kosik, K.S. Intraneuronal compartments of the amyloid precursor protein. J. Neurosci. 13, 3112–3123 (1993).
Sisodia, S.S., Koo, E.H., Hoffman, P.N., Perry, G. & Price, D.L. Identification and transport of full-length amyloid precursor proteins in rat peripheral nervous system. J. Neurosci. 13, 3136–3142 (1993).
Xia, W. et al. A specific enzyme-linked immunosorbent assay for measuring beta-amyloid protein oligomers in human plasma and brain tissue of patients with Alzheimer disease. Arch. Neurol. 66, 190–199 (2009).
Kaether, C., Skehel, P. & Dotti, C.G. Axonal membrane proteins are transported in distinct carriers: a two-color video microscopy study in cultured hippocampal neurons. Mol. Biol. Cell 11, 1213–1224 (2000).
Shankar, G.M. et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat. Med. 14, 837–842 (2008).
Otmakhov, N. et al. Forskolin-induced LTP in the CA1 hippocampal region is NMDA receptor dependent. J. Neurophysiol. 91, 1955–1962 (2004).
Changeux, J.P., Kasai, M. & Lee, C.Y. Use of a snake venom toxin to characterize the cholinergic receptor protein. Proc. Natl. Acad. Sci. USA 67, 1241–1247 (1970).
Small, S.A. & Gandy, S. Sorting through the cell biology of Alzheimer's disease: intracellular pathways to pathogenesis. Neuron 52, 15–31 (2006).
Sheng, J.G., Price, D.L. & Koliatsos, V.E. Disruption of corticocortical connections ameliorates amyloid burden in terminal fields in a transgenic model of Abeta amyloidosis. J. Neurosci. 22, 9794–9799 (2002).
Klyubin, I. et al. Amyloid beta protein dimer-containing human CSF disrupts synaptic plasticity: prevention by systemic passive immunization. J. Neurosci. 28, 4231–4237 (2008).
Jones, I.W., Barik, J., O'Neill, M.J. & Wonnacott, S. Alpha bungarotoxin-1.4 nm gold: a novel conjugate for visualising the precise subcellular distribution of alpha 7* nicotinic acetylcholine receptors. J. Neurosci. Methods 134, 65–74 (2004).
Fabian-Fine, R. et al. Ultrastructural distribution of the alpha7 nicotinic acetylcholine receptor subunit in rat hippocampus. J. Neurosci. 21, 7993–8003 (2001).
Dineley, K.T., Bell, K.A., Bui, D. & Sweatt, J.D. Beta-amyloid peptide activates alpha 7 nicotinic acetylcholine receptors expressed in Xenopus oocytes. J. Biol. Chem. 277, 25056–25061 (2002).
Dani, J.A. & Bertrand, D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 47, 699–729 (2007).
Raschetti, R., Albanese, E., Vanacore, N. & Maggini, M. Cholinesterase inhibitors in mild cognitive impairment: a systematic review of randomized trials. PLoS Med. 4, e338 (2007).
Coan, E.J., Irving, A.J. & Collingridge, G.L. Low-frequency activation of the NMDA receptor system can prevent the induction of LTP. Neurosci. Lett. 105, 205–210 (1989).
Molnár, Z. et al. Enhancement of NMDA responses by beta-amyloid peptides in the hippocampus in vivo. Neuroreport 15, 1649–1652 (2004).
Acknowledgements
We thank members of the Malinow laboratory for helpful discussions, J. Huang for careful reading of the manuscript and N. Dawkins and I. Hunton for expert technical assistance. This work was supported by grants from the US National Institutes of Health (R.M.), the Cure Alzheimer's Fund (R.M.), Eisai (H.H.) and the Leslie C. Quick Fellowship (W.W.).
Author information
Authors and Affiliations
Contributions
W.W., L.N.N., H.W.K. and H.H. designed and conducted the experiments and analyzed data. S.S. and R.M. designed experiments and supervised the project.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 662 kb)
Rights and permissions
About this article
Cite this article
Wei, W., Nguyen, L., Kessels, H. et al. Amyloid beta from axons and dendrites reduces local spine number and plasticity. Nat Neurosci 13, 190–196 (2010). https://doi.org/10.1038/nn.2476
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2476
This article is cited by
-
Synaptic degeneration in Alzheimer disease
Nature Reviews Neurology (2023)
-
Amyloid β-based therapy for Alzheimer’s disease: challenges, successes and future
Signal Transduction and Targeted Therapy (2023)
-
Physiological Roles of β-amyloid in Regulating Synaptic Function: Implications for AD Pathophysiology
Neuroscience Bulletin (2023)
-
Implications of fractalkine on glial function, ablation and glial proteins/receptors/markers—understanding its therapeutic usefulness in neurological settings: a narrative review
Future Journal of Pharmaceutical Sciences (2022)
-
Enhanced activity of Alzheimer disease-associated variant of protein kinase Cα drives cognitive decline in a mouse model
Nature Communications (2022)